Temperature and pH responsive 3D printed scaffolds†
Abstract
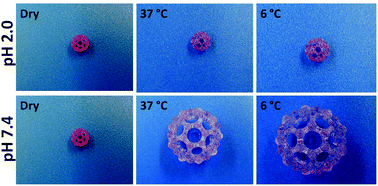
This study focused on developing novel materials for 3D printed reverse thermo-responsive (RTR) and pH-sensitive structures, using the stereolithography (SLA) technique and demonstrates the double responsiveness of the constructs printed. Methacrylated poly(ethylene oxide)–poly(propylene oxide)–poly(ethylene oxide) (PEO–PPO–PEO) triblocks are the basic building blocks of the RTR hydrogels, while acrylic acid is responsible for imparting also pH sensitivity to the 3D printed gels. The water absorption behavior and dimensional changes exhibited by the different 3D printed hydrogel constructs, strongly depended on the temperature and pH, varying also as a function of their composition and concentration. All the hydrogels showed a fast and reversible swelling–deswelling response, as they fluctuated between pH 2.0 and pH 7.4 behavior. Structures comprising two different dually responsive hydrogels were 3D printed and their environmentally sensitive dimensional behavior was reported.


 Please wait while we load your content...
Please wait while we load your content...