Zinc ion mediated synthesis of cuprous oxide crystals for non-enzymatic glucose detection†
Abstract
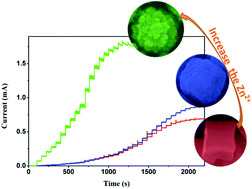
Morphology control is expected to be an effective method to enhance the electrochemical properties of materials. In this work, zinc cation-mediated growth of Cu2O crystals was achieved via an aqueous chemical route at room temperature. Thus, by simply increasing the concentration of Zn2+, concave cube-like (C-Cu2O), porous (P-Cu2O), and hierarchical (H-Cu2O) Cu2O crystals were selectively obtained. The morphologies and structures of the as-prepared Cu2O crystals were characterized by SEM, TEM, XRD and XPS. The three materials were subsequently employed as active materials for the non-enzymatic detection of glucose. The H-Cu2O-based electrode exhibited the highest sensitivity (3076 μA mM−1 cm−2) in virtue of its highest surface area, while the P-Cu2O-based electrode showed the widest linear range (up to 24 mM). The reliability of the Cu2O-based glucose sensors was proved by determining their detection limit, response time, selectivity, and stability characteristics on human serum samples. This work provides a novel strategy for the morphology-controlled Zn2+-mediated fabrication of Cu2O crystals with different glucose sensing performances depending on their structures.


 Please wait while we load your content...
Please wait while we load your content...