Study on a reliable epoxy-based phase change material: facile preparation, tunable properties, and phase/microphase separation behavior†
Abstract
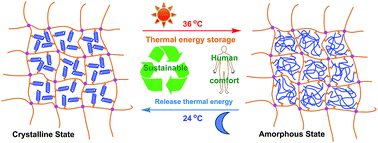
Room-temperature-use phase change materials (PCMs) are of vital importance in combining the sustainable development of energy with human comfort. Here, a series of novel epoxy-based polymeric solid–solid PCMs (SSPCMs) were facilely prepared by UV grafting 1-octadecanethiol (ODT) onto an allyl-based epoxy resin (DADGEBA) via thiol–ene click chemistry followed by the epoxy–amine thermal curing process. Therefore, the grafted ODT can be tightly locked in a reliable three-dimensional crosslinking network of the epoxy resin, which provides the PCMs with excellent shape-stable property. Phase change and mechanical properties characterized by DSC, DMA, hardness test, and tensile test can be easily tuned by adjusting the mass ratio of DGEBA and the ODT-grafted-DADGEBA product (D18), which was characterized by FTIR and NMR. XRD and POM analyses proved the crystallinity of EPD18-X PCMs. The structure and morphology of the EPD18-X PCMs were characterized by visual images, SEM, and POM analyses. Microphase separation was observed in all the EPD18-X PCMs, and an obvious phase separation was observed in the EPD18-25 system. However, the phase separation gradually disappeared with the increasing of the D18 content. Thermal recycling tests showed that EPD18-X PCMs can remain stable after 50 DSC thermal cycles. Due to the unique strong encapsulated epoxy curing networks, EPD18-X PCMs have excellent thermal stability with the onset degradation temperature higher than 250 °C. Tunable EPD18-X systems can be applied for room-temperature-use thermal energy storage applications such as buildings, thermoregulated fabrics, and so on.


 Please wait while we load your content...
Please wait while we load your content...