3D porous nanostructured platinum prepared using atomic layer deposition†
Abstract
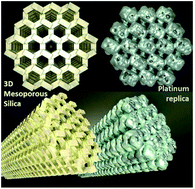
A robust and easy to handle 3D porous platinum structure was created via replicating the 3D channel system of an ordered mesoporous silica material using atomic layer deposition (ALD) over micrometer distances. After ALD of Pt in the silica material, the host template was digested using hydrogen fluoride (HF). A fully connected ordered Pt nanostructure was obtained with morphology and sizes corresponding to that of the pores of the host matrix, as revealed with high-resolution scanning transmission electron microscopy and electron tomography. The Pt nanostructure consisted of hexagonal Pt rods originating from the straight mesopores (11 nm) of the host structure and linking features resulting from Pt replication of the interconnecting mesopore segments (2–4 nm) present in the silica host structure. Electron tomography of partial replicas, made by incomplete infilling of Zeotile-4 material with Pt, provided insight in the connectivity and formation mechanism of the Pt nanostructure by ALD. The Pt replica was evaluated for its potential use as electrocatalyst for the hydrogen evolution reaction, one of the half-reactions of water electrolysis, and as microelectrode for biomedical sensing. The Pt replica showed high activity for the hydrogen evolution reaction and electrochemical characterization revealed a large impedance improvement in comparison with reference Pt electrodes.


 Please wait while we load your content...
Please wait while we load your content...