BCN network-encapsulated multiple phases of molybdenum carbide for efficient hydrogen evolution reactions in acidic and alkaline media†
Abstract
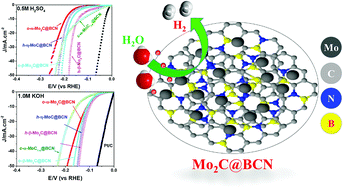
Five phases of molybdenum carbide encapsulated by a boron–carbon–nitrogen (BCN) network are synthesized by decomposition of a Mo–imidazole-borate organometallic complex with slight variations in the imidazole-borate ligand structure. The method relies on the restrained in situ carburization reaction between Mo atoms and imidazole-borate ligands on an atomic scale, thus generating molybdenum carbide nanoparticles encapsulated conformally by the BCN shell. All phases of molybdenum carbide demonstrate excellent electrocatalytic hydrogen evolution reaction (HER) activity and stability in both acidic and basic electrolytes outperforming most of molybdenum carbides reported in the literature. Hexagonal β-Mo2C@BCN consistently exhibits the most outstanding performance under all conditions. The less active cubic α and hexagonal η phases also display enhanced HER activity and stability due to the promotional effect of the BCN shell. The dual natured (electrophilic and nucleophilic) BCN layers can protect molybdenum carbide nanoparticles from corrosion and agglomeration, and improve their electrocatalytic activity by serving as an electron transfer medium and providing ample adsorption sites for water due to enhanced wetting properties.


 Please wait while we load your content...
Please wait while we load your content...