Mechanically robust and shape-memory hybrid aerogels for super-insulating applications†
Abstract
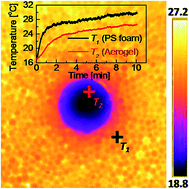
Super-insulating aerogels are promising materials to improve the energy efficiency of buildings. However, fabricating super-insulating yet mechanically robust and shape-memory aerogels remains challenging. Here, we integrate graphene oxide and a block copolymer to fabricate hybrid aerogels with triple networks and systematically study their mechanical and thermal properties. We show that the first network serves as sacrificial bonds and dissipates energy upon deformation, enabling the aerogels to have a high mechanical performance. The second network allows the aerogels to memorize the original permanent shape, and the third network is able to store strain energy and fix the aerogels in a temporary shape by vitrification. Remarkably, the strong phonon-scattering effect generated by the enormous interfaces between the three networks yields an ultra-low solid thermal conductivity of ∼8 mW m−1 K−1. The multi-functionality makes this class of hybrid aerogels particularly suitable for super-insulation applications on complex surfaces and in small spaces of buildings, industry and spacecrafts.


 Please wait while we load your content...
Please wait while we load your content...