One-step synthesis of multilayered 2D Sn nanodendrites as a high-performance anode material for Na-ion batteries†
Abstract
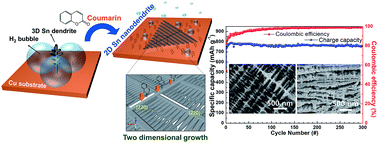
Multilayered 2D Sn nanodendrites were synthesized by a one-step electrodeposition process from an aqueous solution containing coumarin (C9H6O2). Under a high cathodic current density that is necessary for dendritic growth, hydrogen bubbles evolved with Sn reduction. The hydrogen bubbles limited the diffusion path of the Sn ions to space between hydrogen bubbles and concealed the splitting sites for additional branches, which suppressed the development of the branches. The addition of coumarin greatly reduced the departure diameter of the hydrogen bubbles and thus made the growth of the Sn dendrites with a high density of nanosized branches possible. Furthermore, coumarin suppressed Sn crystallite growth along the [001] direction, promoting two-dimensional growth of the 2D Sn nanodendrites along the set of [220] directions that are normal to the [001] direction. These 2D Sn nanodendrites grew layer-by-layer on a substrate, forming a unique multilayered structure. As an anode material for Na-ion batteries, the multilayered 2D Sn nanodendrites exhibited a high specific capacity of 783.88 mA h g−1 and excellent cycle performance with 96.6% capacity retention over 300 cycles. Moreover, the multilayered 2D Sn nanodendrites showed a superior rate capability of 412.84 mA g−1 at 5C. The outstanding performance is attributed to a high density of free space inside the multilayered 2D Sn nanodendrites and the nanosized Sn branches that offer short diffusion paths for Na ions.


 Please wait while we load your content...
Please wait while we load your content...