Pressure-induced abnormal insulating state in triangular layered cobaltite LixCoO2 (x = 0.9)†
Abstract
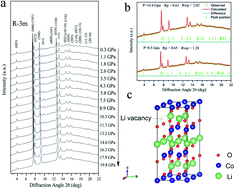
Lithium cobaltite oxides (LixCoO2) have been serving as an important rechargeable battery material with reversible extraction and insertion of lithium ions. During the charge–discharge process, 50% or more amount of lithium can be extracted, and the layered structure remains stable with a semiconductor to metal transition at around x = 0.7. Static high pressure, an effective tool to tune crystal and electronic structure, is utilized herein in the most studied layered compound Li0.9CoO2 to investigate the effects on the structural stability and transport properties by synchrotron X-ray diffraction (XRD), electric resistivity, UV-vis absorption spectroscopy, and ab initio calculations. Up to 19.8 GPa, no structural phase transition was observed, but surprisingly its electric transport behavior changed from a semiconducting state to an insulating state. The detailed XRD and UV-vis spectroscopy analysis reveals that the pressure-induced Co–O bond length shrinkage in the CoO6 octahedron enhances crystal field splitting, which leads to band gap opening, and the decrease in Co–Co distance causes the t2g bands to overlap and the electron holes to be localized. The profoundly different response of the ground state to high pressure indicates an unusual, delicate interplay between the crystal structure and the electronic structure in LixCoO2 and may provide a new route for the development of lithium-ion battery with high performance under the assistance of pressure.


 Please wait while we load your content...
Please wait while we load your content...