Photoactive antimicrobial nanomaterials
Abstract
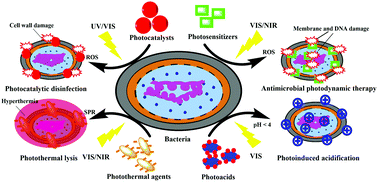
Pathogenic microbes cause infections and are excellent at adapting to the disinfection strategies that address their biochemistry. The growth in number of drug resistant superbugs is alarming, and has attracted attention to alternative solutions. In recent years, researchers have put effort into designing nanomaterials with activatable antimicrobial properties initiated by light irradiation. The underlying mechanisms of these nanomaterials' photoactivatable antimicrobial effect may vary from reactive oxygen species generation, to heat production or pH variation in procedures like photocatalysis, photodynamic therapy, photothermal lysis and photoinduced acidification. In this article, we review the photoactive nanomaterial solutions for fighting against microbial diseases, especially bacterial infections. This Review shines light on the fundamental principles, important developments, promising applications, and limitations of current technologies as well as open questions that these methods may answer or help to answer.
- This article is part of the themed collection: Recent Review Articles


 Please wait while we load your content...
Please wait while we load your content...