Theoretical design of solid electrolytes with superb ionic conductivity: alloying effect on Li+ transportation in cubic Li6PA5X chalcogenides†
Abstract
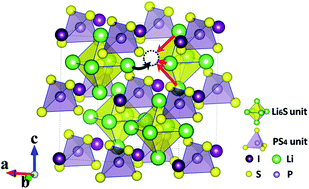
It is of great importance to develop solid inorganic electrolytes with high ionic conductivity, which would thus enable solid-state Li-ion batteries to overcome the notorious safety issues with the current technology due to the use of highly flammable liquid organic electrolytes. On the basis of systematic first-principles modelling, we have formulated new inorganic electrolytes with ultra-low activation energies for the long-distance diffusion of Li+ ions by alloying cubic argyrodite Li6PA5X chalcogenides (A = chalcogen; X = halogen). We found that the long-distance transportation of Li+ ions is dictated by inter-octahedral diffusion, as the activation energy required for Li+ ions to migrate over an Li6A octahedron is minimal. The inter-octahedral diffusion barrier for Li+ ions is largely dependent on their interaction with chalcogen anions in the compound. A radical reduction of the diffusion barrier for Li+ ions can be realized through isovalent substitution of S using elements of lower electronegativity, together with smaller halogen ions at X sites.


 Please wait while we load your content...
Please wait while we load your content...