Shaped stimuli-responsive hydrogel particles: syntheses, properties and biological responses
Bing
Xue
a,
Veronika
Kozlovskaya
a and
Eugenia
Kharlampieva†
*ab
aChemistry Department, University of Alabama at Birmingham, AL 35294, USA
bCenter for Nanomaterials and Biointegration, University of Alabama at Birmingham, AL 35294, USA
First published on 17th November 2016
Abstract
The ability to create nano- and micro-sized hydrogel matrices of well-defined shapes can provide a powerful means not only to mimic the key properties of biological systems, but also to regulate shape-dependent particle biodistribution and cellular association, and to correspondingly optimize drug delivery carriers. This review focuses on stimuli-responsive hydrogel particles of non-spherical shapes ranging from filled porous networks to hollow capsules. We summarize a pool of current experimental approaches and discuss perspectives in the development of the synergistic combination of shape and stimuli-response in particulate hydrogels. Recent advances in the design and synthesis of the pH-, redox-, temperature-sensitive, mechanical force-, magnetic- and enzyme-responsive hydrogel particles of non-spherical shapes are presented. Examples of existing and emerging technologies for creating a variety of shapes with controlled hydrogel composition and size are highlighted. We also discuss the effects of shape on the physiochemical properties of these particles as well as their shape-regulated biological interactions including particle circulation time and biodistribution.
1. Introduction
Hydrogels are three-dimensional polymeric networks with permanent (covalent) or temporary (physical) cross-links that are able to swell upon uptake of large amounts of water and which have demonstrated great utility for drug delivery and tissue engineering applications.1–3 Hydrogels with non-spherical shapes have been receiving increasing attention in biomedical applications due to their ability to mimic the shape of natural biological objects and their shape-regulated biological interactions.4,5 Depending on the distribution of material density in the network, shaped hydrogel particles can be either filled or hollow (capsular) where, in the former case, polymer is distributed throughout the hydrogel volume or, as in the latter, exists only in the particle shell with a hollow interior volume. Filled hydrogel particles can provide expanded surface area for cargo loading, while their environmentally triggered volume transition can provide on-demand delivery of molecular therapeutics.6 In this case, the bulk mechanical modulus is tuned by the chemical composition and cross-linking density and allows imitation of the elasticity ranges of living cell tissues which is useful for advanced tissue engineering.7 Conversely, hollow hydrogels may exhibit a distinguishable hierarchical/heterogeneous architecture, resembling the structure of a cell, which can facilitate the development of versatile artificial cells and help in fundamental understanding of cell behavior in flow.8,9Shaped hydrogel particulates can be produced in macro-, micro- and nano-sizes. Macrogels can be useful as shape memory materials which alter their shape in response to environmental stimuli including pH, ionic strength, and temperature.10–12 For instance, macrogel scaffolds with a well-defined structure have been designed for localized drug delivery and are important for observing and modulating biological activities of cells, e.g., stem cells or bone cells, under various conditions.13,14 In the case of microgels and nanogels, their colloidal nature renders them exceptionally beneficial in solving biomedical challenges. Thus, for example, the small dimensions of micro- or nano-gels constrain their biological interaction to a cellular or subcellular level by which negative side effects to the surrounding tissues are minimized.15
The integration of a well-defined shape into micro/nanoscale soft materials can provide a powerful means not only to mimic the key properties of biological systems but also to regulate shape-dependent particle biodistributions and cellular association, and to correspondingly optimize drug delivery carriers.16–18 For example, bovine serum albumin (BSA) hydrogels of biconcave discoidal shape mimicking that of red blood cells (RBCs) could carry oxygen and reversibly deform to pass through capillaries smaller than the diameter of the hydrogels.16 Microgel particles with low modulus and the size and shape of RBCs showed prolonged circulation time in vivo.17,18 In another example, the discoid shape of polyethylene glycol (PEG) hydrogels promoted better internalization by mammalian epithelial and immune cells in contrast to hydrogel nanorods.19 Moreover, the threshold pressure differential indicating the critical pressure differential at which 50% of particles pass through the capillary tube was demonstrated to vary by four orders of magnitude for hydrogel particles upon changing the shape and cross-link density, where a discoid shape was the least deformable and the S-shape demonstrated the highest flexibility when passing through a constricted channel, which affected the particle circulation time and bio-distribution.20
Recently, engineering of shaped particles has been demonstrated successfully by using methods of Particle Replication In Non-wetting Template (PRINT), Step and Flash Imprint Lithography (S-FIL) and Microfluid Continuous Lithography which provide hydrogel particulates of a precisely controlled size and shape.21–24 However, these methods lack a finer control over hydrogel cross-link densities which may render the generation of stimuli-responsive hydrogels with distinctive reversible changes in dimension or shape challenging. Hydrogel particles obtained by these technologies either may be highly cross-linked and therefore incapable of a stimuli-triggered shape reconfiguration, or their shape is irreversibly dissociated upon environmental triggering. Very recently, layer-by-layer (LBL) assembly of polymers onto/into sacrificial particulate templates has been demonstrated to be useful for yielding shaped hydrogels with pre-programmed stimuli-responsiveness and mechanical robustness allowing precise control over the interior structures and mechanical properties of hydrogel particles of micrometer and sub-micrometer sizes.25,26
The objective of this review is to focus the attention on stimuli-responsive hydrogel particles of non-spherical shapes, the existing and novel emerging methods for their fabrication, and effects of their shape on the physiochemical properties of the particles as well as the biological responses. We highlight recent developments of the pH-, redox-, temperature-sensitive mechanical force-, magnetic- and enzyme-responsive hydrogel particles of non-spherical shapes for drug delivery and biomedical applications. This review illustrates some noteworthy examples and discusses perspectives in the development of the synergistic combination of shape and stimuli-response in particulate hydrogels for the design of novel ‘smart’ drug delivery systems.
2. Synthesis of microgel particles of non-spherical shape
2.1. Particle replication in non-wetting template
Particle Replication In Non-wetting Template (PRINT) is among the most advanced top-down technologies for fabricating hydrogel particles, and it has been utilized frequently for the design and synthesis of monodisperse micro- and nano-particles in large scalable quantities.21–23 As a unique soft lithography technique, PRINT can be used to generate hydrogel particles with precisely defined size, shape, chemical composition, and surface functionalities.27 A classic PRINT particle nano-molding process is illustrated in Fig. 1.28 A silicon substrate template is generated by standard photolithography with a pre-designed pattern on a 2-dimensional (2D) array. The PRINT mold can be obtained by pouring liquid photocurable perfluoropolyethers (PFPEs) onto the template and curing it in specific patterns. The elastomeric PFPE mold can preserve all micro- and nano-featured negative patterns on the template after curing and solidification because of the positive spreading coefficient of the liquid PFPE on all surfaces. Desired materials, including monomers, initiators and cross-linkers can then be filled into the cavities without wetting the surrounding areas. However, photo-sensitive initiators such as diphenyl (2,4,6-trimethylbenzoyl) phosphine oxide (TPO),24 2-hydroxyl-1-[4-(hydroxyl)phenyl]-2-methyl-1-propanone (I2959),29 and 2,2-diethoxyacetophenone (DEAP)30 are necessary to trigger polymerization reactions which are initiated by UV light and yield shaped hydrogel particle arrays on the PFPE templates. Because of the higher surface energy with the counter-sheet film of polyethylene terephthalate (PET), the produced hydrogel particles can then be transferred from PFPE films and harvested upon dissolving the sacrificial films by the roll-to-roll process.21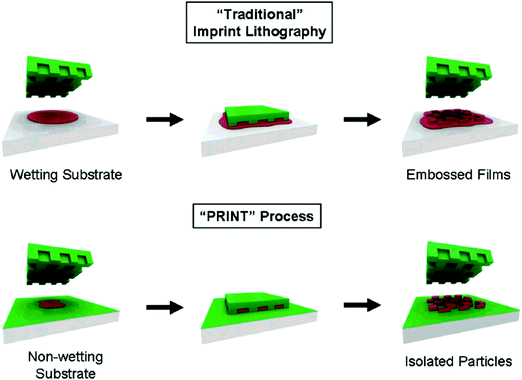 | ||
| Fig. 1 Illustration of the PRINT process in comparison to traditional imprint lithography in which the affinity of the liquid precursor for the surface results in a scum layer. In the PRINT process, the non-wetting nature of fluorinated materials and surfaces (shown in green) confines the liquid precursor inside the features of the mold, allowing for the generation of isolated particles. Reproduced with permission from ref. 28. Copyright © 2005 American Chemical Society. | ||
To date, the majority of hydrogel particles generated by PRINT are cross-linked by poly(ethylene glycol) diacrylate (PEGDA) and poly(silyl ether). The functionality and cargo encapsulation of the hydrogel particle matrix can be tuned by varying the ratio of cross-linkers to monomers as well as particle sizes and the shapes. Thus, for example, DeSimone and colleagues demonstrated the use of the PRINT method for the synthesis of PEG-based rectangular hydrogel nanoparticles from a tetraethylene glycol monoacrylate monomer and a PEGDA cross-linker.28 The size of the hydrogel rectangles was shown to be precisely controlled with the particles having a 80 nm width but a varied length ranging from 180 to 5000 nm.31 The deformability of synthesized hydrogels could be accurately controlled by changing the cross-linker concentration. For instance, the 80 nm wide and 180 nm long particles with 96 wt% PEGDA retained their shape when dried unlike the particles produced with 2 wt% PEGDA which flattened and lost their shape demonstrating enhanced deformability. Atomic force microscopy (AFM) analysis revealed that the particle width decreased while its height increased upon increasing the hydrogel cross-link density.31
The PRINT particles can be imparted with biologically active functionalities by attachment of functional moieties or by introducing functional cross-linkers. For instance, the PEG hydrogels obtained from succinimidyl succinate monomethoxy-modified PEG were used to incorporate siRNA into the hydrogel matrix.32 The siRNA molecules were demonstrated to successfully attach to cylindrical PEG hydrogels having 200 nm length and diameter via free amine groups.32 PRINT hydrogels for tumor targeting therapy were produced from dimethylaminoethyl acrylate (DMAEA) using poly(silyl ether) as a cross-linker.30 In this case, the C–O–Si(R)2–O–C linkage in the bifunctional poly(silyl ether) can be hydrolyzed in acidic environments and therefore can render the particles biodegradable ensuring sustained therapeutic release from the cross-linked hydrogels.
2.2 Step and flash imprint lithography
Step and Flash Imprint Lithography (S-FIL) is a traditional lithography technique that specifically utilizes a quartz imprinting template to generate hydrogel nanoparticles with controlled dimensions.33 In a typical S-FIL process, a sacrificial layer of poly(vinyl alcohol) (PVA) is applied to bottom anti-reflective coating silicon wafers followed by the mixture containing monomers, photo-initiators and cross-linkers. The quartz template is then pressed onto PEGDA followed by exposure to UV light for polymerization. Unreacted PEGDA is removed by rinsing with DMSO after oxygen plasma etching. The polymerized particles are collected by dissolving the PVA sacrificial layer in water. Unlike the PRINT process that mostly relies on shear forces and a high surface energy with the counter sheet, S-FIL technology provides an exceptional convenience by utilizing a water-soluble sacrificial layer to release and harvest the hydrogel particles.34,35A key feature of this method is the ability to vary the cross-link density and composition of the particle matrix using different macromers and macromer concentrations. Varying the concentration or molecular weight of the macromer solution does not significantly affect the size or shape of the particles but affects the dispensing efficiency of the solution. The S-FIL method can produce particles of various shapes with the minimal size down to 50 nm. For example, Roy et al. generated scalable amounts of cubical, discoid and rod-like PEG hydrogel particles with various swelling ratios by adjusting the concentration of PEGDA and using I2959 as an initiator.19,29,33 Apart from functionalizing the hydrogel particles by direct molecule attachment as mentioned for the PRINT particles, the S-FIL approach also provides another modification site, a sacrificial water-soluble polymer layer. Besides the PVA layer, poly(acrylic acid) (PAA) recently has been introduced as a sacrificial layer due to its ion sensitivity in aqueous solutions. For instance, switching from the PAA soluble to insoluble state in sodium and calcium ion solutions, respectively, enables a better release of the produced hydrogel particles from the template and therefore greatly improves the process's scalability with the benefit of being a one-step imprinting technology with high efficiency.34
By combining PRINT and S-FIL approaches, a novel method of sequential patterning with responsive micromolds has been recently developed.36 Temperature-responsive poly(N-isopropylacrylamide) (PNIPAM) microgels were first synthesized through PRINT technology, and then used as dynamic templates for the fabrication of agarose microgels via the imprint method. Using the PRINT technique, a PNIPAM template was molded to different shapes, including micro-grooves, circular and square micro-wells.36 Unlike the static templates used in traditional PRINT and S-FIL methods, the PNIPAM template also exhibited a dimensional change in response to temperature which enabled agarose microgels to achieve multi-compartmental structures (Fig. 2). The core–shell microgels with separate spatial distribution of red or green microbeads were obtained by sequentially adding agarose precursor containing microbeads into PNIPAM templates at a different temperature followed by agarose gelation (Fig. 2). In addition, prepared agarose microgels replicated the shapes of PNIPAM templates leading to differently shaped microstructures, e.g., cylindrical or cubical. This combined approach allows the microgels to acquire more complicated structures without sacrificing their shape.36
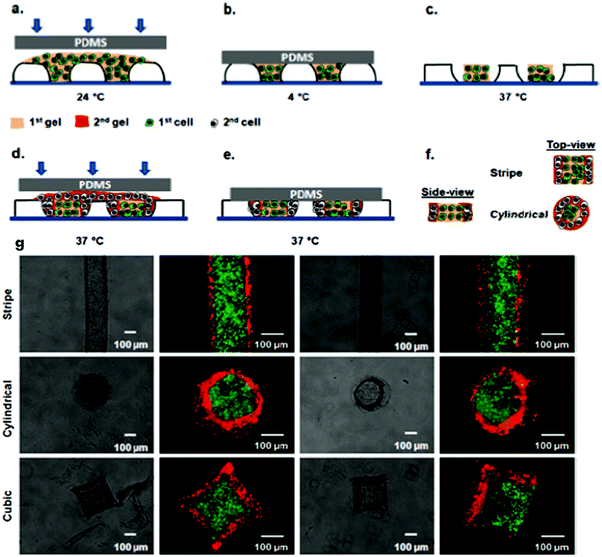 | ||
| Fig. 2 Top: Schematic diagram of sequential patterning of hydrogel microstructures with responsive micromolds. Fluorescent microbeads or cells (3T3 fibroblasts, HepG2 cells, and HUVECs) were encapsulated within agarose microgels during the fabrication process. (a) A gel precursor with encapsulated cells or microbeads is placed on a responsive micromold and molded with a PDMS slab at 24 °C. (b) Gel cross-linking at 4 °C for 15 min. (c) Shrinkage of responsive micromolds at 37 °C for 30 min. (d) A second gel precursor with encapsulated cells or microbeads is placed on a responsive micromold and molded with a PDMS slab at 37 °C. (e) Cross-linking of the second gel at 37 °C for 30 min. (f) Side and top views of obtained hydrogel microstructures. Bottom: (g) Phase contrast and fluorescence images for stripe, cylindrical, and cubic microgels recovered from responsive micromolds. Reproduced with permission from ref. 36. Copyright © American Chemical Society. | ||
2.3 Flow lithography
Microfluidics-based methods have been developed for continuous hydrogel particle synthesis and can be multiplexed for a higher throughput with less effort in collecting samples. The microfluidic continuous flow lithography (CFL) technique provides a single-step route to synthesize monodisperse hydrogel particulates. Instead of exclusively relying on UV light to initiate polymerization used in the PRINT method, CFL provides flexibility by allowing other cross-linking methods.37 In a typical procedure, poly(dimethylsiloxane) (PDMS) is used to form a phase mask with a defined shape to facilitate a 3-dimensional (3D) light intensity and a microfluidic channel for the flow of a pre-polymer solution. The photo-polymerization is initiated by UV light passing through the shaped phase mask which leads to monodisperse hydrogels with a defined 3D structure.38,39Doyle and colleagues demonstrated a wide variety of shaped PEG cross-linked particles obtained from PEGDA mixed with a photosensitive initiator.40 Particle arrays of mask-defined shapes were generated by exposing the flowing oligomer to controlled pulses of UV light. Due to the rapid polymerization kinetics, the hydrogel particles could be formed within 0.1 s, and an oxygen-aided inhibition near the PDMS surfaces favored the particle flow within a non-polymerized oligomer stream. Using this approach, the particle shape can be controlled in two directions. In the x–y plane, a transparency mask with designed features determines the flat particle shape, while the height of the channel can be adjusted in the z-axis to control the particle height dimension.40
Highly curved bullet-shaped PEG hydrogel particles with magnetic functionalities were obtained by Hwang and colleagues using a variation of the microfluidic stop flow lithography (SFL) method.41 The magnetic polymeric hydrogel particles were synthesized from a mixture of PEGDA, the 2-hydroxy-2-methylpropiophenone initiator and a water-based ferrofluid.41 In addition, temperature-sensitive microgels composed of PNIPAM and alginate were achieved by using modified capillary microfluidic devices in which monodisperse spherical PNIPAM/alginate droplets were synthesized in a microfluidic channel and later changed their initial shape during cross-linking in a hot collection solution (45 °C, barium/glycerol). The final shape of the microgels could be tailored to a Janus particulate structure when the initial size of droplets was 150 μm, and to mushroom-like in the case of 300 μm droplets. The Janus particles exhibited distinct surface areas, while mushroom-like particles were shown to possess anisotropic architectures along with variable curvatures. These uniquely structured hydrogels can potentially be used in the design of stimuli-responsive drug delivery carriers for biomedical applications.42
Despite its many achievements, the continuous flow lithography method still has limitations including high costs of microscopy equipment, a decreased resolution of output particles at high flow rates, and a relatively low number of produced particles per light exposure. To address these issues, the Doyle group has advanced this technique further into contact flow lithography to fabricate shaped hydrogel particles efficiently and with lower costs.43 In the improved system, the photomask was tightened to a UV source and the distance between the mask and a microfluidic device was minimized enabling focusing of the UV light on a defined area to yield hydrogel particles of better resolved dimensions (20–50 μm).43 Using contact flow lithography, shaped particles deposited on surfaces or free-floating in the microfluidic channel can be obtained. Remarkably, after finely tuning the microfluidic multi-channel in the contact flow lithography system, in which the long sinuous channel was substituted with grouped short multi-channels while a splitting design was applied to equally split a monomer solution from a single inlet to the parallel channels, the particle synthesis rate increased by 100-fold compared to the standard continuous flow lithography approach without compromising the shape resolution of the prepared hydrogel particles. The triangular, cubical and rectangular particles maintained their sharp edges and straight side walls.43 Overall, this method provides new opportunities for the industrial scale-up of shaped hydrogels of high quality and should be able to further promote their applications.
2.4 Layer-by-layer assembly of polymers
The layer-by-layer (LbL) assembly of polymers at surfaces is a method of creating multilayer coatings at solid/solvent interfaces where one type of polymer chain can interact with another type via ionic-pairing, hydrogen-bonding, host–guest, and covalent and/or hydrophobic interactions when deposited on surfaces in a stepwise manner.44 When this polymer assembly method is applied to surfaces of colloidal organic, inorganic or biological (cells, bacteria) particles which can be later dissolved, polymeric particles replicating the size and shape of a sacrificial template can be obtained (Table 1).45–47 A high degree of control over composition, nanoscale thickness, mechanical and stimuli-responsive properties of the resultant polymeric particles is among the main advantages of this method.48–54 Conventionally, this method of polymer assembly onto particulates relies on centrifugation of the particles to separate excess polymer in solution from coated particulates followed by several rinsing steps with polymer-free solvent. When a desired number of polymer pairs (bilayers) is assembled on particle surfaces, the particulate template is dissolved or decomposed leading to a polymeric replica of the template. Because of the multistep process, this method can be time-consuming in the case when a large number of polymer bilayers is required, and may also suffer from partial colloid coagulation/aggregation resulting in partial loss of the colloids. To circumvent the issues of the time required for the polymer multilayer deposition and for an industrial scale-up, various approaches to automate the polymer deposition and increase the coated particle throughput have been developed by the Caruso group including electrophoretic polymer assembly,55 immersive polymer assembly on immobilized particles,56 a fluidized-bed assembly (Fig. 3),57 and the flow-based assembly using tangential flow filtration (Fig. 4),58 as well as a continuous ionic LbL assembly method using a tubular flow type reactor.59| Shape | Template | Template decomposition | Ref. |
|---|---|---|---|
| Cubical | CdCO3; MnCO3; CaCO3 | 0.1 M HCl | 73–77 |
| Tetrahedral | SnS | 0.5 M HCl | 78 |
| Rod | SiO2 | HF | 79 |
| Polystyrene particles | THF | 80 | |
| Ellipsoid | Bacteria E. coli | NaCl + NaOCl | 45 |
| CaCO3 | HCl | 77 | |
| Discoid | Si particles | HF + HNO3 | 75 and 81 |
| Echinocyte | Red blood cells | NaCl + NaOCl | 45 |
| Dodecahedral | Metal organic frameworks | EDTA | 82 |
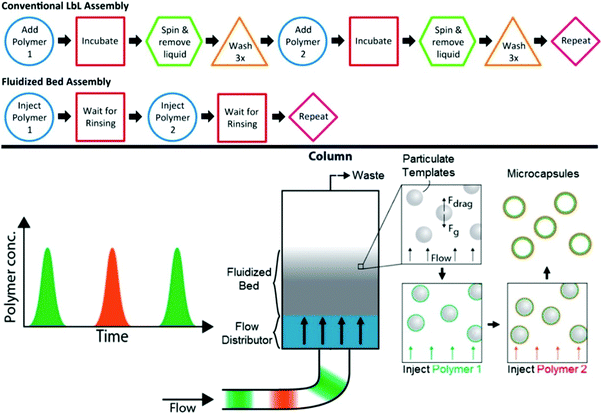 | ||
| Fig. 3 Top: Comparison of the steps required for conventional LbL assembly versus fluidized bed assembly to deposit a polymer bilayer. Bottom: Illustration of the flow stream, fluidized bed and microcapsule formation. The fluidized bed is formed in the column when the force of gravity on the template particles is balanced by the drag force of the flow. Polymer solution can be injected into the flow and is distributed upon entering the column so that the template particles can be uniformly layered. After a sufficiently thick film is generated, the template particles can be removed to yield microcapsules. Reproduced with permission from ref. 57. Copyright © 2014 American Chemical Society. | ||
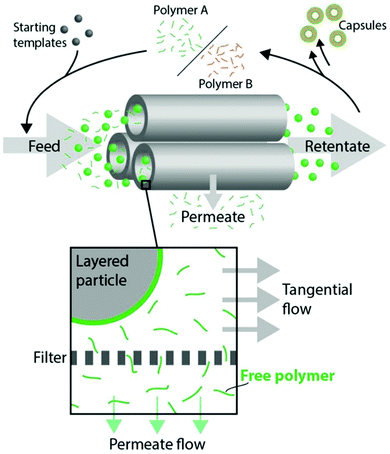 | ||
| Fig. 4 Hollow fiber tangential flow filtration (TFF) LbL assembly of capsules. Particulate templates are mixed with polymer A and, after incubation, purified using TFF. The layered and purified particles can then be mixed with the complementary polymer B to create a second layer. This process of TFF purification and LbL film deposition can then be repeated, yielding core–shell particles or capsules after core dissolution. Reproduced with permission from ref. 58. Copyright © 2015 American Chemical Society. | ||
The polymer LbL assemblies might not fully represent the hydrogel-like structures unless one or both polymer components of the multilayer are covalently linked. In this case, the multilayer can resemble a hydrogel structure when exposed to conditions causing high swelling of polymeric networks. The polyelectrolyte capsule wall in which the polymer chains are held together via temporary physical links can exhibit stimuli-triggered polymer chain rearrangements which can lead to capsule volume alterations, and changes in capsule properties.60 For example, in ionically-paired poly(styrene sulfonate)/poly(allylamine hydrochloride) (PSS/PAH) capsules, electrostatic repulsions between accumulated excess charges lead to wall swelling, softening, increased permeability, and changes in capsule dimensions followed by capsule dissolution.61,62 Hydrogen-bonded systems, such as poly(methacrylic acid)/poly(N-vinyl pyrrolidone) (PMAA/PVPON), are stable at low pH but dissolve at elevated pH due to PMAA ionization. Therefore, in the case of multilayer hydrogel capsules, hydrogen-bonded or ionically paired LbL films as initial platforms are exposed to thermal-, photo-, or chemical cross-linking at the post-assembly step.63,64 Depending on the cross-linking method, single- or multi-polymer component hydrogels can be obtained.65–67 The covalent links stabilize the hydrogel wall upon environmentally-triggered core dissolution, while functional groups which are not involved in binding provide stimuli-responsive behavior.68–70 The resulting multilayer hydrogel capsules represent a unique example of fluid encapsulated by a stimuli-responsive nanothin hydrogel wall.71,72
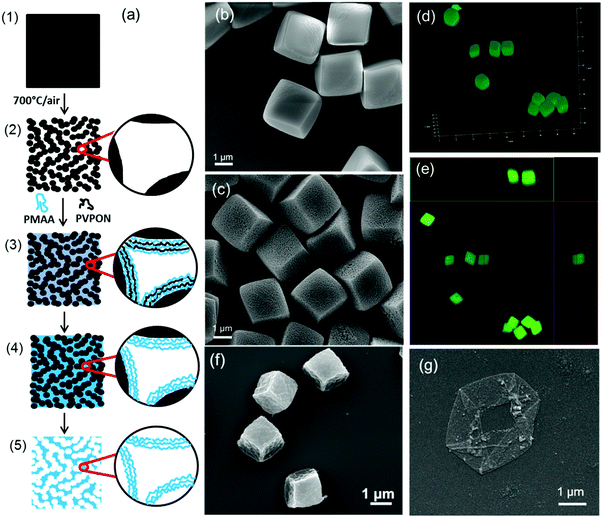 | ||
| Fig. 5 (a) Non-porous MnCO3 cubic particles (1) transformed into porous Mn2O3 cubic particles (2) upon heating at 700 °C in air. Hydrogen-bonded PMAA/PVPON complexes formed inside pores of PEI-coated templates (3) were cross-linked with ethylenediamine to form an interconnected PMAA hydrogel network (4). After core dissolution, responsive hydrogel cubes of interconnected PMAA networks are formed (5). SEM images of MnCO3 (b) and porous Mn2O3 (c) cubic inorganic templates. Three-dimensional reconstructions of consecutive focal plane confocal microscopy images of 2 μm cubic hydrogels of PMAA (d) with their corresponding orthogonal (side panel) view (e) imaged in 0.01 M phosphate buffer solutions at pH = 7.4. SEM images of cubic (PMAA)5 multilayer hydrogel particles (f) and capsules (g). Adopted with permission from ref. 25, Copyright © 2014 Royal Society of Chemistry and from ref. 26, Copyright © 2015 American Chemical Society. | ||
In contrast to multilayer hydrogel capsules which collapse upon drying and lose their non-spherical solution shape, the PMAA network of a multilayer-derived hydrogel particle is ‘packed’ throughout the volume of the particle, therefore providing an enormous area of interaction for various species in contrast to that in a capsule which has only a thin hydrogel shell. Moreover, these hydrogel particles preserve their three-dimensional structure when dried because of their enhanced rigidity (Fig. 5f and g).25,26 It is worth noting that when multilayer hydrogel capsules and particles are done through LBL, cross-linkers are not limited to photo-sensitive molecules like in the PRINT method which may broaden their future applications in biomedicine and biotechnology.
3. Stimuli-sensitive properties of non-spherical hydrogel particles
3.1. pH Sensitivity
Acidity in biological environments can vary significantly which is widely exploited for designing pH-sensitive particulate hydrogels for the delivery of therapeutics. For example, the extracellular pH decreases in the tumor space due to the high rate of aerobic glycolysis down to pH ∼ 6.1–5.6.90,91 In addition, the acidic pH can render tumors resistant to some anticancer drugs because of increased drug polarity which may prevent their crossing of biological barriers92 and therefore may warrant their encapsulation into delivery vehicles.49,93 Also, intracellular release of therapeutics from pH-sensitive hydrogel drug carriers can be designed based on acidification of cellular organelles when they progress along the endocytic pathway from early to late endosomes, and further to late lysosomes with the corresponding acidity values decreasing from pH = 6.5 to pH < 6.0, and to pH < 5.5, respectively.94 These pH variations are used to design pH-responsive hydrogel particles for site-specific drug delivery.The typical structure of a pH-sensitive hydrogel contains ionizable pendant groups which undergo reversible ionization at different pH values leading to pH-dependent particle size changes.95 In general, synthetic polymer-based anionic hydrogels are synthesized from acidic polyacids including poly(acrylic acid) (PAA), PMAA, and poly(ethacrylic acid) (PEAA) or their copolymers, which swell at pH greater than their pKa values due to the osmotic pressure of ions.96 Cationic hydrogels derived from poly(N,N-dimethylaminoethyl methacrylate) or other primary amine-containing polymers become polar and swell at pH < pKa.97,98 The hydrogel volume changes induced by the environmental pH shifts can be used for drug loading and release under hydrogel swelling and collapse, respectively.
In one example, cubic PMAA multilayer hydrogel microparticles showed pH-dependent swelling ratios of 1.7, defined as the ratio of swollen to deswollen hydrogel particle size.25 Hydrogel cubes made from 21 kDa PMAA increased in size from 1.9 ± 0.2 μm to 3.2 ± 0.1 μm when the solution pH was changed from pH = 5 to pH = 7.4 (Fig. 6). Similarly, cubic hydrogels of 360 kDa PMAA changed from 1.9 ± 0.2 μm to 3.1 ± 0.2 μm, resembling the previous pH variations and indicating no effect of PMAA molecular weight on cubic hydrogel pH-responsive behavior. The pH-triggered volume changes were completely reversible. Remarkably, these hydrogels retained their cubic shape upon pH change and preserved their cubic geometry in the swollen state at pH = 7.4 indicating uniform swelling/shrinkage of the networks. Both cubic and spherical hydrogels underwent a nearly 2-fold reversible swelling/shrinkage response to pH variations and retained their shape while increasing in size, providing remarkable examples of pH-responsive shaped hydrogels. The swelling behavior of these cubic and spherical hydrogel particles is controlled by the network structure, which can be regulated by the PMAA molecular weight and the template properties.
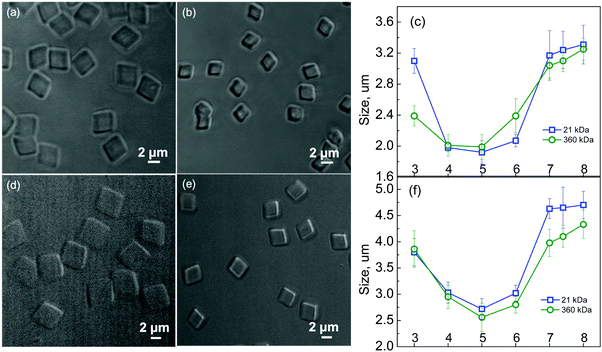 | ||
| Fig. 6 Left: Optical microscopy images of 2 μm cubic (21 kDa PMAA) (a and b), 4 μm cubic (21 kDa PMAA) (d and e) particles imaged at pH = 7.4 (a and d) and at pH = 5 (b and e). Right: pH-Induced size variations of 2 μm cubic (c) and 4 μm cubic PMAA hydrogel particles (f) produced from 21 kDa or 360 kDa PMAA. The size measurements were performed using confocal microscopy. Reproduced with permission from ref. 25. Copyright © 2014 Royal Society of Chemistry. | ||
Apart from using ionizable polymers, the use of pH-cleavable network linkers can confer pH-responsiveness along with pH-induced degradability. The DeSimone group has demonstrated the synthesis of silyl ether-containing monomers which were molded into cubic or hexnut microgel particles using the PRINT technique (Fig. 7).30 The particle degradation and the encapsulated drug release rate were shown to be precisely controlled by introducing various substituent groups to the Si atom. All hydrogel particles underwent pH-induced degradation showing a significant acceleration in degradation rates at pH = 5 compared to those at pH = 7 regardless of the substituent groups used for the modification. The bulkiness of the substituent groups, however, affected the microgel particle degradation at a fixed pH leading to a prolonged degradation time. For example, by using dimethyl, diethyl and diisopropyl substituent groups, the degradation of PRINT hydrogel cubes could be tuned from several hours to several weeks at pH = 5.30 Notably, the silyl ether hydrogels swelled and then degraded to lose their distinctive hexnut shape after internalization by HeLa cells while non-degradable hexnut hydrogels retained their original size and shape inside the cells. The silyl ether chemistry was used to conjugate a drug molecule to the shaped PRINT hydrogels for controlled drug delivery, and the release of the drug was also regulated by changing the solution pH and the substituents around the Si atom (Fig. 8).99 The controlled intracellular drug release was further verified through the apoptosis and proliferation assessment of LNCaP cells. The ethyl-substituted silyl ether prodrug-hydrogel exhibited comparable cytotoxicity as the free drug, indicating successful drug transport into cancer cells after 72 h incubation. In contrast, the tert-butyl substitution on the Si atom led to negligible drug release from the hydrogel, resulting in nontoxicity to LNCaP cells at the same incubation time (Fig. 8).99
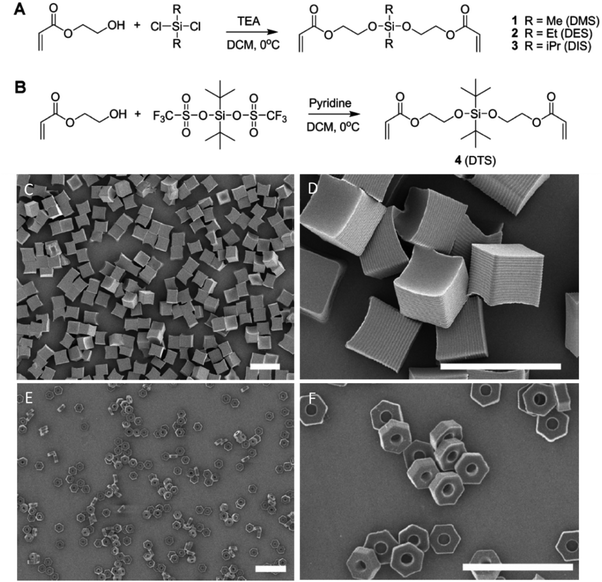 | ||
| Fig. 7 (A and B) Synthesis of bifunctional silyl ether cross-linkers. Scanning electron micrographs of (C and D) 5 μm cubes and (E and F) 3 μm hexnut particles fabricated using the PRINT process (scale bars are 10 μm). Reproduced with permission from ref. 30. Copyright © 2010 American Chemical Society. | ||
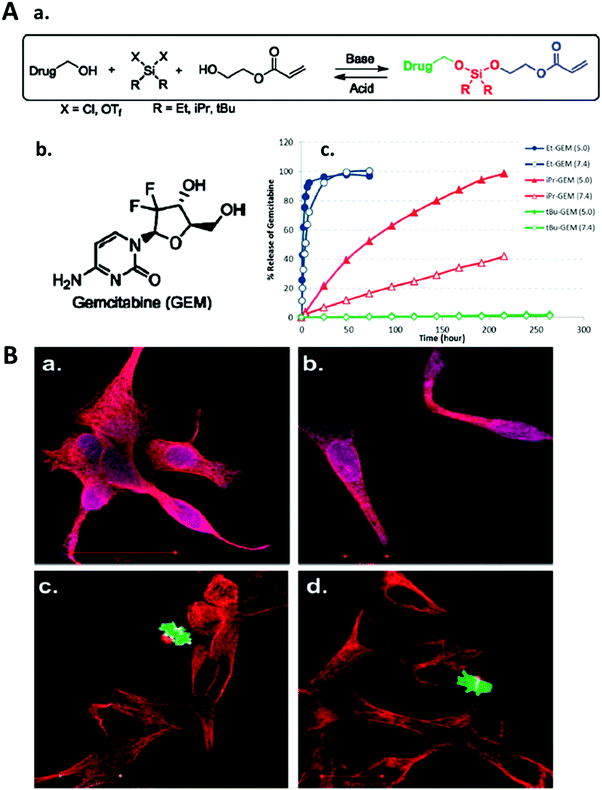 | ||
| Fig. 8 (A) (a) Asymmetric bifunctional silyl ether prodrugs of gemcitabine; (b) structure of gemcitabine; (c) percent release of gemcitabine versus time for 200 × 200 nm PRINT nanoparticles fabricated with Et-GEM (blue, Et: ethyl), iPr-GEM (red, iPr: isopropyl), and tBu-GEM (green, tBu: tert-butyl) prodrugs. Closed symbols represent particles degraded at pH 5.0 and open symbols represent particles degraded at pH 7.4. (B) Confocal microscopy images of LNCaP cells stained with the PathScan apoptosis and proliferation kit. The cells were separately dosed with (a) free gemcitabine, (b) Et-GEM particles, (c) tBu-GEM particles, and (d) blank particles. Red indicates a healthy cell containing fundamental cytosolic fibers important in meiotic/mitotic chromosome alignment. Green indicates a healthy cell undergoing microtubule assembly during mitosis. Purple indicates cytoskeleton proteins and nuclear protein experiencing an apoptotic event. Reproduced with permission from ref. 99. Copyright © 2010 American Chemical Society. | ||
Similarly, the pH-sensitive degradation of ester bonds in a highly cross-linked diacrylate PLA-b-PEG-b-PLA water-swollen gel network that degraded through the hydrolysis of ester bonds in the PLA block was utilized in microgel particles made by stop-flow lithography from this bulk-erodable polymer with cubical and triangular shapes and tunable time-degradation properties.100
Related to hydrogel size consideration, experimental and theoretical results on monodisperse hydrogel particles with various lengths but with the same cross-sectional dimensions of 800 nm by 100 nm by 100 nm, 400 nm by 100 nm by 100 nm, and 100 nm by 100 nm by 100 nm made from PEGDA of various percent polymers 10–50% (v/v) using the S-FIL technique demonstrated that geometric (size) and mechanical (surface attachment) constraints may result in anisotropic and inhomogeneous swelling for hydrogel particles.33 For example, it was reported that the swelling ratio of bulk versus surface-attached particles is comparable when the particle length is longer than 400 nm, but the surface-attached swelling ratio becomes significantly larger than the bulk value when all particle dimensions are less than or equal to 100 nm. Theoretical analysis also confirmed that the highly cross-linked PEGDA hydrogels could not swell to the point where the shape and size of these particles would be altered for particle sizes larger than 100 nm. These findings may provide important insights into dimensional parameters of hydrogel particles capable of dynamic shape changes.
Unlike bulk microscopic networks, hydrogel microcapsules have much larger free volume due to the capsule interior and the ultrathin (<100 nm) hydrogel wall which can result in much larger swelling ratios compared to those of bulk hydrogels of the same chemistry. In addition, because the swelling rate is inversely proportional to the square of network dimensions,104 nanoscale capsule hydrogel walls can exhibit an extremely fast response to external stimuli, unlike microscopic hydrogels.105,106 For instance, cubical single-component (PMAA)14 multilayer hydrogel capsules cross-linked with ethylenediamine74 and (PMAA)20 capsules obtained from multilayers of the PMAA-co-((3-aminopropyl)methacrylamide) statistical copolymer became bulged into sphere-like structures after exposure to pH = 8. The bulging was attributed to the stress in the capsule wall resulting from uncompensated electrostatic repulsion between COO− groups at pH = 8 and small counter ions penetrating the hydrogel to compensate negative charges. The shape, however, was not completely reversed when the (PMAA)20 cubical capsules were exposed to acidic pH and the PMAA hydrogel collapsed.
Increasing the capsule wall thickness and/or the cross-link density should lead to reversibility in the capsule shape change or even to the ability of the non-spherical capsules to maintain their shape upon size increase when the hydrogel wall swells. Indeed, when the second component was retained within the hydrogel capsule wall by chemical cross-linking of PVPON-co-((3-aminopropyl)methacrylamide) (PVPON–NH2) to PMAA layers using a water-soluble carbodiimide, the two-component (PMAA–PVPON)5 cubical hydrogel capsules did not bulge and remained cubical, increasing in size instead when solution acidity was increased from pH = 3 to pH = 8.85 These distinctively different pH-triggered shape responses of (PMAA) and (PMAA–PVPON) multilayer hydrogel capsules were achieved by controlling the capsule shell rigidity.85
Hydrogel rigidity and capsule geometry are key factors in regulating the shape response of a hydrogel capsule. As reported in a computational study, faceted capsules can display inhomogeneous strain/stress distributions along the edges, while vertices provide mechanical reinforcement to edges restricting their expansion/contraction.107 Thus, pH-induced stress may release inhomogeneously through mechanically weak facets resulting in anisotropic swelling of non-spherical capsules. For example, our group demonstrated two types of discoid multilayer hydrogel capsules mimicking the shape of red blood cells which exhibited different shape transitions in response to pH changes.81 The (PMAA)15 and (PMAA–PVPON)5 discoidal capsules demonstrated various degrees of out-of-plane swelling of the discoidal circular faces, i.e., circular face bulging at pH = 7.4. The spherical (PMAA)15 capsules displayed a dramatic 19-fold volume increase when the solution pH was altered from 4 to 7.4. Unlike the 19-fold swelling of (PMAA)15 systems, two-component (PVPON–PMAA)5 capsules underwent only a 2.9-fold increase in their volume. This difference in pH-triggered variations in capsule size was rationalized by comparing free volume within the capsule hydrogel shell. The (PMAA)5 multilayer planar hydrogel displayed a 2-fold greater water uptake than (PMAA–PVPON)5 at pH = 3 with water content values of 41% and 19% for (PMAA)5 and (PMAA–PVPON)5, respectively.81 Apparently, the presence of the second component reduced the free volume in the dual-component network suppressing the capsule swelling.
Importantly, pH-triggered dimensional changes of (PMAA)15 and (PMAA–PVPON)5 discoidal capsules were demonstrated to be drastically different. The (PMAA)15 showed dramatic volume transitions of 24-fold; while a moderate 2.3-fold volume change was found for (PMAA–PVPON)5 discoidal capsules upon the pH shifts. Both radial and axial dimensions of (PMAA) capsules were 1.8-fold greater than those for (PMAA–PVPON) capsules at pH = 7.4. The larger expansion of the one-component hollow hydrogel discs was consistent with the greater swelling of the corresponding hydrogel spheres and surface-attached hydrogel films as compared to their dual-component counterparts. However, the aspect ratios of two types of discoidal capsules, i.e., length-to-height ratios, remained similar at pH = 7.4 indicating that both types of hydrogels expanded in a similar way in the ionized state. Those values were smaller than the aspect ratio for their solid templates which indicated the preferential expansion in the axial direction for both types of capsules upon core dissolution and exposure to neutral pH.81
The pH-triggered change in capsule dimensions resulted in anisotropic swelling/shrinkage leading to discoidal-to-ellipsoidal shape transformations with the degree of this shape transition depending on the wall composition. The observed variations in pH-dependent aspect ratios for the two types of the discoid hydrogel capsules were explained by a greater degree of the out-of-plane swelling of (PMAA) capsules compared to that of (PVPON–PMAA) systems at pH = 7.4. Quantifying the degree of swelling in the axial and radial directions revealed a negligible difference between the ratios for both types of the discoidal capsules which implied no preferential swelling in axial or radial directions upon pH variations.81
Apart from the shape anisotropy, anisotropic structures of a hydrogel composed of passive (non-swellable) and active (highly swellable) hydrogel layers on top of each other can also be used to induce an overall shape change using a pH trigger. For example, a dual hydrogel composed of a poly(2-hydroxyethyl methacrylate) (PHEMA) layer on top of a poly(2-hydroxyethyl methacrylate-co-acrylic acid) P(HEMA-co-AA) layer prepared in the shape of a planar circle using photolithography quickly (within a minute) formed a microcapsule with the PHEMA as an inner coating of the particle shell when exposed to a pH = 9 solution.108 The reversible shape transformation occurred due to P(HEMA-co-AA) layer swelling at pH = 9 which could swell up to 1000 v/v% while the PHEMA layer remained de-swollen leading to two-dimensional bending due to the highly flexible hydrogel matrix. This result is particularly interesting demonstrating the importance of softness and mechanical robustness of the hydrogel matrix to induce particle shape reconfiguration.
3.2. Redox sensitivity
There is a significant difference in the redox potential between intracellular and extracellular environments. Glutathione (GSH), a tripeptide enzyme in cells, can reduce disulfide bonds to the corresponding thiols as an electron donor.109 The intracellular GSH concentration of 2–10 mM is 1000 times higher than that of 2–20 μM in the extracellular environment and some tumor cells have GSH concentration several times more than normal cells.110 Furthermore, the endosomes/lysosomes contain a high content of the reducing enzyme γ-interferon-lysosomal thiol reductase (GILT) that can reduce protein disulfide bonds at low pH, and of cysteine which may also exhibit reducing properties.111 Therefore, the hydrogel particles with an engineered redox response can facilitate a rapid intracellular drug release while being stable in the extracellular media, which can inhibit drug resistance.112–116 The disulfide bond is among the most widely used to impart redox responsiveness to gel particles while other redox-sensitive linkers are still overlooked, most likely due to the challenge of their incorporation into particles in aqueous solution.117Redox-sensitive hydrogel particulates, similar to other types of hydrogels, swell in aqueous solutions owing to the presence of hydrophilic moieties. Cross-linkers that have disulfide centers can be used to establish and stabilize the three-dimensional structure of a gel particle under physiological conditions which can rapidly disassemble upon the cleavage of disulfide bonds in the presence of increased concentrations of reducing agents present in intracellular or tumor sites, resulting in the fast and complete release of encapsulated therapeutics.26,118–121
A variety of factors including hydrogel geometry, cross-link density, and the type of reducing agents present in solution can affect the degradation profile of the redox-sensitive non-spherical hydrogels. For example, redox-labile PRINT cubes 2 μm in size reported by DeSimone and coworkers demonstrated a different anticancer effect compared to those with non-degradable linkers.122 After incubation of the PRINT cubes loaded with doxorubicin (DOX) in a 100 mM dithiothreitol solution for 48 hours, a ∼20% decrease in DOX fluorescence was observed for the reductively-labile cubes indicating DOX release in the reducing environment unlike non-degradable cubes where there was an absence of DOX in the bulk solution.122 The reduction of the disulfide bonds was believed to cause a decrease in the particle mesh density which allowed DOX to diffuse out. The viability of cancer HeLa cells also decreased for the reductively-labile PRINT cubes compared to the particles with non-cleavable linkers, confirming a redox-triggered drug release in the cells. In addition, the redox-sensitive PRINT cubes were surface-functionalized with avidin for further incorporation of biotinylated targeting ligands for targeted delivery.122
Apart from stabilizing the hydrogel network, disulfide-containing linkers can also be used not only to conjugate therapeutic agents to a non-spherical hydrogel but also to control the rate of drug release as well as to protect therapeutics from premature release or even destruction by active molecules in serum. For example, cylindrical hydrogel rods (with the aspect ratio of 4) bearing primary amines were synthesized first and thiol-modified siRNA was conjugated to the hydrogels via succinimidyl 3-(2-pyridyldithio)propionate (SPDP) chemistry with the loading ratio of 18 wt% (Fig. 9).123 The siRNA molecules attached to the hydrogel cylinders were stable after incubation in 30% fetal bovine serum (FBS) solution for 24 hours in contrast to naked unprotected siRNA molecules which were completely degraded by FBS under the same incubation conditions. This pro-drug strategy of conjugating siRNA into the shaped cylindrical hydrogel through disulfide bonds used for gene delivery also prevented siRNA leakage from the pro-siRNA hydrogel under physiological conditions and resulted in increased stability of the siRNA. The in vivo study further indicated that the coagulation factor VII (FVII) siRNA was successfully delivered to liver tissues through hydrogel particles and was able to exert its bio-function, leading to a significant reduction in FVII mRNA expression in a dose-dependent manner (Fig. 9).123
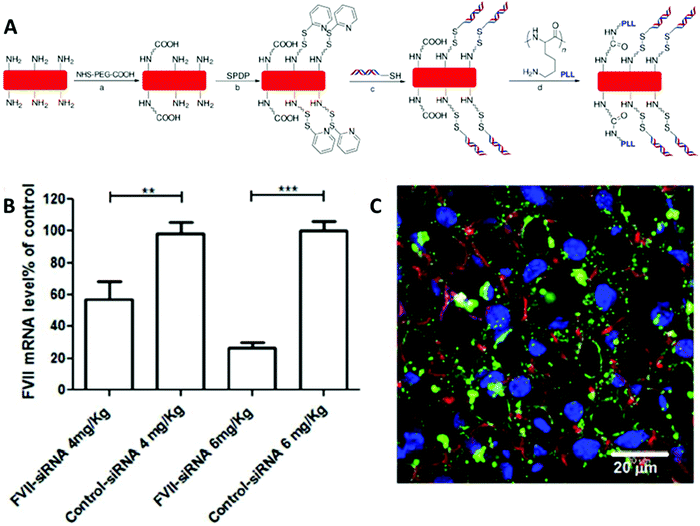 | ||
| Fig. 9 (A) Surface modification of PRINT hydrogel cylinders with post-fabricated siRNA: (a) NHS–PEG–COOH, DMF, pyridine; (b) (succinimidyl 3-(2-pyridyldithio)propionate) (SPDP), PBS/CH3CN; (c) siRNA-SH, PBS; (d) poly-L-lysine, 1-ethyl-3-(3-(dimethylamino)propyl)carbodiimide hydrochloride (EDC), sulfo-NHS, PBS. (B) Coagulation factor VII (FVII) mRNA level in the liver with qRT-PCR assay. (C) Confocal micrograph of liver tissue (24 h after intravenous administration of nanoparticles) from mice treated with DyLight 680 labeled nanoparticles (green). Cellular actin cytoskeleton was stained with phalloidin (red), macrophages with MARCO (magenta), and nuclei with DAPI (blue). Reproduced with permission from ref. 123. Copyright © 2015 American Chemical Society. | ||
Using simultaneous copolymerization of the siRNA macromonomer with the hydrogel matrix components, a larger degree of loading of the nucleic acid into a PRINT cylindrical particle could be achieved. For instance, when a degradable disulfide siRNA pro-drug macromer (where siRNA was derivatized with a photopolymerizable acrylate) with a degradable disulfide center was covalently incorporated inside PRINT hydrogel particles during the network formation of loosely cross-linked cationic PRINT discoid cylinders, the siRNA loading ranged from 15 to 35% depending on the content of 2-aminoethylmethacrylate hydrochloride incorporated within the hydrogel during polymerization (Fig. 10).124 The siRNA inside the particles was also demonstrated to be protected from degradation by RNAses for 48 hours when incubated in serum, while a naked macro-monomer siRNA was rapidly degraded in serum under the same conditions.124
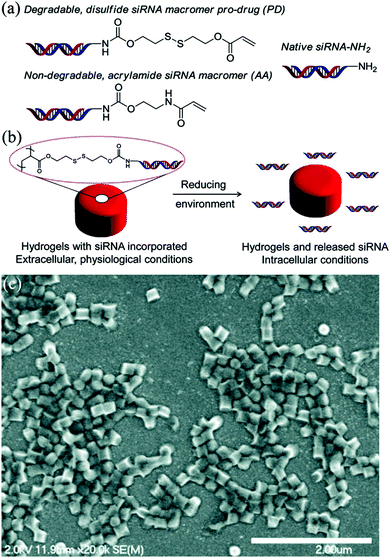 | ||
| Fig. 10 (a) Structures of degradable and nondegradable siRNA macromers as well as native siRNA, (b) illustration of pro-siRNA hydrogel behavior under physiological and intracellular conditions, and (c) SEM micrograph of pro-siRNA, 200 × 200 nm cylindrical nanoparticles (scale bar = 2 μm). Reproduced with permission from ref. 124. Copyright © 2012 American Chemical Society. | ||
Since hydrogel degradation and subsequent release of therapeutics depend on particle surface area, size, and other dimensional characteristics, it would be highly desirable to explore the effect of shape on the degradation behavior of non-spherical hydrogel particles. This is especially interesting as hydrogels of complex shapes can produce complex multistep degradation profiles due to variations in thicknesses as well as changes in particle shape which follow the degradation process.
For example, a slightly faster degradation of nano-porous hydrogel spheres compared to cubes made of (PMAA)5 multilayer hydrogels and cross-linked with cystamine was observed in the presence of intracellular concentrations of glutathione as measured using suspension turbidity changes.26 The relative turbidity, defined as the ratio of the (PMAA)5 hydrogel particle solution scattering intensity at certain time points to the initial scattering intensity, was measured in the presence of 5 mM GSH solution in PBS (pH = 7.4, 37 °C) using fluorescence spectrophotometry. With no GSH in the hydrogel suspension, the relative turbidity of (PMAA)5 hydrogels did not change with time indicating the chemical stability of the hydrogel particles while GSH treatment provided complete hydrogel particle degradation in 3 hours. The faster degradation of the spherical multilayer hydrogels versus that of cubical shaped hydrogels was observed within the first hour with the relative turbidities of 40 ± 3 and 50 ± 2%, respectively, and was attributed to a 1.9-fold larger hydrogel cube volume compared to that of spheres of the same size. Despite the micrometer size of the hydrogels, the reduction of the disulfide groups within the cystamine-cross-linked (PMAA) multilayer hydrogel cubes or spheres to the corresponding thiols in the cytoplasm still releases only short polymer chains capable of rapid renal clearance.
The thiolated PMAA obtained by modification of the polyacid carboxylic groups with pyridine dithioethylamine hydrochloride using carboddimide chemistry has been developed and widely used by the Caruso group for the fabrication of redox degradable spherical multilayer hydrogel capsules using LbL assembly. This technique was also applied to fabricate rod-like multilayer hydrogel capsules with various aspect ratios ranging from 1 to 4 (Fig. 11).79 Due to the coexistence of PMAA and disulfide bonds, the hydrogel capsules could both swell at physiological pH and degrade after cellular internalization via endocytotic pathways.
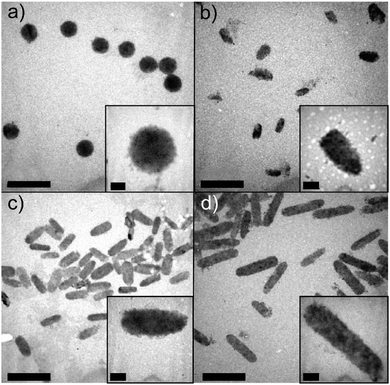 | ||
| Fig. 11 TEM images of PMAA hollow hydrogel capsules with aspect ratios of (a) AR = 1, (b) AR = 2, (c) AR = 3, and (d) AR = 4, obtained by using silica rod templates. Scale bars are 2 μm. Scale bars in the insets are 0.2 μm. Reproduced with permission from ref. 79. Copyright © 2013 American Chemical Society. | ||
3.3. Temperature sensitivity
Temperature-sensitive hydrogels exhibit volume-phase transitions in response to temperature change in the surroundings, which can be classified into two main categories: (1) those composed of polymers which have an upper critical solution temperature and (2) those composed of polymers which have a lower critical solution temperature (LCST). Among the LCST polymers, special interest is devoted to hydrogel particles made of PNIPAM and poly(N-vinylcaprolactam) (PVCL) with their LCSTs close to the physiological temperature range. For PNIPAM- or PVCL-based hydrogels the phase transition is described by a volume phase transition temperature when the hydrophobicity of the cross-linked polymer chains increases and water molecules are expelled from the gel interior. Even though PNIPAM and PVCL have similar LCSTs in the range of 32–36 °C, the mechanism and thermodynamics of the phase transition are different and therefore require different methods for the LCST variation. While PNIPAM has an LCST independent of polymer molecular weight, with environmental conditions such as pH and composition controlling its LCST, PVCL exhibits an LCST which follows classical Flory–Huggins thermoresponsive phase diagram behavior125 with the LCST value being highly dependent on the polymer molecular weight and concentration.126,127An amazing example of hydrogel particle shape control via reaction temperature during a micro-emulsion polymerization was demonstrated by Zhu and coworkers who synthesized temperature-sensitive PNIPAM/chitosan/Fe3O4 microgels with a spindle-shaped, cuboid, and spherical morphologies by increasing the reaction temperature from 28 to 34, and to 40 °C, respectively.128 This polymerization is based on the precipitation of polymeric chains as particles as they grow and become insoluble in a solvent. During polymerization, the growing polymer chain phase separates from the solvent and forms a nucleic aggregate by capturing newly formed polymer chains. Interestingly, the corresponding PNIPAM/chitosan microgels produced at corresponding temperatures were all of spherical shape. In this case the various degrees of stabilization provided by Fe2O3 nanoparticles affected both the polymer chain conformation during polymerization (from more extended to more collapsed) and the subsequent particle shape. The temperature sensitivity of these hydrogel particles was measured by dynamic light scattering and it was found that a sharp decrease in the hydrodynamic diameter resulting from collapsed PNIPAM chains occurred when the temperature was increased above 32 °C. The temperature-sensitivity study, however, was limited to spherical hydrogels and the effect of the temperature-induced volume changes on the shape of non-spherical hydrogels was not reported.128
The effect of temperature-triggered hydrogel volume changes on the shape of non-spherical hydrogel structures was recently explored and reported by Zhang et al.129 and Crassous et al.130 In the first case, a temperature-sensitive dendrite-shaped hybrid hydrogel particle composed of PNIPAM/dextran-allyl isocyanate (PNIPAM/Dex-AI) in which Dex-AI served as a precursor and a biodegradable cross-linker was fabricated through a precipitation polymerization.129 The swollen hydrogel particles had cotton ball-like morphology and each particle had a central mass with many spikes protruding in a dendritic fashion. An average dendrite-like particle exhibited significant geometry changes over the temperature range of 10 to 60 °C. The volume of hydrogel particles decreased by 11% from 10 to 22 °C and could reach up to 39% shrinkage at 60 °C due to the temperature sensitivity of the PNIPAM component. The freeze-dried PNIPAM/Dex-AI hydrogel particles had the appearance of coral with a heterogeneous porous microstructure. In the second case, PNIPAM-based, core–shell composite ellipsoidal, faceted, and bowl-like microgels composed of a polystyrene core and a cross-linked PNIPAM shell were reported.130 The specific particle shapes were achieved through different techniques, including uniaxial stretching for ellipsoidal shapes, phase separation in dodecane for faceted gels, and freeze-drying for bowl-shaped gel particles. These (PS core–PNIPAM hydrogel shell) particles exhibited shape-dependent temperature responsiveness. For example, the volume-phase transition of the spherical hydrogels occurred at 42 °C whereas it shifted to 38 °C for the ellipsoidal and to 45 °C for the faceted hydrogels. The bowl-shaped hydrogels had a volume-phase transition similar to that of the spherical particles at 42 °C. The shape-dependent variation of the volume-phase transition was shown to originate from various deformation processes during particle formation resulting in different stiffness of the particle shell.
Our group recently reported on cubical hollow capsules with a shell made of a PVCL multilayer hydrogel which showed distinctive and reversible temperature-responsive behavior (Fig. 12).131 These cubical PVCL hydrogel cages were produced by selective cross-linking of PVCL–NH2 copolymer layers within LbL hydrogen-bonded multilayer films of PMAA and PVCL–NH2 which were assembled on monodisperse spherical silica and cubical manganese carbonate templates, followed by the release of PMAA from the PVCL multilayer hydrogel at basic pH and dissolution of the inorganic template particle in hydrochloric acid.131 In contrast to the above-mentioned hydrogel particles where temperature changes during or after particle synthesis could affect and/or alter a hydrogel particle shape to some degree, these PVCL hydrogel cubical capsules could shrink in size when the temperature of the capsule solution was elevated from 25 to 50 °C but maintained their cubical shape. Numerically, the cubical (PVCL)7 capsules decreased in size by 21 ± 1%. The fact that the capsules retained their cubical shape at the elevated temperature indicated a uniform decrease in the capsule size upon heating. Spherical (PVCL)7 hydrogel capsules of the same size demonstrated similar shrinkage of 23 ± 1%. The temperature-triggered size changes of both types of capsules were completely reversible. The degree of the temperature-triggered shrinkage was controlled by varying the cross-link density from 9 to 23 cross-links per chain for the hydrogels derived from PVCL–NH2-n with n = 7 and n = 14, respectively, where n denotes the molar percentage of amine group-containing polymer units. The hydrogel films made from PVCL–NH2-7 exhibited a shrinkage of 1.9 ± 0.1. An increase in the cross-link density in PVCL–NH2-14 hydrogels significantly suppressed the shrinkage ratio down to 1.3 ± 0.1.
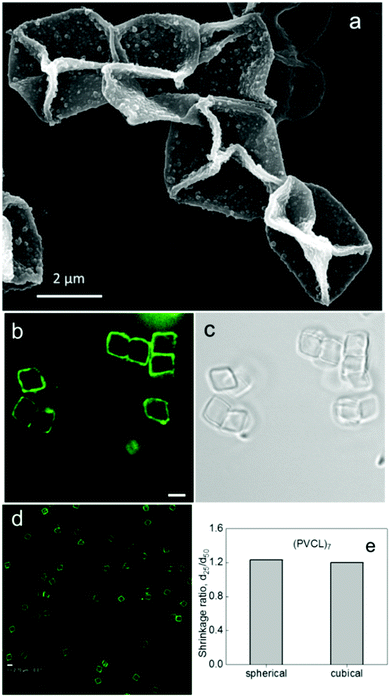 | ||
| Fig. 12 (a) SEM image of (PVCL)15 hydrogel cubical hollow capsules dried on a Si wafer from aqueous solution at pH = 3. (b and c) Hollow cubical (PVCL)7 multilayer hydrogel capsules in aqueous solution at T = 25 °C. (d) Cubical (PVCL)7 capsules imaged after 25 °C/50 °C/25 °C/50 °C temperature cycles. Scale bar is 2.7 μm. (e) The shrinkage ratios (the ratio of the capsule size at 25 °C to that at 50 °C, d25/d50) of (PVCL)7 multilayer hydrogel hollow spherical and cubical capsules. Reproduced with permission from ref. 131. Copyright © 2012 American Chemical Society. | ||
3.4. Enzyme sensitivity
Enzyme-responsive hydrogel particles usually contain ester or peptide linkages in their network and can be cleaved by intracellular esterases and proteases. Similar to the redox-sensitive hydrogels, the enzyme-responsive hydrogels can lose their cross-linked structure in response to enzymatic degradation which can be highly advantageous for fast and site-specific drug delivery. Unlike pH- or redox-sensitive hydrogels, the enzymatically-sensitive hydrogel particles may be designed to release encapsulated therapeutics at a particular diseased tissue or inside a specific cellular compartment. This specificity provided by selective enzymes should yield a significant improvement in the delivery of highly toxic chemotherapeutics or drugs with a short half-life.In this regard, Roy and colleagues fabricated enzymatically degradable hydrogels of square, triangular and pentagonal shapes using the S-FIL technique.29 The hydrogels were composed of hydrophilic PEGDA, acrylated peptide, Gly-Phe-Leu-Gly-Lyse, and a diacrylated GFLGK peptide cross-linker which served as a cleavable moiety. The Gly-Phe-Leu-Gly can be selectively degraded in the presence of several lysosomal thiol proteases, and it is particularly sensitive to Cathepsin B which is overexpressed in the lungs, ovarian and colorectal tumor cells and is present extracellularly in tumor tissues.132 After incubation with Cathepsin B, the shaped hydrogels obtained from the mixture of PEGDA and acrylated GFLGK with a molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 started to degrade within 30 minutes and completely disassembled after 48 hours. To confirm that the prepared hydrogels exhibited the enzyme-triggered drug behavior, an antibody and small DNA were used as model drugs which were loaded into hydrogels during S-FIL fabrication. In both cases, major drug release occurred only after exposure to the Cathepsin B enzyme indicating the highly controlled, disease-specific release of drugs.
1 started to degrade within 30 minutes and completely disassembled after 48 hours. To confirm that the prepared hydrogels exhibited the enzyme-triggered drug behavior, an antibody and small DNA were used as model drugs which were loaded into hydrogels during S-FIL fabrication. In both cases, major drug release occurred only after exposure to the Cathepsin B enzyme indicating the highly controlled, disease-specific release of drugs.
3.5. Other stimuli
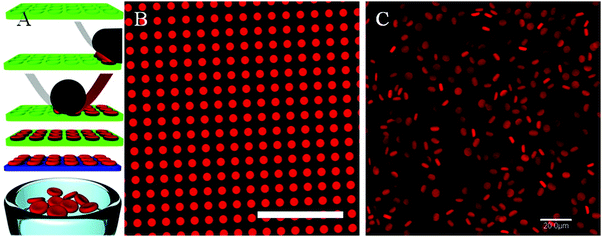 | ||
| Fig. 13 (A) The PRINT process used to fabricate RBC-shaped microgels: an elastomeric fluoropolymer mold (green) with disk-shaped wells was covered by an aliquot of the prepolymer liquid (red). The mold was passed through a pressured nip (black) covered by a high-surface energy sheet (gray), wicking away excess liquid from the mold surface while filling the wells. The filled mold was cured photochemically, yielding crosslinked particles, which were harvested from the mold by laminating a PVA film (blue) on top of the mold through a pressured nip and peeling way the mold. Dissolving the poly(vinyl alcohol) film resulted in a suspension of hydrogel particles. (B) Fluorescence micrograph of polymerized particles in the mold, and (C) fully hydrated particles free of the mold, suspended in PBS. Scale bars = 20 μm. Reprinted with permission from ref. 133. Copyright © 2012 American Chemical Society. | ||
The use of a filamentous shape in PRINT hydrogel particles was shown to circumvent the problem of large particle stiffness for passing through pores with much smaller diameters than the particle size.134 When the hydrogel particle recovery was assessed by measuring the percentage of particles that could squeeze through a filter with 0.2 μm pores, it was found that the recovery of 80 nm particles with varied lengths was largely independent of the cross-linker concentration for lengths of up to 2 μm and was in the range of 93 to 77%.134 However, the lowest particle recovery of 29% was found for larger 80 × 5000 nm particles when the cross-linker concentration was greater than 50% which was attributed to decreased flexibility and deformability (Fig. 14).
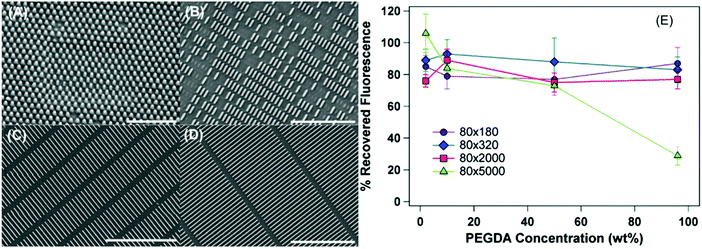 | ||
| Fig. 14 SEM images of the PEG-based hydrogel particles 80 nm in diameter. Lengths of the particles are (A) 180 nm, (B) 320 nm, (C) 2000 nm, and (D) 5000 nm. Scale bar values are (A) 1 μm, (B) 3 μm, and (C and D) 5 μm. (E) Percent recovered fluorescence for 80 nm particles as a function of cross-linker concentration. The percentage values also refer to the percentage of particles recovered following filtration. Reproduced with permission from ref. 134. Copyright © 2012 American Chemical Society. | ||
The mechanical flexibility necessary for a hydrogel particle to successfully navigate through size constrictions of blood vasculature under pressure differentials observed in healthy and diseased human capillaries can be regulated by varying only the shape of the hydrogel vehicle with the same nominal dimensions. For instance, squishy hydrogel disks, rings, crosses, and S-shapes of 8 μm in size and 2 μm in thickness obtained by polymerization of 700 Da PEGDA using the stop flow lithography technique demonstrated different deformabilities as a function of their microstructure.20 The threshold pressure differential for the hydrogels with a 10% cross-linker for passage of disks through 4 μm × 4 μm constrictions was 2.8 mmHg, while being only 0.05 mmHg for S-shapes. Ranking the four shapes by increasing flexibility gives disks, followed by rings, crosses, and then S-shapes as the most flexible. Interestingly, disk-, ring-, or cross-shaped hydrogel particles which were trapped within the constriction adopted a folded-over shape, while in the case of S-shapes, the two ends of the particle twisted in opposite directions resulting in only minimal bending.20
Unlike the discoidal PRINT or SFL hydrogel particles discussed above, a different deformation behavior was observed for discoidal PAH hydrogel capsules produced by LbL assembly and cross-linked with glutaraldehyde.135 Statistical analyses of the 6.7 μm hydrogel discoidal capsules squeezed through a glass capillary tip of 5 μm revealed that 90% of the discoidal capsules not only passed through the capillary constriction but also recovered their original discoidal shape. The remarkable fact is that the elasticity modulus of this discoidal capsule was in the order of hundreds of MPa, which is much larger than the passage threshold for the discoidal PRINT hydrogels, indicating that the hollow hydrogel structure can exhibit larger flexibility than that of a filled hydrogel of the same shape.18,20 This difference can be attributed to the fact that the hollow capsule interior, which resembles the RBC structure of a vesicle with fluid wrapped by a flexible cell membrane, facilitates better shape recovery by a more fluid-like deformation of the capsule membrane than an uninterrupted hydrogel of the PRINT and SFL hydrogel disks.18 The discoidal hydrogel capsule shape also allowed for better shape recovery when passing through pores with sizes smaller than those of the spherical hydrogel capsules which showed only 63% recovery ratio of the same (PAH)10 composition and size (6.7 μm).135
In another example,136 2 μm-sized multilayer cubical capsules made of hydrogen-bonded PVPON and tannic acid with a lower elasticity of 0.6 MPa were demonstrated to be able to extravasate through membrane pores of 0.8 μm under 18 psi of flow pump-controlled pressure. In contrast, filled PRINT hydrogels of a filamentous shape 80 nm in diameter with various lengths were able to cross 200 nm pores under 40 psi of manual pressure.31 Confocal microscopy analysis revealed that 80% of the cubical shells which penetrated the membrane fenestrations could maintain their initial cubical shape (Fig. 15).
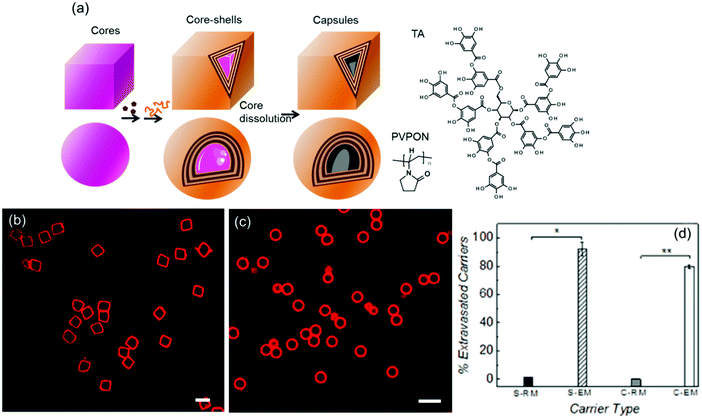 | ||
| Fig. 15 (a) Rigid core–shells and soft shells are produced using LbL assembly of TA and PVPON on sacrificial inorganic manganese carbonate and silicon dioxide cores of cubic and spherical shapes, respectively. Confocal microscopy images of (b) cubical and (c) spherical hollow capsules obtained via dissolution of the cores. The scale bar is 5 μm. (d) Percentage of the particles able to traverse through the pores. The labels denote S = spherical, C = cubical, RM = rigid core–shell, EM = soft capsule. Reproduced with permission from ref. 136. Copyright © 2015 Wiley. | ||
The cell-line dependent intracellular force exerted by cells on the particles upon their internalization can also serve as guidelines for the synthesis of mechano-responsive shaped hydrogel drug carriers for cell-specific drug delivery. For example, in a recent study, the Caruso group137 discovered that a different mechanical force could be generated by cells during internalization of soft PMAA hydrogel capsules of spherical and cylindrical shapes in a cell line-dependent way. This led to different deformations of capsules distributed within cell interiors. For both spherical and cylindrical hydrogel capsules, the highest capsule deformation occurred in HeLa cells (human epithelial cell line) when the capsules were highly deformed and lost their initial shape. In contrast, in differentiated human monocyte-derived macrophage cells, a large amount of capsules could maintain their distinguishable shape after cellular internalization.137
In one example, magnetic hydrogel particles of spherical, dicoidal and plug-like shapes were synthesized by photopolymerization of an iron oxide-containing PEGDA solution in a microfluidic device.139 These non-spherical hydrogels assembled into long chains upon exposure to uniform external magnetic fields due to attractive interactions between the particles parallel to the field direction. Unlike the magnetic spherical hydrogels, the discoidal and plug-like particles exhibited a directional preference in the external magnetic field because of their shape anisotropy. The non-spherical hydrogels flipped up perpendicular to the plane of view and aligned along the field direction. This unique magnetic response and the ability of these anisotropic particles to form more complex patterns can be further exploited for field-induced pattern formation.
In another example, the inclusion of superparamagnetic iron oxide nanoparticles into electrosprayed cellulose-based RBC-shaped particles provided a magnetic-field response and a magnetic resonance imaging (MRI) signal contrast.140 Interestingly, the observed formation of a discoidal shape was believed to be due to the sheet-like molecular structure of the cellulose polymer. During the electrospraying process, the formed discoid was believed to become dented in its center due to electrostatic repulsions between the surface charge and the resistance between the nozzle and the collector.140 Due to the unique assembly possibilities and potential for imaging capability, magnetic field sensitivity enables shaped microparticles to be promising platforms for bio-imaging and in vivo drug delivery.
4. Effect of shape of non-spherical hydrogel particles on biological responses
Cell association with drug carriers is among the most crucial parameters for controlled drug delivery, and both the geometry and rigidity of delivery vehicles can regulate the cellular internalization efficiency in a cell type-dependent way.141 Remarkably, the upper size limit for cell internalization of hydrogel colloids by non-phagocytotic cells is much higher than that of rigid inorganic or polymeric nanoparticles which are excluded from cellular uptake at sizes greater than 150–200 nm, while softer and more flexible hydrogel particulates with sizes up to 3–5 μm can be internalized by cells.26,79,81,136,142Apart from the conventional factors such as size, surface charge, and hydrogel rigidity that control cellular interactions of hydrogel particles, the shape of non-spherical hydrogel particles has undoubtedly been found to be critical for cell interactions including the rate and percentage of internalization. With regard to the hydrogel shape, the impact of the aspect ratio of the particle on cellular interactions has been studied on both filled, uninterrupted hydrogels made via PRINT technology142 as well as on hollow hydrogel particles, i.e., multilayer hydrogel capsules made via the LbL approach.79 In the former case, cylindrical PEG-based PRINT hydrogels with a high aspect ratio of 3 (length = 450 nm, width = 150 nm) were internalized by HeLa cells more rapidly and efficiently than those with an aspect ratio of 1 (length = width = 200 nm) (Fig. 16a). This difference in cellular uptake favoring the high-aspect ratio hydrogel cylinders was attributed to more cationic interactions with cells available with the higher-aspect-ratio particles due to the larger surface areas in contact with the cell. In contrast to those results, increasing the aspect ratio of hollow PMAA hydrogel capsules diminished the efficiency of the capsule internalization by HeLa cells (Fig. 16b). Increasing the aspect ratio in the case of cylindrical PMAA hydrogel capsules decreased their internalization by the cells from 91 to 82 and to 77% when the capsule aspect ratio increased from 1 to 3, and to 4, respectively, after 24 h of incubation.79 The differences in cell internalization between high-aspect-ratio filled and hollow hydrogel particles could also be related to their different rigidities as well as their net-surface charges. In other words, the negative surface charge of the high-aspect-ratio hydrogel capsules could play a negative role in the cell association/internalization process, and is worthy of continued studies in the future.
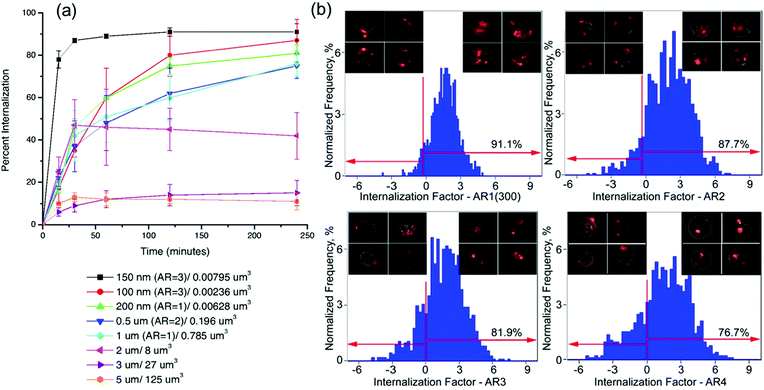 | ||
| Fig. 16 (a) Internalization of PRINT PEG-based hydrogels with HeLa cells over a 4 h incubation period at 37 °C. Legend depicts the particle diameter per particle volume. Reproduced with permission from ref. 142. Copyright 2008 National Academy of Sciences. (b) Internalization of PMAA hydrogel capsules in HeLa cells quantified by imaging flow cytometry. The degree of internalization is expressed as the internalization factor (IF). An overlay of the bright-field and fluorescence images of cells is shown in the insets for two areas: capsules bound with the cell membrane (negative IF) and capsules internalized within cells (positive IF). Reproduced with permission from ref. 79. Copyright 2012 American Chemical Society. | ||
The same study by the Caruso group showed that the cylindrical hydrogel capsule shape is less prone to interaction with HeLa cells compared to the spherical shape.79 In this work, they found that spherical 1100 nm PMAA hydrogel capsules which had greater volume than the cylindrical capsules with an aspect ratio of 4 demonstrated almost 2-fold greater cellular association over 24 h of incubation. This finding agreed with the earlier observations by Crespy and colleagues143 that cellular internalization of high-aspect-ratio colloids required extended membrane wrapping time during the process. Despite the differences in the aspect ratio, both spherical and rod-like capsules accumulated within cellular lysosomes indicating that intracellular capsule distribution was independent of capsule shape.
The discoidal shape of the hydrogel capsules was also shown to decrease the capsule association with cells compared to their spherical counterparts.81 When spherical and discoidal (PMAA–PVPON)5 hydrogel capsules were incubated with J744.A1 macrophages, human microvascular endothelial cells (HMVEC), and 4T1 breast cancer cells, the hydrogel capsules showed a negligible uptake by the J774A.1 macrophages. Also, cell association with discoidal capsules was shown to be 60% lower than that of spherical capsules. The internalization of discoidal hydrogel capsules by 4T1 breast cancer cells was found to be 5-fold smaller than that of the spherical ones. This finding correlates with the studies on rigid polymeric particles,144 which explained the decreased cellular uptake of polymeric discs by stronger cell surface binding due to their shape as compared to spheres. Indeed, the PMAA–PVPON hydrogel capsules could be regarded as discoidal plates with a high aspect ratio of 5 at pH = 4 and of 3 at pH = 7.4.81
A recent computational study by Gompper and co-workers145 analyzed the effects of shape, aspect ratio, and orientation of rigid rod and cubical nanoparticles on cellular membrane bending and wrapping and predicted the extent and kinetics of the endocytosis based on particle geometry. The study revealed that to sufficiently determine particle uptake, local geometrical properties such as the local mean curvature along with particle size and aspect ratio are extremely important factors in predicting the membrane wrapping behavior. They found that both particle adhesion to the cell and wrapping of the cell membrane around it are controlled by the competition between the adhesion energy gain for particle-cell membrane contact and the energy cost for the lipid bilayer deformation. Therefore, membrane wrapping can be a crucial mode of entry for particle uptake (Fig. 17). For example, enhanced binding but lower uptake can occur for ellipsoidal particles when oriented parallel to the membrane compared with spherical ones. However, reorientation from the parallel to perpendicular state may suppress particle uptake because wrapping the highly curved tip of an ellipsoid will cost a high bending energy per area.145 Also, it was shown that for cubical particles, the adhesion strength for a cube should be 3-fold more than that of a sphere to compensate the membrane deformation energy at the upper edges of the cube.145 Because of a higher wrapping energy barrier for cubes compared to that for spheres, rigid spheres can be internalized faster than cubes.
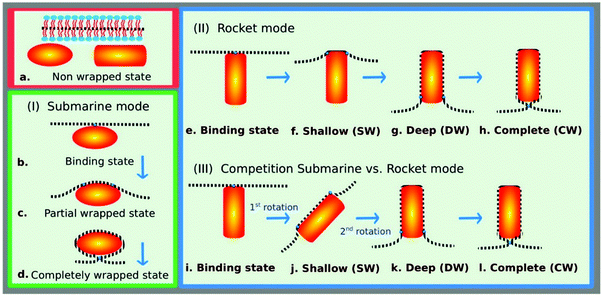 | ||
| Fig. 17 Modes of entry for nanoparticle uptake by membrane wrapping: (I) submarine mode with the long axis of the particles oriented parallel to the membrane, (II) rocket mode with the long axis oriented perpendicular to the membrane, and (III) competition between the submarine and rocket modes as observed for rod-like particles with high aspect ratios. The completely wrapped particle is connected by an infinitely small neck to the membrane; the particle orientation in this state is irrelevant. Reprinted with permission from ref. 145. Copyright © 2014 American Chemical Society. | ||
Indeed, the effect of the cubical hydrogel shape on the cell internalization was observed within the first 10 hours of the incubation of redox-sensitive PMAA multilayer hydrogel cubes and spheres with HeLa cells (Fig. 18).26 The DOX-loaded hydrogel spheres exhibited 12% higher cell cytotoxicity when incubated with HeLa cells for 10 hours as compared to that of hydrogel cubes, which indicated a more rapid internalization of spherical hydrogels, indicating an important role of the hydrogel particle shape in the membrane adhesion process in the initial steps of cell internalization. However, since both DOX-loaded (PMAA)5 spheres and cubes demonstrated similar cytotoxicity to HeLa cancer cells after longer incubation times of 24 and 48 hours, and given their similar DOX payload, long-term amounts of the hydrogel cubes or spheres internalized by HeLa cancer cells seemed to be similar.
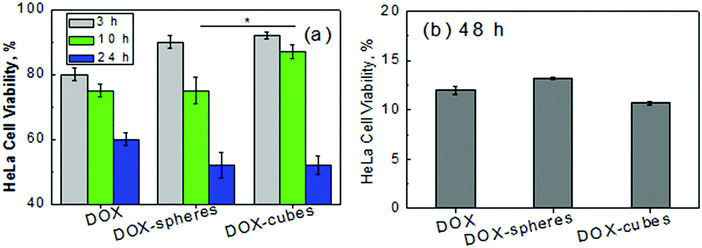 | ||
| Fig. 18 Viability of HeLa cells (% of negative control) after (a) 3, 10 and 24 and (b) 48 hours of incubation with free DOX, and DOX-loaded (PMAACS)5 cubes and spheres. Particle-free supernatants from suspensions of the DOX-loaded cubes and spheres were used as a negative control with the same volume as that of DOX-loaded particle suspensions. Each data point represents an average of four replicates ± std dev (*p < 0.05). Reproduced with permission from ref. 26. Copyright © 2016 American Chemical Society. | ||
Conversely, the cubical geometry of soft multilayer capsules was shown to promote the interaction of the particles with breast cancer cells.136 The cellular uptake of the cubical shells was demonstrated to be five-fold more efficient by human microvascular endothelial cells and six-fold and 2.5-fold more efficient by MDA-MB-231 and by SUM159 breast cancer cells, respectively, compared to the spherical capsules.136 This difference in the uptake efficiency was attributed to a softer structure of the cubical capsules.
Besides cellular internalization, the immunological cell responses have also been demonstrated to be modulated by particle shape. Although most studies on the effect of particle shape on immune responses have been conducted with solid inorganic particles, very recently soft PMAA hydrogel capsules have been demonstrated to induce shape-dependent cytokine secretion by human monocyte-derived macrophages (dTHP-1 cells).146 Unlike previously reported interactions with HeLa cancer cells, the thiolated (PMAA) hydrogel capsules with spherical, short rod- and long rod-shapes prepared by LbL assembly were internalized slightly differently by the dTHP-1 professional phagocytes showing slightly increased internalization levels of 90.6, 96, and 96.8% with increasing capsule aspect ratios.146 However, hydrogel capsules shaped like short rods promoted a stronger, 1.7-fold in TNF-α and 2.1-fold in IL-8, synthesis of pro-inflammatory cytokines by the macrophages compared to spherical and by 2.8- and 2.0-fold in TNF-α and IL-8 cytokines, respectively, compared to the long rod-shaped hydrogel capsules (Fig. 19).146 The exact mechanism for the observed effect of the hydrogel capsule shape on the release of these cytokines by the macrophage cells remains unclear, although this phenomenon could be utilized for the rational design of the hydrogel delivery vehicles.
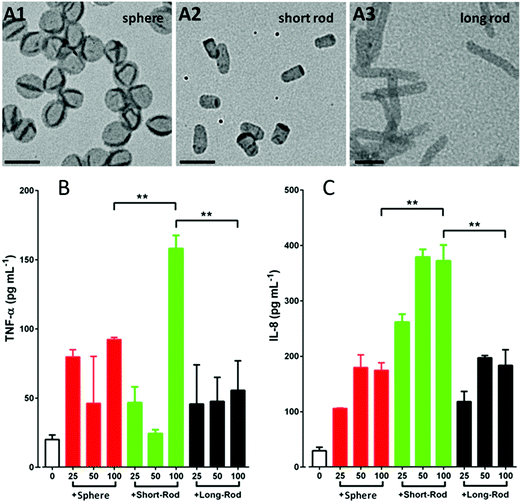 | ||
| Fig. 19 Transmission electron microscopy images of thiolated PMAA hydrogel capsules with spherical (A1) short rod (A2) and long rod (A3) shapes. Scale bar is 1 μm. (B) TNF-α and (C) IL-8 secretion from dTHP-1 cells treated with PMAA hydrogel capsules at capsule-to-cell ratios of 0, 25, 50, and 100 for 24 h (red: spherical capsules; green: short rod-shaped capsules; black: long rod-shaped capsules). Reproduced with permission from ref. 146. Copyright © 2016 American Chemical Society. | ||
5. Conclusion and outlook
This review summarizes a pool of experimental approaches for the design and synthesis of non-spherical hydrogel particles with well-defined shapes. Multiple scientific reports have indicated the great potential of environmentally responsive hydrogel biomaterials which utilize specific well-defined shapes. Biomedical applications including targeted drug delivery, selective cellular interactions, and controlled bio-distributions require reliable methods of particulate synthesis of well-defined shapes with imparted on-demand stimuli responsiveness. These particles additionally need to be able to navigate in biological fluids and interact with biological tissues in a controllable programmed way. These challenges are being faced with the development of new synthetic approaches and methods using various lithography techniques as well as expanding polymer assembly protocols. Strategies for the fabrication of non-spherical hydrogel particles are being developed to mimic the shape and flexibility of innate biological species in order to mimic and incorporate the architectural peculiarities essential to their biological functions. An emerging class of hydrogel particulates with a variety of shapes based on LbL assembly can be uniquely suitable for creating either hollow or filled porous particles of multilayer hydrogel-like networks with well-defined, precisely controlled size and shape. Considering the potentially regulated mechanical properties and interior structures of the resultant hydrogel particles, the multilayer assembly approach can be utilized for systematic investigation of fundamental relationships between the particle's architectural features and their effects on the non-spherical particle's behavior in flow and biological responses.Despite some progress in studies of non-spherical hydrogel particulates, the area of research still has many uncharted territories. For example, there is still need for the development of new strategies to scale-up the production of stimuli-responsive hydrogels and to further investigate their interaction with various tissues and cells. Also, there are only a few reports attempting to study the effect of temperature on shape changes of temperature-sensitive hydrogel particles which is especially intriguing in the case of hollow temperature-responsive particulates. The development of and studies on shape changes of soft hydrogel particulates induced by magnetic fields and other biomedically and biologically relevant triggers should be of further interest for researchers in the field of nanotechnology and nanomedicine.
Acknowledgements
This work was supported by the NSF CAREER Award #1350370. We also thank Mr Aaron Alford for technical assistance.References
- J. M. Knipe and N. A. Peppas, Multi-Responsive Hydrogels for Drug Delivery and Tissue Engineering Applications, Regener. Biomater., 2014, 57–65 CrossRef PubMed.
- S. Merino, C. Martín, K. Kostarelos, M. Prato and E. Vázquez, Nanocomposite Hydrogels: 3D Polymer Nanoparticle Synergies for On-Demand Drug Delivery, ACS Nano, 2015, 9, 4686–4697 CrossRef CAS PubMed.
- E. M. White, J. Yatvin, J. B. Grubbs III, J. A. Bilbrey and J. Locklin, Advances in Smart Materials: Stimuli-Responsive Hydrogel Thin Films, J. Polym. Sci., Part B: Polym. Phys., 2013, 51, 1084–1099 CrossRef CAS.
- X. Hu, J. Zhou, M. Vatankhah-Varnosfaderani, W. F. M. Daniel, Q. Li, A. P. Zhushma, A. V. Dobrynin and S. S. Sheiko, Programming Temporal Shapeshifting, Nat. Commun., 2016 DOI:10.1038/ncomms12919.
- J. Zhou and S. S. Sheiko, Reversible Shape-Shifting in Polymeric Materials, J. Polym. Sci., Part B: Polym. Phys., 2016, 54, 1365–1380 CrossRef CAS.
- H. Bysell, R. Månsson, P. Hansson and M. Malmsten, Microgels and Microcapsules in Peptide and Protein Drug delivery, Adv. Drug Delivery Rev., 2011, 63, 1172–1185 CrossRef CAS PubMed.
- S. Saxena, C. E. Hansen and L. A. Lyon, Microgel Mechanics in Biomaterial Design, Acc. Chem. Res., 2014, 47, 2426–2434 CrossRef CAS PubMed.
- B. Städler, A. D. Price, R. Chandrawati, L. Hosta-Rigau, A. N. Zelikin and F. Caruso, Polymer Hydrogel Capsules; En Route Toward Synthetic Cellular Systems, Nanoscale, 2009, 1, 68–73 RSC.
- L. K. Fiddes, E. W. Young, E. Kumacheva and A. R. Wheeler, Flow of Microgel Capsules through Topographically Patterned Microchannels, Lab Chip, 2007, 7, 863–867 RSC.
- B. Q. Y. Chan, Z. W. K. Low, S. J. W. Heng, S. Y. Chan, C. Owh and X. J. Loh, Recent Advances in Shape Memory Soft Materials for Biomedical Applications, ACS Appl. Mater. Interfaces, 2016, 8, 10070–10087 CAS.
- W. Nan, W. Wang, H. Gao and W. Liu, Fabrication of a Shape Memory Hydrogel Based on Imidazole-Zinc Ion Coordination for Potential Cell-Encapsulating Tubular Scaffold Application, Soft Matter, 2013, 9, 132–137 RSC.
- A. Yasin, W. Zhou, H. Yang, H. Li, Y. Chen and X. Zhang, Shape Memory Hydrogel Based on a Hydrophobically-Modified Polyacrylamide (HMPAM)/α-CD Mixture via a Host–Guest Approach, Macromol. Rapid Commun., 2015, 36, 845–851 CrossRef CAS PubMed.
- Y. Han, T. Bai and W. Liu, Controlled Heterogeneous Stem Cell Differentiation on a Shape Memory Hydrogel Surface, Sci. Rep., 2014, 4, 5815 CAS.
- M. Xie, L. Wang, J. Ge, B. Guo and P. X. Ma, Strong Electroactive Biodegradable Shape Memory Polymer Networks Based on Star-Shaped Polylactide and Aniline Trimer for Bone Tissue Engineering, ACS Appl. Mater. Interfaces, 2015, 7, 6772–6781 CAS.
- B. R. Saunders, N. Laajam, E. Daly, S. Teow, X. Hu and R. Stepto, Microgels: From Responsive Polymer Colloids to Biomaterials, Adv. Colloid Interface Sci., 2009, 147, 251–262 CrossRef PubMed.
- N. Doshi, A. S. Zahr, S. Bhaskar, J. Lahann and S. Mitragotri, Red Blood Cell-Mimicking Synthetic Biomaterial Particles, Proc. Natl. Acad. Sci. U. S. A., 2009, 106, 21495–21499 CrossRef CAS PubMed.
- T. J. Merkel, K. Chen, S. W. Jones, A. A. Pandya, S. Tian, M. E. Napier, W. E. Zamboni and J. M. DeSimone, The effect of particle size on the biodistribution of low-modulus hydrogel PRINT particles, J. Controlled Release, 2012, 162, 37–44 CrossRef CAS PubMed.
- T. J. Merkel, S. W. Jones, K. P. Herlihy, F. R. Kersey, A. R. Shields, M. Napier, J. C. Luft, H. Wu, W. C. Zamboni, A. Z. Wang, J. E. Bear and J. M. DeSimone, Using Mechanobiological Mimicry of Red Blood Cells to Extend Circulation Times of Hydrogel Microparticles, Proc. Natl. Acad. Sci. U. S. A., 2011, 108, 586–591 CrossRef CAS PubMed.
- R. Agarwal, V. Singh, P. Jurney, L. Shi, S. V. Sreenivasan and K. Roy, Mammalian Cells Preferentially Internalize Hydrogel Nanodiscs over Nanorods and Use Shape-Specific Uptake Mechanisms, Proc. Natl. Acad. Sci. U. S. A., 2013, 110, 17247–17252 CrossRef CAS PubMed.
- R. Haghgooie, M. Toner and P. S. Doyle, Squishy Non-Spherical Hydrogel Microparticles, Macromol. Rapid Commun., 2010, 31, 128–134 CAS.
- J. Xu, D. H. Wong, J. D. Byrne, K. Chen, C. Bowerman and J. M. DeSimone, Future of the Particle Replication in Nonwetting Templates (PRINT) Technology, Angew. Chem., Int. Ed., 2013, 52, 6580–6589 CrossRef CAS PubMed.
- J. Y. Kelly and J. M. DeSimone, Shape-Specific, Monodisperse Nano-Molding of Protein Particles, J. Am. Chem. Soc., 2008, 130, 5438–5439 CrossRef CAS PubMed.
- J. L. Perry, K. P. Herlihy, M. E. Napier and J. M. DeSimone, PRINT: A Novel Platform Toward Shape and Size Specific Nanoparticle Theranostics, Acc. Chem. Res., 2011, 44, 990–998 CrossRef CAS PubMed.
- K. G. Reuter, J. L. Perry, D. Kim, J. C. Luft, R. Liu and J. M. DeSimone, Targeted PRINT Hydrogels: The Role of Nanoparticle Size and Ligand Density on Cell Association, Biodistribution, and Tumor Accumulation, Nano Lett., 2015, 15, 6371–6378 CrossRef CAS PubMed.
- V. Kozlovskaya, J. Chen, C. Tedjo, X. Liang, J. Campos-Gomez, J. Oh, M. Saeed, C. T. Lungu and E. Kharlampieva, pH-Responsive Hydrogel Cubes for Release of Doxorubicin in Cancer Cells, J. Mater. Chem. B, 2014, 2, 2494–2507 RSC.
- B. Xue, V. Kozlovskaya, F. Liu, J. Chen, J. Williams, J. Campos-Gomez, M. Saeed and E. Kharlampieva, Intracellular Degradable Hydrogel Cubes and Spheres for Anticancer Drug Delivery, ACS App. Mater. Interfaces, 2015, 7, 13633–13644 CrossRef CAS PubMed.
- D. A. Canelas, K. P. Herlihy and J. M. DeSimone, Top-Down Particle Fabrication: Control of Size and Shape for Diagnostic Imaging and Drug Delivery, Wiley Interdiscip. Rev.: Nanomed. Nanobiotechnol., 2009, 1, 391–404 CrossRef CAS PubMed.
- J. P. Rolland, B. W. Maynor, L. E. Euliss, A. E. Exner, G. M. Denison and J. M. DeSimone, Direct Fabrication and Harvesting of Monodisperse, Shape-Specific Nanobiomaterials, J. Am. Chem. Soc., 2005, 127, 10096–10100 CrossRef CAS PubMed.
- L. C. Glangchai, M. Caldorera-Moore, L. Shi and K. Roy, Nanoimprint lithography based fabrication of shape-specific, enzymatically-triggered smart nanoparticles, J. Controlled Release, 2008, 125, 263–272 CrossRef CAS PubMed.
- M. C. Parrott, J. C. Luft, J. D. Byrne, J. H. Fain, M. E. Napier and J. M. DeSimone, Tunable Bifunctional Silyl Ether Cross-linkers for the Design of Acid-Sensitive Biomaterials, J. Am. Chem. Soc., 2010, 132, 17928–17932 CrossRef CAS PubMed.
- F. R. Kersey, T. J. Merkel, J. L. Perry, M. E. Napier and J. M. DeSimone, Effect of Aspect Ratio and Deformability on Nanoparticle Extravasation Through Nanopores, Langmuir, 2012, 28, 8773–8781 CrossRef CAS PubMed.
- S. S. Dunn, S. Tian, S. Blake, J. Wang, A. L. Galloway, A. Murphy, P. D. Pohlhaus, J. P. Rolland, M. E. Napier and J. M. DeSimone, Reductively Responsive siRNA-Conjugated Hydrogel Nanoparticles for Gene Silencing, J. Am. Chem. Soc., 2012, 134, 7423–7430 CrossRef CAS PubMed.
- M. Caldorera-Moore, M. K. Kang, Z. Moore, V. Singh, S. V. Sreenivasan, L. Shi, R. Huang and K. Roy, Swelling Behavior of Nanoscale, Shape- and Size-Specific, Hydrogel Particles Fabricated Using Imprint Lithography, Soft Matter, 2011, 7, 2879–2887 RSC.
- R. Agarwal, V. Singh, P. Jurney, L. Shi, S. V. Sreenivasan and K. Roy, Scalable Imprinting of Shape-Specific Polymeric Nanocarriers Using a Release Layer of Switchable Water Solubility, ACS Nano, 2012, 6, 2524–2531 CrossRef CAS PubMed.
- J. M. Williford, J. L. Santos, R. Shyam and H. Q. Mao, Shape Control in Engineering of Polymeric Nanoparticles for Therapeutic Delivery, Biomater. Sci., 2015, 3, 894–907 RSC.
- H. Tekin, T. Tsinman, J. G. Sanchez, B. J. Jones, G. Camci-Unal, J. W. Nichol, R. Langer and A. Khademhosseini, Responsive Micromolds for Sequential Patterning of Hydrogel Microstructures, J. Am. Chem. Soc., 2011, 133, 12944–12947 CrossRef CAS PubMed.
- T. S. Shim, S. H. Kim and S. M. Yang, Elaborate Design Strategies Toward Novel Microcarriers for Controlled Encapsulation and Release, Part. Part. Syst. Charact., 2013, 30, 9–45 CrossRef CAS.
- J. H. Jang, D. Dendukuri, T. A. Hatton, E. L. Thomas and P. S. Doyle, A Route to Three-Dimensional Structures in a Microfluidic Device: Stop-Flow Interference Lithography, Angew. Chem., Int. Ed., 2007, 119, 9185–9189 CrossRef.
- K. W. Bong, D. C. Pregibon and P. S. Doyle, Lock Release Lithography for 3D and Composite Microparticles, Lab Chip, 2009, 9, 863–866 RSC.
- D. Dendukuri, D. C. Pregibon, J. Collins, T. A. Hatton and P. S. Doyle, Continuous-Flow Lithography for High-Throughput Microparticle Synthesis, Nat. Mater., 2006, 5, 365–369 CrossRef CAS PubMed.
- N. Hakimi, S. S. Tsai, C. H. Cheng and D. K. Hwang, One-Step Two-Dimensional Microfluidics-Based Synthesis of Three-Dimensional Particles, Adv. Mater., 2014, 26, 1393–1398 CrossRef CAS PubMed.
- Y. Hu, S. Wang, A. Abbaspourrad and A. M. Ardekani, Fabrication of Shape Controllable Janus Alginate/PNIPAAm Microgels via Microfluidics Technique and Off-Chip Ionic Cross-linking, Langmuir, 2015, 31, 1885–1891 CrossRef CAS PubMed.
- G. C. Le Goff, J. Lee, A. Gupta, W. A. Hill and P. S. Doyle, High-Throughput Contact Flow Lithography, Adv. Sci., 2015, 2, 1500149 CrossRef.
- Multilayer Thin Films: Sequential Assembly of Nanocomposite Materials, ed. G. Decher and J. B. Schlenoff, Wiley, 2nd edn, 2012 Search PubMed.
- B. Neu, A. Voigt, R. Mitlöhner, S. Leporatti, C. Y. Gao, E. Donath, H. Kiesewetter, H. Möhwald, H. J. Meiselman and H. Bäumler, Biological Cells as Templates for Hollow Microcapsules, J. Microencapsulation, 2001, 18, 385–395 CrossRef CAS PubMed.
- J. Cui, M. P. van Koeverden, M. Müllner, K. Kempe and F. Caruso, Emerging Methods for the Fabrication of Polymer Capsules, Adv. Colloid Interface Sci., 2014, 207, 14–31 CrossRef CAS PubMed.
- F. Caruso, Hollow Capsule Processing Through Colloidal Templating and Self-Assembly, Chem. – Eur. J., 2000, 6, 413–419 CrossRef CAS.
- N. Elsner, V. Kozlovskaya, S. A. Sukhishvili and A. Fery, pH-Triggered Softening of Cross-linked Hydrogen-Bonded Capsules, Soft Matter, 2006, 2, 966–972 RSC.
- J. Cui, J. J. Richardson, M. Björnmalm, M. Faria and F. Caruso, Nanoengineered Templated Polymer Particles: Navigating the Biological Realm, Acc. Chem. Res., 2016, 49, 1139–1148 CrossRef CAS PubMed.
- A. L. Becker, A. P. R. Johnston and F. Caruso, Layer-by-Layer-Assembled Capsules and Films for Therapeutic Delivery, Small, 2010, 6, 1836–1852 CAS.
- M. Matsusaki and M. Akashi, Functional Multilayered Capsules for Targeting and Local Drug Delivery, Expert Opin. Drug Delivery, 2009, 6, 1207–1217 CrossRef CAS PubMed.
- L. L. Del Mercato, P. Rivera-Gil, A. Z. Abbasi, M. Ochs, C. Gnas, I. Zins, G. Sönnichsen and W. J. Parak, LbL Multilayer Capsules: Recent Progress and Future Outlook for Their Use in Life Sciences, Nanoscale, 2010, 2, 458–467 RSC.
- M. Delcea, H. Möhwald and A. G. Skirtach, Stimuli-responsive LbL Capsules and Nanoshells for Drug Delivery, Adv. Drug Delivery Rev., 2011, 63, 730–747 CrossRef CAS PubMed.
- Y. Jang, J. Seo, B. Akgun, S. Satija and K. Char, Molecular Weight Dependence on the Disintegration of Spin-Assisted Weak Polyelectrolyte Multilayer Films, Macromolecules, 2013, 46, 4580–4588 CrossRef CAS.
- J. J. Richardson, H. Ejima, S. L. Lçrcher, K. Liang, P. Senn, J. Cui and F. Caruso, Preparation of Nano- and Microcapsules by Electrophoretic Polymer Assembly, Angew. Chem., Int. Ed., 2013, 52, 6455–6458 CrossRef CAS PubMed.
- J. J. Richardson, K. Liang, K. Kempe, H. Ejima, J. Cui and F. Caruso, Immersive Polymer Assembly on Immobilized Particles for Automated Capsule Preparation, Adv. Mater., 2013, 25, 6874–6878 CrossRef CAS PubMed.
- J. J. Richardson, D. Teng, M. Björnmalm, S. T. Gunawan, J. Guo, J. Cui, G. V. Franks and F. Caruso, Fluidized Bed Layer-by-Layer Microcapsule Formation, Langmuir, 2014, 30, 10028–10034 CrossRef CAS PubMed.
- M. Björnmalm, A. Roozmand, K. F. Noi, J. Guo, J. Cui, J. J. Richardson and F. Caruso, Flow-Based Assembly of Layer-by-Layer Capsules through Tangential Flow Filtration, Langmuir, 2015, 31, 9054–9060 CrossRef PubMed.
- I. S. Elizarova and P. F. Luckham, Fabrication of Polyelectrolyte Multilayered Nano-Capsules using a Continuous Layer-by-Layer Approach, J. Colloid Interface Sci., 2016, 470, 92–99 CrossRef CAS PubMed.
- P. Lavalle, J.-C. Voegel, D. Vautier, B. Senger, P. Shaah and V. Ball, Dynamic Aspects of Films prepared by a Sequential Deposition of Species: Perspective for Smart and Responsive Materials, Adv. Mater., 2011, 23, 1191–1221 CrossRef CAS PubMed.
- C. Dejugnat and G. B. Sukhorukov, pH-Responsive Properties of Hollow Polyelectrolyte Microcapsules Templated on Various Cores, Langmuir, 2004, 20, 7265–7269 CrossRef CAS PubMed.
- T. Mauser, C. Dejugnat and G. B. Sukhorukov, Balance of Hydrophobic and Electrostatic Forces in the pH Response of Weak Polyelectrolyte Capsules, J. Phys. Chem. B, 2006, 110, 20246–20253 CrossRef CAS PubMed.
- S. Y. Yang and M. F. Rubner, Micropatterning of Polymer Thin Films with pH-Sensitive and Cross-linkable Hydrogen-Bonded Polyelectrolyte Multilayers, J. Am. Chem. Soc., 2002, 124, 2100–2101 CrossRef CAS PubMed.
- V. Kozlovskaya, S. Ok, A. Sousa, M. Libera and S. A. Sukhishvili, Hydrogen-Bonded Polymer Capsules Formed by Layer-by-Layer Self-Assembly, Macromolecules, 2003, 36, 8590–8592 CrossRef CAS.
- D. Lee, M. F. Rubner and R. E. Cohen, Formation of Nanoparticle-Loaded Microcapsules Based on Hydrogen-Bonded Multilayers, Chem. Mater., 2005, 17, 1099–1105 CrossRef CAS.
- S. Y. Yang, D. Lee, R. E. Cohen and M. F. Rubner, Bioinert Solution-Cross-linked Hydrogen-Bonded Multilayers on Colloidal Particles, Langmuir, 2004, 20, 5978–5981 CrossRef CAS PubMed.
- J. Kim, H.-J. Lim, Y. K. Hwang, H. Woo, J. W. Kim and K. Char, Template-Free Uniform-Sized Hollow Hydrogel Capsules with Controlled Shell Permeation and Optical Responsiveness, Langmuir, 2012, 28, 11899–11905 CrossRef CAS PubMed.
- E. Kharlampieva, V. Kozlovskaya and S. A. Sukhishvili, Layer-by-Layer Hydrogen-Bonded Polymer Films: From Fundamentals to Applications, Adv. Mater., 2009, 21, 3053–3065 CrossRef CAS.
- A. N. Zelikin, Q. Li and F. Caruso, Disulfide-Stabilized Poly(methacrylic acid) Capsules: Formation, Cross-linking, and Degradation behavior, Chem. Mater., 2008, 20, 2655–2661 CrossRef CAS.
- S.-F. Chong, J. H. Lee, A. N. Zelikin and F. Caruso, Tuning the Permeability of Polymer Hydrogel Capsules: An Investigation of Cross-linking Density, Membrane thickness, and Cross-linkers, Langmuir, 2011, 27, 1724–1730 CrossRef CAS PubMed.
- G. K. Such, A. P. R. Johnston and F. Caruso, Engineered Hydrogen-Bonded Polymer Multilayers: From Assembly to Biomedical Applications, Chem. Soc. Rev., 2011, 40, 19–29 RSC.
- Q. H. Zhao and B. Y. Li, pH-Controlled Drug Loading and Release from Biodegradable Microcapsules, Nanomed. Nanotech. Biol. Med., 2008, 4, 302–310 CrossRef CAS PubMed.
- V. Kozlovskaya, S. Yakovlev, M. Libera and S. A. Sukhishvili, Surface Priming and the Self-Assembly of Hydrogen-Bonded Multilayer Capsules and Films, Macromolecules, 2005, 38, 4828–4836 CrossRef CAS.
- V. Kozlovskaya, W. Higgins, J. Chen and E. Kharlampieva, Switching Shape of Layer-by-layer Hydrogel Microcontainers, Chem. Commun., 2011, 47, 8352–8354 RSC.
- V. Kozlovskaya, J. Baggett, B. Godin, X. Liu and E. Kharlampieva, Hydrogen-bonded Multilayers of Silk Fibroin: From Coatings to Cell-mimicking Shaped Microcontainers, ACS Macro Lett., 2012, 1, 384–387 CrossRef CAS PubMed.
- X. Liang, V. Kozlovskaya, Y. Chen, O. Zavgorodnya and E. Kharlampieva, Thermosensitive Multilayer Hydrogels of Poly(N-vinylcaprolactam) as Nanothin Films and Shaped Capsules, Chem. Mater., 2012, 24, 3707–3719 CrossRef CAS PubMed.
- A. Yashchenok, B. Parakhonskiy, S. Donatan, D. Kohler, A. Skirtach and H. Möhwald, Polyelectrolyte Multilayer Microcapsules Templated on Spherical, Elliptical and Square Calcium Carbonate Particles, J. Mater. Chem. B, 2013, 1, 1223–1228 RSC.
- O. Shchepelina, V. Kozlovskaya, E. Kharlampieva, W. Mao, A. Alexeev and V. V. Tsukruk, Anisotropic Micro- and Nano-Capsules, Macromol. Rapid Commun., 2010, 31, 2041–2046 CrossRef CAS PubMed.
- O. Shimoni, Y. Yan, Y. Wang and F. Caruso, Shape-Dependent Cellular Processing of Polyelectrolyte Capsules, ACS Nano, 2012, 7, 522–530 CrossRef PubMed.
- X. Zan, A. Garapaty and J. A. Champion, Engineering Polyelectrolyte Capsules with Independently Controlled Size and Shape, Langmuir, 2015, 31, 7601–7608 CrossRef CAS PubMed.
- V. Kozlovskaya, J. Alexander, Y. Wang, T. Kuncewicz, X. Liu, B. Godin and E. Kharlampieva, Internalization of Red Blood Cell-mimicking Hydrogel Capsules with pH-triggered Shape Responses, ACS Nano, 2014, 8, 5725–5737 CrossRef CAS PubMed.
- H. Ejima, N. Yanai, J. P. Best, M. Sindoro, S. Granick and F. Caruso, Near-Incompressible Faceted Polymer Microcapsules from Metal-Organic Framework Templates, Adv. Mater., 2013, 25, 5767–5771 CrossRef CAS PubMed.
- P. J. Flory, Principles of Polymer Chemistry, Cornell University Press, Ithaca, NY, 1953 Search PubMed.
- G. Gao, E. Donath, S. Moya, V. Dudnik and H. Möhwald, Elasticity of Hollow Polyelectrolyte Capsules Prepared by the Layer-by-Layer Technique, Eur. Phys. J. E: Soft Matter Biol. Phys., 2001, 5, 21–27 CrossRef.
- V. Kozlovskaya, Y. Wang, W. Higgins, J. Chen, Y. Chen and E. Kharlampieva, pH-Triggered Shape Response of Cubical Ultrathin Hydrogel Capsules, Soft Matter, 2012, 8, 9828–9839 RSC.
- F. Liu, V. Kozlovskaya, O. Zavgorodnya, C. Martinez-Lopez, S. Catledge and E. Kharlampieva, Encapsulation of Anticancer Drug with Hydrogen-Bonded Multilayers of Tannic Acid, Soft Matter, 2014, 10, 9237–9247 RSC.
- Y. Wang and F. Caruso, Template Synthesis of Stimuli-Responsive Nanoporous Polymer-Based Spheres via Sequential Assembly, Chem. Mater., 2006, 18, 4089–4100 CrossRef CAS.
- Y. Wang, A. D. Price and F. Caruso, Nanoporous Colloids: Building Blocks for a New Generation of Structured Materials, J. Mater. Chem., 2009, 19, 6451–6464 RSC.
- Y. Yan, Z. W. Lai, R. J. A. Goode, J. Cui, T. Bacic, M. M. J. Kamphuis, E. C. Nice and F. Caruso, Particles on the Move: Intracellular Trafficking and Asymmetric Mitotic Partitioning of Nanoporous Polymer Particles, ACS Nano, 2013, 7, 5558–5567 CrossRef CAS PubMed.
- R. Cairns, I. Papandreou and N. Denko, Overcoming Physiologic Barriers to Cancer Treatment by Molecularly Targeting the Tumor Microenvironment, Mol. Cancer Res., 2006, 4, 61–70 CrossRef CAS PubMed.
- P. Vaupel, F. Kallinowski and P. Okunieff, Blood Flow, Oxygen and Nutrient Supply, and Metabolic Microenvironment of Human Tumors: A Review, Cancer Res., 1989, 49, 6449–6465 CAS.
- B. P. Mahoney, N. Raghunand, B. Baggett and R. J. Gillies, Tumor Acidity, Ion Trapping and Chemotherapeutics. I. Acid pH Affects the Distribution of Chemotherapeutic Agents In Vitro, Biochem. Pharmacol., 2003, 66, 1207–1218 CrossRef CAS PubMed.
- E. Fleige, M. A. Quadir and R. Haag, Stimuli-Responsive Polymeric Nanocarriers for the Controlled Transport of Active Compounds: Concepts and Applications, Adv. Drug Delivery Rev., 2012, 64, 866–884 CrossRef CAS PubMed.
- N. Demaurex, pH Homeostasis of Cellular Organelles, News Physiol. Sci., 2002, 17, 1–5 CAS.
- M. C. Koetting, J. T. Peters, S. D. Steichen and N. A. Peppas, Stimulus-Responsive Hydrogels: Theory, Modern Advances, and Applications, Mater. Sci. Eng., R, 2015, 93, 1–49 CrossRef PubMed.
- J. Klier, A. B. Scranton and N. A. Peppas, Self-Associating Networks of Poly(methacrylic acid-g-ethylene glycol), Macromolecules, 1990, 23, 4944–4949 CrossRef CAS.
- L. Deng, Y. Zhai, X. Lin, F. Jin, X. He and A. Dong, Investigation on Properties of Re-dispersible Cationic Hydrogel Nanoparticles, Eur. Polym. J., 2008, 44, 978–986 CrossRef CAS.
- S. V. Vinogradov, T. K. Bronich and A. V. Kabanov, Nanosized Cationic Hydrogels for Drug Delivery: Preparation, Properties and Interactions with Cells, Adv. Drug Delivery Rev., 2002, 54, 135–147 CrossRef CAS PubMed.
- M. C. Parrott, M. Finniss, J. C. Luft, A. Pandya, A. Gullapalli, M. E. Napier and J. M. DeSimone, Incorporation and Controlled Release of Silyl Ether Prodrugs from PRINT Nanoparticles, J. Am. Chem. Soc., 2012, 134, 7978–7982 CrossRef CAS PubMed.
- D. K. Hwang, J. Oakey, M. Toner, J. A. Arthur, K. S. Anseth, S. Lee, A. Zeiger, K. J. Van Vliet and P. S. Doyle, Stop-Flow Lithography for the Production of Shape-Evolving Degradable Microgel Particles, J. Am. Chem. Soc., 2009, 131, 4499–4504 CrossRef CAS PubMed.
- S. Y. Chou, P. R. Krauss and P. J. Renstrom, Imprint Lithography with 25-Nanometer Resolution, Science, 1996, 272, 85–87 CAS.
- S. Q. Xu, Z. H. Nie, M. Seo, P. Lewis, E. Kumacheva, H. A. Stone, P. Garstecki, D. B. Weibel, I. Gitlin and G. M. Whitesides, Generation of Monodisperse Particles by Using Microfluidics: Control over Size, Shape, and Composition, Angew. Chem., Int. Ed., 2005, 44, 724–728 CrossRef CAS PubMed.
- M. Geissler and Y. N. Xia, Patterning: Principles and Some New Developments, Adv. Mater., 2004, 16, 1249–1269 CrossRef CAS.
- T. Tanaka and D. J. Fillmore, Kinetics of Swelling of Gels, J. Chem. Phys., 1979, 70, 1214–1218 CrossRef CAS.
- M. T. Am Ende, D. Hariharan and N. A. Peppas, Factors Influencing Drug and Protein Transport and Release from Ionic Hydrogels, React. Polym., 1995, 25, 127–137 CrossRef CAS.
- V. Kozlovskaya, E. Kharlampieva, I. Erel and S. A. Sukhishvili, Multilayer-Derived, Ultrathin, Stimuli-Responsive Hydrogels, Soft Matter, 2009, 5, 4077–4087 RSC.
- O. Shchepelina, V. Kozlovskaya, E. Kharlampieva, W. Mao, A. Alexeev and V. V. Tsukruk, Cubic and Tetrahedral Anisotropic Hollow Micro- and Submicron Capsules Based on Tannic Acid, Macromol. Rapid Commun., 2010, 31, 2041–2046 CrossRef CAS PubMed.
- T. S. Shim, S. H. Kim, C. J. Heo, H. C. Jeon and S.-M. Yang, Controlled Origami Folding of Hydrogel Bilayers with Sustained Reversibility for Robust Microcarriers, Angew. Chem., Int. Ed., 2012, 51, 1420–1423 CrossRef CAS PubMed.
- O. Shimoni, A. Postma, Y. Yan, A. M. Scott, J. K. Heath, E. C. Nice, A. N. Zelikin and F. Caruso, Macromolecule Functionalization of Disulfide-Bonded Polymer Hydrogel Capsules and Cancer Cell Targeting, ACS Nano, 2012, 6, 1463–1472 CrossRef CAS PubMed.
- R. Cheng, F. Feng, F. H. Meng, C. Deng, J. Feijen and Z. Y. Zhong, Glutathione-Responsive Nano-Vehicles as a Promising Platform for Targeted Intracellular Drug and Gene Delivery, J. Controlled Release, 2011, 152, 2–12 CrossRef CAS PubMed.
- B. Arunachalam, U. T. Phan, H. J. Geuze and P. Cresswell, Enzymatic Reduction of Disulfide Bonds in Lysosomes: Characterization of a Gamma-Interferon-Inducible Lysosomal Thiol Reductase (GILT), Proc. Natl. Acad. Sci. U. S. A., 2000, 97, 745–750 CrossRef CAS.
- W. Chen, Y. Zou, F. H. Meng, R. Cheng, C. Deng, J. Feijen and Z. Y. Zhong, Functional Poly(ε-caprolactone)s via Copolymerization of ε-Caprolactone and Pyridyl Disulfide-Containing Cyclic Carbonate: Controlled Synthesis and Facile Access to Reduction-Sensitive Biodegradable Graft Copolymer Micelles, Macromolecules, 2013, 46, 699–707 CrossRef CAS.
- Y. Yan, Y. Wang, J. K. Heath, E. C. Nice and F. Caruso, Cellular Association and Cargo Release of Redox-Responsive Polymer Capsules Mediated by Exofacial Thiols, Adv. Mater., 2011, 23, 3916–3921 CrossRef CAS PubMed.
- A. N. Zelikin, Q. Li and F. Caruso, Degradable Polyelectrolyte Capsules Filled with Oligonucleotide Sequences, Angew. Chem., Int. Ed., 2006, 45, 7743–7745 CrossRef CAS PubMed.
- Y. C. Wang, F. Wang, T. M. Sun and J. Wang, Redox-Responsive Nanoparticles from the Single Disulfide Bond-Bridged Block Copolymer as Drug Carriers for Overcoming Multidrug Resistance in Cancer Cells, Bioconjugate Chem., 2011, 22, 1939–1945 CrossRef CAS PubMed.
- N. Gao, S. Lü, C. Gao, X. Wang, X. Xu, X. Bai, C. Feng and M. Liu, Injectable Shell-Cross-linked F127 Micelle/Hydrogel Composites with pH and Redox Sensitivity for Combined Release of Anticancer Drugs, Chem. Eng. J., 2016, 287, 20–29 CrossRef CAS.
- M. Huo, J. Yuan, L. Tao and Y. Wei, Redox-Responsive Polymers for Drug Delivery: From Molecular Design to Applications, Polym. Chem., 2014, 5, 1519–1528 RSC.
- F. Cavalieri, G. L. Beretta, J. Cui, J. A. Braunger, Y. Yan, J. J. Richardson, S. Tinelli, M. Folini, N. Zaffaroni and F. Caruso, Redox-Sensitive PEG–Polypeptide Nanoporous Particles for Survivin Silencing in Prostate Cancer Cells, Biomacromolecules, 2015, 16, 2168–2178 CrossRef CAS PubMed.
- N. Kamaly, B. Yameen, J. Wu and O. C. Farokhzad, Degradable Controlled-Release Polymers and Polymeric Nanoparticles: Mechanisms of Controlling Drug Release, Chem. Rev., 2016, 116, 2602–2663 CrossRef CAS PubMed.
- J. Xu, J. Wang, J. C. Luft, S. Tian, G. Owens Jr, A. A. Pandya, P. Berglund, P. Pohlhaus, B. W. Maynor, J. Smith, B. Hubby, M. E. Napier and J. M. DeSimone, Rendering protein-based particles transiently insoluble for therapeutic applications, J. Am. Chem. Soc., 2012, 134, 8774–8777 CrossRef CAS PubMed.
- J. Xu, J. C. Luft, X. Yi, S. Tian, G. Owens, J. Wang, A. Johnson, P. Berglund, J. Smith, M. E. Napier and J. M. DeSimone, RNA Replicon Delivery via Lipid-Complexed PRINT Protein Particles, Mol. Pharmaceutics, 2013, 10, 3366–3374 CrossRef CAS PubMed.
- R. A. Petros, P. A. Ropp and J. M. DeSimone, Reductively Labile PRINT Particles for the Delivery of Doxorubicin to HeLa Cells, J. Am. Chem. Soc., 2008, 130, 5008–5009 CrossRef CAS PubMed.
- D. Ma, S. Tian, J. Baryza, J. C. Luft and J. M. DeSimone, Reductively Responsive Hydrogel Nanoparticles with Uniform Size, Shape, and Tunable Composition for Systemic siRNA Delivery in Vivo, Mol. Pharmaceutics, 2015, 12, 3518–3526 CrossRef CAS PubMed.
- S. S. Dunn, S. Tian, S. Blake, J. Wang, A. L. Galloway, A. Murphy, P. D. Pohlhaus, J. P. Rolland, M. E. Napier and J. M. DeSimone, Reductively Responsive siRNA-Conjugated Hydrogel Nanoparticles for Gene Silencing, J. Am. Chem. Soc., 2012, 134, 7423–7430 CrossRef CAS PubMed.
- A. S. Dubivik, E. Makhaeva, V. Y. Grinberg and A. R. Khokhlov, Energetics of Cooperative Transitions of N-Vinylcaprolactam Polymers in Aqueous Solutions, Macromol. Chem. Phys., 2005, 206, 915–928 CrossRef.
- J. Ramos, A. Imaz and J. Forcada, Temperature-Sensitive Nanogels: Poly(N-vinylcaprolactam) versus poly(N-isopropylacrylamide), Polym. Chem., 2012, 3, 852–856 RSC.
- N. A. Cortez-Lemus and A. Licea-Claverie, Poly(N-vinylcaprolactam), A Comprehensive Review on a Thermoresponsive Polymer Becoming Popular, Prog. Polym. Sci., 2015, 53, 1–51 CrossRef.
- M. Sun, A. Zhu, Q. Zhang, M. Ye and Q. Liu, Smart Shape-Controlled Synthesis of Poly(N-isopropylacrylamide)/Chitosan/Fe3O4 Microgels, Eur. Polym. J., 2015, 66, 569–576 CrossRef CAS.
- X. Z. Zhang, G. M. Sun and C. C. Chu, Temperature Sensitive Dendrite-Shaped PNIPAAm/Dex-AI Hybrid Hydrogel Particles: Formulation and Properties, Eur. Polym. J., 2004, 40, 2251–2257 CrossRef CAS.
- J. J. Crassous, A. M. Mihut, L. K. Månsson and P. Schurtenberger, Anisotropic Responsive Microgels with Tuneable Shape and Interactions, Nanoscale, 2015, 7, 15971–15982 RSC.
- X. Liang, V. Kozlovskaya, Y. Chen, O. Zavgorodnya and E. Kharlampieva, Thermosensitive Multilayer Hydrogels of Poly(N-vinylcaprolactam) as Nanothin Films and Shaped Capsules, Chem. Mater., 2012, 24, 3707–3719 CrossRef CAS PubMed.
- K. Ulbrich, T. Etrych, P. Chytil, M. Jelınková and B. Říhová, HPMA copolymers with pH-controlled release of doxorubicin: in vitro cytotoxicity and in vivo antitumor activity, J. Controlled Release, 2003, 87, 33–47 CrossRef CAS PubMed.
- K. Chen, T. J. Merkel, A. Pandya, M. E. Napier, J. C. Luft, W. Daniel, S. Sheiko and J. M. DeSimone, Low Modulus Biomimetic Microgel Particles with High Loading of Hemoglobin, Biomacromolecules, 2012, 13, 2748–2759 CrossRef CAS PubMed.
- F. R. Kersey, T. J. Merkel, J. L. Perry, M. E. Napier and J. M. DeSimone, Effect of Aspect Ratio and Deformability on Nanoparticle Extravasation through Nanopores, Langmuir, 2012, 28, 8773–8781 CrossRef CAS PubMed.
- S. She, Q. Li, B. Shan, W. Tong and C. Gao, Fabrication of Red-Blood-Cell-Like Polyelectrolyte Microcapsules and Their Deformation and Recovery Behavior Through a Microcapillary, Adv. Mater., 2013, 25, 5814–5818 CrossRef CAS PubMed.
- J. F. Alexander, V. Kozlovskaya, J. Chen, T. Kuncewicz, E. Kharlampieva and B. Godin, Cubical Shape Enhances the Interaction of Layer-by-Layer Polymeric Particles with Breast Cancer Cells, Adv. Healthcare Mater., 2015, 4, 2657–2666 CrossRef CAS PubMed.
- X. Chen, J. Cui, H. Sun, M. Müllner, Y. Yan, K. F. Noi, Y. Ping and F. Caruso, Analysing Intracellular Deformation of Polymer Capsules Using Structured Illumination Microscopy, Nanoscale, 2016, 8, 11924–11931 RSC.
- B. Yu, H. Cong, X. Liu, Y. Ren, J. Wang, L. Zhang, J. Tang, Y. Ma and T. Akasaka, Preparation of Monodisperse PEG Hydrogel Composite Microspheres via Microfluidic Chip with Rounded Channels, J. Micromech. Microeng., 2013, 23, 095016 CrossRef.
- D. K. Hwang, D. Dendukuri and P. S. Doyle, Microfluidic-based synthesis of non-spherical magnetic hydrogel microparticles, Lab Chip, 2008, 8, 1640–1647 RSC.
- K. Hayashi, K. Ono, H. Suzuki, M. Sawada, M. Moriya, W. Sakamoto and T. Yogo, Electrosprayed Synthesis of Red-Blood-Cell-Like Particles with Dual Modality for Magnetic Resonance and Fluorescence Imaging, Small, 2010, 6, 2384–2391 CrossRef CAS PubMed.
- Y. Yan, M. Björnmalm and F. Caruso, Assembly of Layer-by-Layer Particles and Their Interactions with Biological Systems, Chem. Mater., 2013, 26, 452–460 CrossRef.
- S. E. A. Gratton, P. A. Ropp, P. D. Pohlhaus, J. C. Luft, V. J. Madden, M. E. Napier and J. M. DeSimone, The effect of particle design on cellular internalization pathways, Proc. Natl. Acad. Sci. U. S. A., 2008, 105, 11613–11618 CrossRef CAS PubMed.
- L. Florez, C. Herrmann, J. M. Cramer, C. P. Hauser, K. Koynov, K. Landfester, D. Crespy and V. Mailänder, How Shape Influences Uptake: Interactions of Anisotropic Polymer Nanoparticles and Human Mesenchymal Stem Cells, Small, 2012, 8, 2222–2230 CrossRef CAS PubMed.
- Y. Zhang, S. Tekobo, Y. Tu, Q. Zhou, X. Jin, S. A. Dergunov, E. Pinkhassik and B. Yan, Permission to Enter Cell by Shape: Nanodisk vs Nanosphere, ACS Appl. Mater. Interfaces, 2012, 4, 4099–4105 CAS.
- S. Dasgupta, T. Auth and G. Compper, Shape and Orientation Matter for the Cellular Uptake of Nonspherical Particles, Nano Lett., 2014, 14, 687–693 CrossRef CAS PubMed.
- X. Chen, Y. Yan, M. Müllner, Y. Ping, J. Cui, K. Kempe, C. Cortez-Jugo and F. Caruso, Shape-Dependent Activation of Cytokine Secretion by Polymer Capsules in Human Monocyte-Derived Macrophages, Biomacromolecules, 2016, 17, 1205–1212 CrossRef CAS PubMed.
Footnote |
| † Chemistry Department, University of Alabama at Birmingham, 901 14th St South, CHEM 279, Alabama, 35294, USA. E-mail: ekharlam@uab.edu |
| This journal is © The Royal Society of Chemistry 2017 |



