Cobalt phosphide nanowire array as an effective electrocatalyst for non-enzymatic glucose sensing†
Yiwei
Liu
ab,
Xiaoqin
Cao
ab,
Rongmei
Kong
c,
Gu
Du
d,
Abdullah M.
Asiri
e,
Qun
Lu
*a and
Xuping
Sun
 *b
*b
aDepartment of Chemistry and Chemical Engineering, School of Life Science and Engineering, Southwest Jiaotong University, Chengdu 610031, Sichuan, China. E-mail: luqun1125@home.swjtu.edu.cn
bCollege of Chemistry, Sichuan University, Chengdu 610064, Sichuan, China. E-mail: sunxp@scu.edu.cn
cCollege of Chemistry and Chemical Engineering, Qufu Normal University, Qufu 273165, Shandong, China
dChengdu Institute of Geology and Mineral Resources, Chengdu 610081, Sichuan, China
eChemistry Department, King Abdulaziz University, Jeddah 21589, Saudi Arabia
First published on 13th February 2017
Abstract
It is of significant importance for the design of electrodes to construct enhanced electrochemical sensing platforms, and a nanoarray offers an ideal architecture for the detection of molecules. In this communication, we demonstrate that cobalt phosphide nanowire array grown in situ on titanium mesh (CoP NA/TM) exhibits high catalytic activity towards electrooxidation of glucose. As a non-enzymatic electrochemical glucose sensor, this CoP NA/TM catalytic electrode possesses superior analytical performance with a short response time of less than 5 s, a wide linear range of 0.0005–1.5 mM, a low detection limit of 0.1 μM (S/N = 3), a high sensitivity of 5168.6 μA mM−1 cm−2, and a remarkable selectivity and long-term stability for glucose detection. We further demonstrate the successful use of such glucose biosensors in human blood serum and fruit juice.
The modern investigation of pathology and medicine has demonstrated that in patients with diabetes, the risk of renal, retinal and neural complications is directly related to the chronic elevation of blood glucose.1 Thus, it is of considerable importance to develop fast and reliable methods for glucose determination. The first enzymatic electrochemical glucose biosensor was reported by Clark and Lyons in 1962.2 Despite the high sensitivity and selectivity demonstrated, such sensors suffer from the high cost of the enzyme, the difficult enzyme immobilization process, and the degradation of the activity.3 Noble metals and their metal alloys possess high catalytic activity for non-enzymatic glucose electrooxidation,4–9 but the high cost of such catalysts limits their widespread applications. Therefore, it is highly attractive to develop earth-abundant nanostructures for electrocatalytic glucose sensing.
Cobalt is an interesting transition metal with catalytic activity towards glucose electrooxidation. In recent years, Co-based oxides and hydroxides have received extensive attention for glucose detection.10–13 An ideal glucose electrocatalyst should satisfy the following three requirements: (1) good electrical conductivity for enhanced electronic transfer; (2) an abundance of exposed catalytic sites for efficient catalysis; (3) easy accessibility for the electrolyte and target molecules. Transition metal phosphides are interstitial alloys with superior electrical conductivity and cobalt phosphide nanostructures have been intensively studied as remarkable electrocatalysts for water splitting.14–16 Recently, Sun et al. have reported the immobilization of CoP nanorods on a glassy carbon electrode using a polymer binder for electrochemical glucose detection, but this sensor shows a high detection limit of 9 μM.17 It is well-established that the direct growth of a nanoarray catalyst on a current collector offers a 3D catalytic electrode with obvious advantages, including strong mechanical adhesion, effective electrical connection between the catalyst and current collector, and exposure of more active sites.14,15 Moreover, the polymer binder-free feature for such a nanoarray electrode also effectively avoids blocking of the active sites.18 It is thus expected that a CoP nanoarray would provide superior sensing performance over reported CoP nanorods.17 However, this has not been previously explored.
In this communication, we demonstrate that CoP nanowire array on Ti mesh (CoP NA/TM) possesses high catalytic activity towards glucose electrooxidation. As a 3D non-enzymatic glucose sensor, this CoP NA/TM catalyst electrode shows a short response time of less than 5 s, a wide linear range of 0.0005–1.5 mM, a low detection limit of 0.1 μM (S/N = 3), and a high sensitivity of 5168.6 μA mM−1 cm−2, as well as satisfactory reproducibility, stability and selectivity. Furthermore, it also performs efficiently for glucose detection both in a human blood sample and fruit juice.
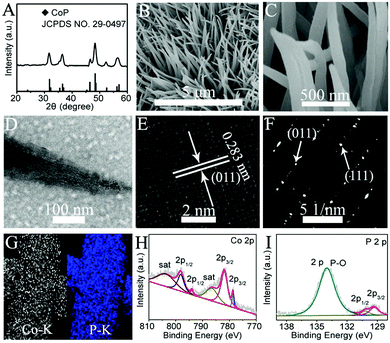
Fig. 1A shows the XRD pattern of the phosphide product scratched down from the Ti mesh. These peaks located at 31.6°, 32.0°, 35.3°, 36.3°, 36.7°, 46.2°, 48.1°, 48.4°, 52.3°, 56.0°, and 56.8° can be indexed to the (011), (002), (200), (111), (102), (112), (211), (202), (103), (020), and (301) crystal planes of CoP (JCPDS No. 29-0497), respectively. The low-magnification SEM image of CoP NA/TM shows that the entire surface of the TM was uniformly coated with CoP nanowire (Fig. 1B). The high-magnification SEM image (Fig. 1C) further reveals that such nanowires have diameters of about 100–200 nm and are a few micrometers in length. TEM analysis (Fig. 1D) suggests that the CoP nanowire is porous with a rough surface. The high-resolution TEM (HRTEM) image taken from such a nanowire reveals well-resolved lattice fringes with an interplane spacing of 0.283 nm corresponding to the crystal face of (011) planes of CoP (Fig. 1E). The diffraction spots in the selected area electron diffraction (SAED) pattern (Fig. 1F) recorded from the CoP nanowire can be identified as the (111) and (011) planes of an orthorhombic CoP structure.19 The energy-dispersive X-ray spectroscopy (EDX) elemental mapping images (Fig. 1G) reveal the uniform distribution of both Co and P elements. The XPS spectra in the Co 2p (Fig. 1H) and P 2p (Fig. 1I) regions are quite consistent with previous reports.20–25
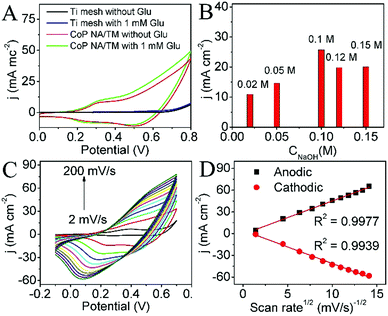
CoP NA/TM was directly utilized as a 3D working electrode for electrochemical glucose detection with platinum wire as the counter electrode and Hg/HgO as the reference electrode. Fig. 2A shows the cyclic voltammograms (CVs) of the bare titanium mesh and CoP NA/TM in 0.1 M NaOH with the absence and presence of glucose at a scan rate of 50 mV s−1 in the potential range from 0 to 0.8 V. In the absence of glucose, no significant redox peaks are observed for bare TM (black curve), which suggests that it is electrochemically silent in the potential range. In contrast, two pairs of redox peaks (red curve) are attributed to the redox reaction of CoP on the electrode surface, which could be assigned to the Co2+/Co3+ and Co3+/Co4+ redox couples. Previous work reported that anodic scanning leads to CoOx on the CoP surface in basic media.26 It is reasonable to conclude that the above peaks arise from the surface faradaic reaction between CoOx and CoOOH. Indeed, the XPS analysis in Fig. S1 (ESI†) demonstrates the formation of a cobalt ion. In the presence of 1 mM glucose, the bare titanium mesh causes a negligible oxidation current density change (blue curve) while CoP NA/TM produces a notable increase in the anodic peak current density (green curve). These observations suggest that CoP NA/TM is efficient for the electrooxidation of glucose. The current related to the redox couple Co2+/Co3+ remains nearly constant in the presence of glucose whereas the anodic peak current related to the oxidation of Co3+ species increases, suggesting that the electrooxidation of glucose is mainly catalyzed by CoOOH/CoO2 rather than Co(OH)2/CoOOH. The mechanism may be expressed according to the following reactions:27,28
| CoOOH + OH− → CoO2 + H2O + e− | (1) |
| CoO2 + glucose + H2O → CoOOH + OH− + gluconolactone | (2) |
Fig. 2B shows the electrochemical responses of CoP NA/TM towards 1 mM glucose in different concentration of NaOH. With an increase of NaOH concentration from 0.02 M to 0.1 M, the peak current increases continuously, and then decreases when the concentration increases from 0.1 M to 0.15 M. Thus, 0.1 M NaOH was chosen as the optimal electrolyte solution in our experiments. The CVs for CoP NA/TM at different scan rates were acquired in 0.1 M NaOH containing 1 mM glucose. It is shown that both the anodic and cathodic peak currents increase in the range of 2 to 200 mV s−1 (Fig. 2C). Fig. 2D shows a good liner relationship between the anodic and cathodic peak currents and the square root of the scan rate, indicating a diffusion-controlled process of glucose oxidation on CoP NA/TM.29,30
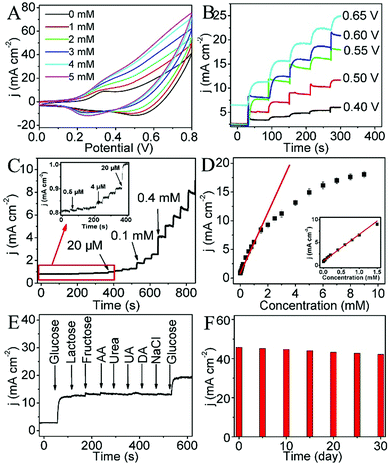
Fig. 3A shows the CVs of the CoP NA/TM electrode in 0.1 M NaOH solution containing different concentrations of glucose (0–5 mM) at a scan rate of 50 mV s−1. Upon the successive addition of glucose, a remarkable increase of current and potential for anodic peaks is observed. The increase of the anodic peak currents contributes to the excellent electrocatalytic activity of CoP NA/TM towards glucose oxidation. Fig. 3B shows the amperometric current response of CoP NA/TM in 0.1 M NaOH with the continuous addition of 0.5 mM glucose at different potentials (ranging from 0.40 to 0.65 V). Remarkable current response was observed with the increase of the applied potential, but the background current is obvious at 0.65 V. Thus, we chose 0.6 V as the optimum potential in the following experiments. The amperometric responses of CoP NA/TM were measured and are shown in Fig. 3C with the successive addition of glucose in 0.1 M NaOH. This electrode exhibited a fast amperometric response toward glucose and achieved steady state current density within 5 s. The slight baseline drift after multiple injections of glucose may be ascribed to small variation of the local pH, faster consumption of glucose than its diffusion, or the adsorption of intermediates on the active sites. The calibration curve of this glucose sensor (Fig. 3D) exhibited a linear range of 0.0005–1.5 mM, and the sensor had a sensitivity of 5168.6 μA mM−1 cm−2 and a detection limit of 0.1 μM at a signal-to-noise ratio of 3 (S/N = 3). These results compare favourably to the behaviours of most reported electrochemical glucose sensors (Table S1, ESI†).
Selectivity is an important parameter for glucose sensors because human blood contains many compounds including lactose, fructose, ascorbic acid (AA), urea, uric acid (UA), dopamine (DA) and NaCl. The interference experiment is performed by successive addition of 1 mM glucose and 2 mM of other interferences in 0.1 M NaOH. It can be clearly seen in Fig. 3E that CoP NA/TM has a remarkable response for glucose but negligible response towards the interfering species, and the current density increases again with another addition of glucose. These results demonstrate the superior selectivity of CoP NA/TM towards glucose. Long-time stability of CoP NA/TM has also been investigated. After being exposed to air for one month, the CoP NA/TM electrode shows about 92% of its initial response current (Fig. 3F), therefore demonstrating a good long-time stability. The reproducibility of the CoP NA/TM electrode was tested by measuring the response of five CoP NA/TM parallel electrodes with 1 mM glucose in 0.1 M NaOH. The relative standard deviation of the anode peak current densities is 3.4%, suggesting an excellent reproducibility.
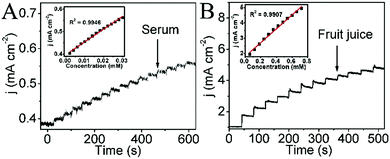
To verify its feasibility for routine analysis, the sensor was applied to the determination of glucose in human blood serum samples and fruit juice (the human blood sample was obtained from a blood bank in Sichuan provincial people's hospital (Sichuan, Chengdu)). Since the blood glucose levels of normal persons are in the range of 4 to 6 mM, the original glucose concentration of a human blood sample is hypothesized as 5 mM. The original glucose concentration of fruit juice is about 600 mM according to the nutritional information. Fig. 4A and B show the amperometric responses of CoP NA/TM with the successive addition of human blood serum and fruit juice in 0.1 M NaOH, and the insets exhibit the corresponding calibration curve, respectively. All of the above indicate that the object electrode is promising in practical analysis.
In summary, the CoP nanoarray has been demonstrated as a 3D catalytic electrode for the effective electrooxidation of glucose. This non-enzymatic glucose sensor shows a short response time, a low limit of detection, a wide linear range and high sensitivity, as well as long-term stability, good reproducibility and high selectivity. Its application for real sample analysis has also been demonstrated successfully. This study provides us with an attractive, low-cost nanoarray, which can be used as an efficient 3D electrochemical sensor for detection of glucose and other small molecules.31–34
References
- A. P. Turner, B. Chen and S. Piletsky, Clin. Chem., 1999, 45, 1596–1601 CAS.
- L. C. Clark and C. Lyons, Ann. N. Y. Acad. Sci., 1962, 102, 29–45 CrossRef CAS PubMed.
- S. Peak, H. Boo and T. D. Chung, Anal. Chim. Acta, 2006, 556, 46–57 CrossRef PubMed.
- G. Chang, H. Shu, Q. Huang, M. Oyama, K. Ji, X. Liu and Y. He, Electrochim. Acta, 2015, 157, 149–157 CrossRef CAS.
- H. Shu, K. G. Chang, J. Su, L. Cao, Q. Huang, Y. Zhang, T. Xia and Y. He, Sens. Actuators, B, 2015, 220, 331–339 CrossRef CAS.
- C. Shan, H. Yang, D. Han, Q. Zhang, A. Ivaska and L. Niu, Biosens. Bioelectron., 2010, 25, 1070–1074 CrossRef CAS PubMed.
- Y. Song, C. Zhu, H. Li, D. Du and Y. Lin, RSC Adv., 2015, 5, 82617–82622 RSC.
- J.-S. Ye, C.-W. Chen and C.-L. Lee, Sens. Actuators, B, 2015, 208, 569–574 CrossRef CAS.
- J. Yang, X. Liang, L. Cui, H. Liu, J. Xie and W. Liu, Biosens. Bioelectron., 2016, 80, 171–174 CrossRef CAS PubMed.
- Z. Gao, L. Zhang, C. Ma, Q. Zhou, Y. Tang, Z. Tu, W. Yang, L. Cui and Y. Li, Biosens. Bioelectron., 2016, 80, 511–518 CrossRef CAS PubMed.
- K. Khun, Z. Ibupoto, X. Liu, V. Beni and M. Willander, Mater. Sci. Eng., B, 2015, 194, 94–100 CrossRef CAS.
- T. Chen, X. Li, C. Qiu, W. Zhu, H. Ma, S. Chen and O. Meng, Biosens. Bioelectron., 2014, 53, 200–206 CrossRef CAS PubMed.
- Q. Wang, Y. Ma, X. Jiang, N. Yang, Y. Coffinier, H. Belkhalfa, N. Dokhane, M. Li, R. Boukherroub and S. Szunerits, Electroanalysis, 2016, 28, 119–125 CrossRef CAS.
- P. Jiang, Q. Liu, Y. Liang, J. Tian, A. M. Asiri and X. Sun, Angew. Chem., Int. Ed., 2014, 53, 12855–12859 CrossRef CAS PubMed.
- J. Tian, Q. Liu, A. M. Asiri and X. Sun, J. Am. Chem. Soc., 2014, 136, 7587–7590 CrossRef CAS PubMed.
- Q. Liu, J. Tian, W. Cui, P. Jiang, N. Cheng, A. M. Asiri and X. Sun, Angew. Chem., Int. Ed., 2014, 53, 6710–6714 CrossRef CAS PubMed.
- Q. Sun, M. Wang, S. Bao, Y. Wang and S. Gu, Analyst, 2016, 141, 256–260 RSC.
- J. D. Roy-Mayhew, G. Boschloo, A. Hagfeldt and I. A. Aksay, ACS Appl. Mater. Interfaces, 2012, 4, 2794–2800 CAS.
- Y. Li, M. A. Malik and P. O'Brien, J. Am. Chem. Soc., 2005, 127, 16020–16021 CrossRef CAS PubMed.
- A. P. Grosveno, S. D. Wik, R. G. Cavell and A. Mar, Inorg. Chem., 2005, 44, 8988–8998 CrossRef PubMed.
- T. I. Korányi, Appl. Catal., A, 2003, 239, 253–267 CrossRef.
- S. J. Sawhill, K. A. Layman, D. R. Van Wyk, M. H. Engelhard, C. Wang and M. E. Bussell, J. Catal., 2005, 231, 300–313 CrossRef CAS.
- H. Li, P. Yang, D. Chu and H. Li, Appl. Catal., A, 2007, 325, 34–40 CrossRef CAS.
- Practical Surface Analysis by Auger and X-ray Photoelectron Spectroscopy, ed. D. Briggs and M. P. Seah, John Wiley & Sons, New York, 1983 Search PubMed.
- A. W. Burns, K. A. Layman, D. H. Bale and M. E. Bussell, Appl. Catal., A, 2008, 343, 68–76 CrossRef CAS.
- J. Chang, Y. Xiao, M. Xiao, J. Ge, C. Liu and W. Xing, ACS Catal., 2015, 5, 6874–6878 CrossRef CAS.
- H. Yu, J. Jin, X. Jian, Y. Wang and G. Qi, Electroanalysis, 2013, 25, 1665–1674 CrossRef CAS.
- Y. Song, C. Wei, J. He, X. Li, X. Lu and L. Wang, Sens. Actuators, B, 2015, 220, 1056–1063 CrossRef CAS.
- S. S. Mahshid, S. Mahshid, A. Dolati, M. Ghorbani, L. Yang, S. Luo and Q. Cai, J. Alloys Compd., 2013, 554, 169–176 CrossRef CAS.
- M. Ranjani, Y. Sathishkumar, Y. S. Lee, D. J. Yoo, A. R. Kim and G. Gnana kumar, RSC Adv., 2015, 5, 57804–57814 RSC.
- T. Chen, D. Liu, W. Lu, K. Wang, G. Du, A. M. Asiri and X. Sun, Anal. Chem., 2016, 88, 7885–7889 CrossRef CAS PubMed.
- Z. Wang, X. Cao, D. Liu, S. Hao, G. Du, A. M. Asiri and X. Sun, Chem. Commun., 2016, 52, 14438–14441 RSC.
- D. Liu, T. Chen, W. Zhu, L. Cui, A. M. Asiri, Q. Lu and X. Sun, Nanotechnology, 2016, 27, 33LT01 CrossRef PubMed.
- C. Tang, R. Zhang, W. Lu, Z. Wang, D. Liu, S. Hao, G. Du, A. M. Asiri and X. Sun, Angew. Chem., Int. Ed., 2017, 56, 842–846 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental section and supplementary figure. See DOI: 10.1039/c6tb02882a |
| This journal is © The Royal Society of Chemistry 2017 |