A new perspective of the ruthenium ion: a bifunctional soluble catalyst for high efficiency Li–O2 batteries†
Abstract
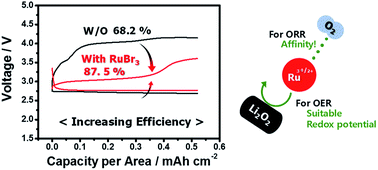
The slow kinetics of Li–O2 batteries cause a large overpotential, which leads to low round-trip efficiency and poor cyclability. Applying a suitable bifunctional catalyst can be an effective way to solve this problem. We anticipated that the ruthenium ion dissolved in the electrolyte will not only overcome the disadvantages of solid phase catalysts, but also reduce the overpotential for both charge and discharge. This is possible due to the suitable redox potential of the ruthenium ion, which effectively reduced the oxygen evolution reaction (OER) overpotential, and the affinity between the ruthenium ion and oxygen, which facilitated the oxygen reduction reaction (ORR) and suppressed the degradation of the cathode. Here, we propose a new soluble catalyst, ruthenium bromide, for Li–O2 batteries. The battery using ruthenium bromide clearly exhibited enhanced cycling performance, increasing round-trip efficiency (from 68.2% to 80.5%) and rate capability. A new understanding and application of the soluble ruthenium catalyst, which is commonly used as a solid catalyst, will not only overcome the existing problems but also provide a promising platform for Li–O2 batteries.


 Please wait while we load your content...
Please wait while we load your content...