Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
The bakery of high-end sorption carbons: sugar–urea doughs as processable precursors for functional carbons†
Regina
Rothe
,
Markus
Antonietti
and
Nina
Fechler
 *
*
Max Planck Institute of Colloids and Interfaces, Department of Colloid Chemistry, Research Campus Golm, 14424 Potsdam, Germany. E-mail: nina.fechler@mpikg.mpg.de
First published on 5th May 2017
Abstract
Via utilizing a phenomenon that is usually observed in the food industry, a scalable, safe, and cheap synthesis method for processable and functional porous carbons has been presented. Using simple sugars in combination with urea, low melting liquids can be formed that are also stable at room temperature without recrystallization. Due to low vapor pressure, these melts can be easily stored and handled for further use as carbon precursors. The resulting carbon materials possess high nitrogen content and are obtained in high yield. The liquid precursor state also allows the addition of further substances, and highly porous carbon monoliths can be formed by the introduction of a salt/fiber mixture. Herein, cellulose was found to serve as an efficient additive, which resulted in processable viscoelastic doughs without the alteration of the final carbon properties. This method is of special importance with regard to industrial processes as compared to standard carbonization, and it becomes possible to handle intermediates in a green body fashion. Eventually, compared to pre-baked bread rolls, storable intermediates can be processed which inherently contain already all the final carbon properties. The carbon cookies were stable against oxidation and were shown to be highly suitable as a sorption material, which was demonstrated via dye-removal from an aqueous solution.
Introduction
The amorphous state of sugars is omnipresent in the pharmaceutical and food industry as it is easily processable, cheap, available, and natural.1 The amorphous state of sugar in pharmacy is deliberately produced via standard processes to effectively encapsulate, stabilize, and release active materials.2–4 As is known, carbohydrates, such as in candy, are indeed good glass-formers and have comparably high glass transition temperatures.5 Moreover, the disaccharides sucrose and trehalose are oftenly used as they have glass transition temperatures of 319 K or 368 K, respectively, whereas glucose as a typical monosaccharide has a somewhat lower value of Tg = 295 K.6Less scientific, candy floss is presumably one of the earliest encounters of young scientists due to its glassy sugar state. It illustrates how the plasticity and processability of glasses can create valuable materials with new effects. Sugar glass, also called breakaway glass, is used to simulate glass in movies, photographs, and plays, and the mechanical weakness makes it an excellent choice for stunts. The production of candy floss from sugar can be compared to glass manufacturing. Herein, upon the dissolution of sugar in water and heat treatment upto at least the hard crack stage (approx. at 150 °C), a sugar glass was formed with only minor amounts of water. Practically, the addition of starch or corn syrup prevented sugar recrystallization. It was found that all the ingredients required for these technologies were cheap, scalable, and sustainable in terms of processing, machinery, and handling of the glasses. Eventually, the well-developed processes from food industry can be adopted which allows for process costs well below ordinary lab ware.
The transformation of sugars into carbons via extended baking is not intended process during cooking, however, takes place very efficient. In Germany, activated carbon was even called Zuckerkohle/sugar charcoal, as the first sorption carbons for medical purposes were made from sugar. This material is comparably clean, poor in foreign elements, cheap, and results in high carbonization yields.7 We believe from a synthesis viewpoint that a significant processing advantage in carbonization can be realized by applying glassy or liquid sugar precursor mixtures. Since these mixtures are either slightly volatile or nonvolatile, they can be regarded to be similar to ionic liquids or deep eutectic mixtures.8–10 Because of the fluidity, shaping or coating can be applied. The same is true for structure-directing agents, which can be introduced based on polarity and miscibility with the liquid precursor. The use of liquid precursors, furthermore, allows the convenient addition of co-monomers and dopants such as nitrogen and other heteroatoms.11 In a previous study, we described the synthesis of heteroatom-doped nanostructured carbons based on supramolecularly pre-organized, deep eutectic precursor mixtures composed of phenols/ketones and urea.12 Herein, the processability and homogeneity of the liquids could be combined with the well-defined structure of the pre-aligned liquid crystals. This finding complemented other studies carried out using DES for carbon synthesis, which, however, previously resulted in isotropic precursor mixtures and carbons with comparably low nitrogen content.13
In the present study, we attempted to combine the glassy carbohydrate approach with eutectic-urea-doping. This formed a solvent-poor carbon precursor in a liquid state suitable for further processing. As is shown, urea is able to slightly lower the glass transition temperature of sugars, which is already quite low, but it greatly improves the processability and stability of the glassy state without recrystallization. When heated, new condensation chemistry is precoded. Herein, the sugar/urea reaction is well known in the food industry as non-enzymatic browning and is, for instance, applied for the browning of pretzels (also known from the roasting of coffee, cacao, cereals, but then at higher temperatures). This sugar/urea liquid is then combined with ZnCl2·1H2O as a porogen and cellulose as a processing agent, leading to a simple, convenient, and very efficient baking-approach towards heteroatom-doped microporous carbon monoliths. While the saccharides serve as a carbon precursor, urea not only acts as a source of doping atoms, but also cross-links the carbohydrates before carbonization to a green body, which allows carbonization without further melting, i.e. preservation of shape.
Results and discussion
In the first step, the carbohydrate, herein fructose, glucose, xylose, or galactose, was mixed with urea. This powder mixture was then gently heated under continuous stirring to elevated temperatures (90–135 °C) to form a clear melt. Even when this liquid was cooled down again to room temperature, it remained a clear, viscous liquid (Fig. 1). This observation indicates the successful suppression of sugar or urea recrystallization, i.e. the formation of a eutectic melt. These honey-like liquids can then be pre-fabricated and stored, in the kilogram scale, for further processing.To support the suppression of recrystallization, differential scanning calorimetry (DSC) data were obtained according to the dependence on the composition of the glass mixture (Table 1). DSC further reveals the absence of the crystallization peaks as well as the location of the glass transition temperature. Taking glucose as an example, the addition of urea lowers the glass transition temperature by about 25 K, i.e. the processing window is expanded, and the viscosity at room temperature is remarkably lowered.
Sugar![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) urea urea |
Molar ratio | Melting point | N [wt%] | C [wt%] | H [wt%] | Yield [wt%] | Carbon yield [wt%] |
|---|---|---|---|---|---|---|---|
| Fructose | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
87.8 | 12.95 | 75.13 | 1.65 | 28.76 | 61.73 |
| Fructose | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 1.5 |
85.15 | 14.63 | 74.75 | 1.84 | 23.30 | 52.25 |
| Fructose | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
98.3 | 15.91 | 71.65 | 1.72 | 21.33 | 53.49 |
| Glucose | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 1.5 |
74.3 | 13.37 | 76.13 | 1.50 | 23.62 | 53.94 |
| Glucose | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
104.3 | 14.49 | 75.67 | 1.69 | 19.15 | 48.30 |
| Xylose | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 1.5 |
101.7 | 14.70 | 75.14 | 1.71 | 22.44 | 50.58 |
| Galactose | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
113.3 | 12.62 | 76.98 | 1.69 | 28.21 | 62.04 |
| Galactose | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 1.5 |
111.3 | 14.47 | 75.35 | 1.74 | 26.33 | 59.52 |
| Galactose | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
111.7 | 14.83 | 72.32 | 1.56 | 20.83 | 52.72 |
Among the chosen systems, it was observed that galactose and fructose show highest glass transition temperature, whereas xylose shows lowest temperature. As typical for the glass transition temperature, mixtures of two monosaccharides do not result in a further lowering of Tg, but only further inhibits the crystallization. On reaching a molar sugar![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) urea ratio of 1
urea ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, slow crystallization of urea from the glassy melt is observed, which means that under these conditions, processing of the sample has to be faster or elevated temperatures need to be applied.
2, slow crystallization of urea from the glassy melt is observed, which means that under these conditions, processing of the sample has to be faster or elevated temperatures need to be applied.
These melts were then used for carbon synthesis and, therefore, heated in crucibles to the desired temperature. Herein, as an example, a carbonization temperature of 800 °C was chosen. All the samples derived from the viscous melts turn into silver-shiny, fluffy solids, and the metallic appearance indicate the special characteristics such as increased electron densities and electrical conductivity of carbons with structural nitrogen heteroatoms. Elemental analysis indeed shows that among all the sugar–urea mixtures, carbons with high nitrogen content can be obtained in good yield (Table 1). Since all carbon compositions are similar, the synthesis can be considered to be applicable to a rather broad range of natural educts. Note that the final nitrogen content in carbon depends on the accessibility of the functional groups inside the precursor mixture, i.e. once an optimal ratio is reached, the nitrogen content remains comparable. Besides nitrogen, the samples also contain oxygen due to the defect and termination sites. Since combustion even in an oxygen atmosphere in incomplete, which is typical for this type of carbon material, the residual oxygen is estimated to be around 10 wt%.
The liquid state of the precursors allows convenient mixing with further ingredients. As the bulk carbons were found to be non-porous via nitrogen sorption, salt was added into the process to introduce pores.14 Herein, to the sugar–urea liquid, a mixture of ZnCl2·1H2O is added as a porogen for pore formation in the approach of salt templating (Fig. SI-1†). It is important to mix both liquids – glucose/urea and ZnCl2·H2O/urea – both heated to 50–60 °C as this results in a homogeneous mixture. Moreover, at high urea concentrations, homogeneous mixtures can be ensured by mixing additional urea to the ZnCl2·H2O solution at 90 °C. Then, the porous samples were prepared using a glucose–urea mixture. First, a mixture of glucose–urea with a molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 was prepared to provide a liquid phase. Second, a separate solution of ZnCl2·H2O was prepared from a ZnCl2 salt and H2O (molar ratio 1
1.5 was prepared to provide a liquid phase. Second, a separate solution of ZnCl2·H2O was prepared from a ZnCl2 salt and H2O (molar ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). To the latter, the corresponding amount of urea was added to reach concentrations of urea above the eutectic point. Subsequently, both liquids were mixed at 60 °C.
1). To the latter, the corresponding amount of urea was added to reach concentrations of urea above the eutectic point. Subsequently, both liquids were mixed at 60 °C.
To obtain carbon, this dough is pre-baked at 100 °C, which can indeed be carried out in an ordinary oven. This results in the effective cross-linking of the sugars with urea under the partial release of CO2 and H2O, which transfers the viscoelastic dough into a cross-linked polymeric material. This is followed by a comparison of the FT-IR measurements of the pure ingredients and the resulting baked dough (Fig. 2).
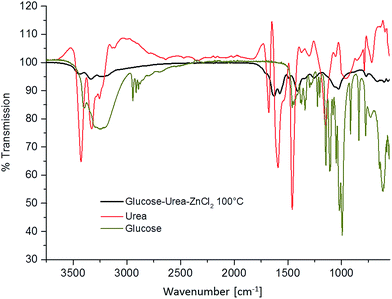 | ||
| Fig. 2 IR spectra of glucose (green), urea (red), and the eutectic mixture of glucose–urea–zinc chloride baked at 100 °C for 1 hour (black). | ||
Compared to the educts urea and glucose, the melt heated to 100 °C in the presence of salt already reveals a very different FT-IR pattern. The characteristic OH-vibrations of glucose vanish, whereas the amine ρ(NH2)- and δ(NH2)-vibrations of urea at 1150 cm−1 and 1674 cm−1 as well as the ν(NH2)-vibrations of urea at 3257 cm−1 and 3427 cm−1 are shifted during condensation with glucose. This indicates the chemical reaction of urea and sugar at comparatively low temperatures to form a cross-linked intermediate polymer, which is essential for the efficient formation of carbon materials in the next steps.
Afterwards, this baked precursor dough was carbonized in a nitrogen atmosphere at 500 °C, 700 °C, and 800 °C. Finally, the porogen salt was removed by washing with water and diluted acid. After simple vacuum drying, carbon appears as a black monolith and is obtained in a high yield of up to over 60% (Table 2).
| Sample | Mass ratio | Carb.-temp. | N [wt%] | C [wt%] | H [wt%] | Yield [wt%] | Carbon yield [wt%] | Surface area [m2 g−1] |
|---|---|---|---|---|---|---|---|---|
| Glucose–urea | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
500 | 17.9 | 68.1 | 3.1 | 23.4 | 53.1 | — |
| Glucose–urea–salt | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
500 | 13.4 | 61.7 | 3.6 | 16.1 | 33.1 | 941 |
| Glucose–urea–salt–cellulose_16 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
500 | 13.8 | 67.5 | 3.9 | 20.7 | 41.6 | 1090 |
| Glucose–urea–salt–cellulose_39 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
500 | 12.9 | 70.0 | 4.1 | 25.4 | 48.6 | 979 |
| Glucose–urea | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
700 | 15.4 | 73.2 | 1.9 | 21.2 | 51.7 | — |
| Glucose–urea–salt | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
700 | 12.3 | 57.9 | 2.2 | 15.2 | 29.3 | 600 |
| Glucose–urea–salt–cellulose_16 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
700 | 13.0 | 67.5 | 3.0 | 20.3 | 40.8 | 641 |
| Glucose–urea–salt–cellulose_39 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
700 | 12.4 | 70.1 | 3.1 | 24.7 | 47.4 | 802 |
| Glucose–urea | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
800 | 14.5 | 75.7 | 1.7 | 19.1 | 48.3 | — |
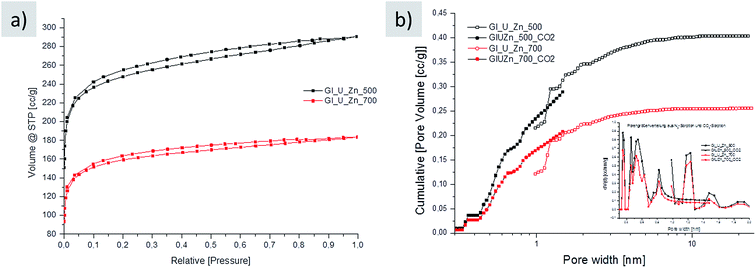
Nitrogen sorption of the final washed carbons indeed reveals high porosity at relatively low carbonization temperatures, representatively shown for carbons prepared at 500 °C (941 m2 g−1) and 700 °C (600 m2 g−1) and supporting the idea of a well-developed, stiff aromatic framework of N-containing heterocycles (Fig. 3a). Note that the surface area of carbon prepared at lower temperatures is higher. This is due to higher amount of micropores in the carbon prepared at 500 °C as revealed by the analysis of the pore size distribution (Fig. 3b). Generally, the high nitrogen uptake of this carbon is a remarkable result as the introduction of pores in these resins is usually challenging because of a comparably soft polymer structure. The relatively high nitrogen content is an essential functional property (Table 2) as well. Usually, there is a trade-off between high surface area, commonly derived at higher carbonization temperatures, and high nitrogen content, which is normally obtained at lower carbonization temperatures. Note that weight-wise, the reaction is practically completed at 500 °C due to the described elimination schemes, whereas further heating certainly improves aromatization and rearrangement of the condensed aromatics towards extended 2d-sheets, however, without significant change in the mass and nitrogen content at higher carbonization temperatures: this hints to the stable incorporation of nitrogen sites into the aromatic resin at 500 °C. Note that the synthesis in the presence of the zinc salt results in an increase in the oxygen content of about 5 wt% due to carbothermal reduction. Eventually, the synthesis presented herein also allows the preparation of resin-like materials with both high porosity and high functionality, which are important with regard to applications.
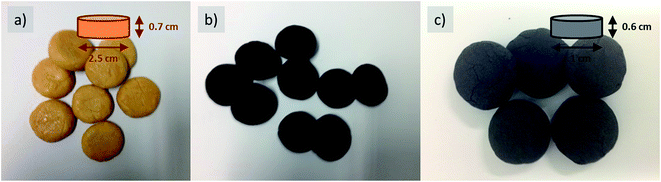
To provide the precursor mixture with more elastic cohesion, i.e. to achieve more shape-retaining dough and easier processing, e.g. into beads or solid pellets (cookies), cellulose was added as a binder, keeping the ratio of the remaining ingredients constant (Fig. 4). Herein, the viscosity can be easily tuned by the amount of added cellulose fibers, again a fact well known in food processing.
As the resulting objects are indistinguishable from food with regard to appearance and haptics, we called them cookies, and after final carbonization, they were termed carbon cookies, respectively (Fig. 4b and c). The intermediate can be stored and afterwards further carbonized at high temperatures in different surroundings. This is of special interest as conventional impregnation methods require immediate further processing, which is difficult with regard to scaled processes. This stability for transport and storage is attributed to the ability of cellulose to gel the system via secondary interactions.
The baked pellets were then carbonized in a nitrogen oven, which resulted in black monoliths (Fig. 4c). The macroscopic size of the final carbon slightly shrinks compared to that of the initial raw cookie; however, the monolithic form is retained throughout the process. After washing with simple diluted hydrochloric acid and water, herein, nitrogen-doped carbons with high porosity were obtained (Fig. 5).
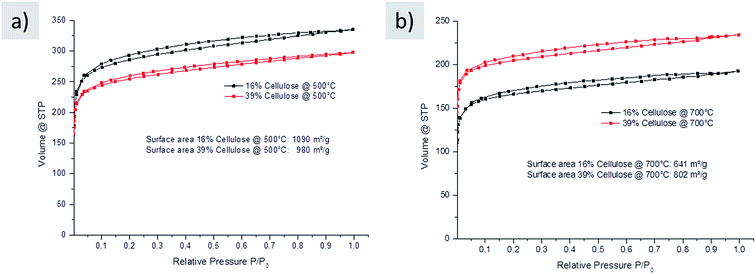 | ||
| Fig. 5 Nitrogen sorption of carbons made from glucose–urea–salt with different amounts of cellulose (black 16% and red 39%) carbonized at (a) 500 °C and (b) 700 °C in nitrogen after washing. | ||
These results reveal high surface areas and porosity for carbons prepared with the additional cellulose filler. This is in agreement with the texture observed in scanning electron microscopy (SEM), which reveals smooth and homogeneous carbon structures (Fig. SI-3†). The comparison with the carbons prepared without additional cellulose showed that the porosity and composition of the final carbon prepared with the filler did not significantly change, which could be somewhat expected. The cellulose indeed mainly serves as an additive to address the current processing issues rather than being a main part of the carbon material.
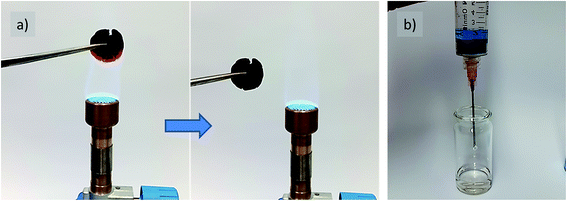
It is a special feature of nitrogen-containing carbons that the heteroatom incorporation can improve the oxidation stability of the carbon materials.15,16 Herein, our carbon cookie made at a low carbonization temperature of 500 °C and with 39 wt% of cellulose was then used to test the thermal stability of the final material under harsh conditions. For this, the cookie was repeatedly placed into the flame of a butane/propane (70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30) gas burner (Fig. 6a, Video ESI†). Even after several cycles, when a red blaze in the central flame (∼800 °C) was achieved, carbon remained in its macroscopic shape (24% weight loss after 6 heating/cooling cycles for 3 s). Considering the very low carbonization temperature and high porosity, this is a remarkable result keeping in mind the usually fast ignition of the untreated cotton or paper (cellulose). This indicates the efficient and homogeneous mixing of the liquid precursors, leading to water elimination and N-heterocycle formation at comparably low temperatures. This essentially is a reflection of the low mass loss revealed by TGA measurement, which is mainly due to the gases released from cellulose and/or carbon, which otherwise promote the flaming phase, whereas the release of water under the formation of an oxidation stable N-doped carbon causes the observed fire inertness (Fig. SI-4†). Of course, this makes the material suitable as a catalyst support or for high temperature thermal insulation based on the micropore content.
30) gas burner (Fig. 6a, Video ESI†). Even after several cycles, when a red blaze in the central flame (∼800 °C) was achieved, carbon remained in its macroscopic shape (24% weight loss after 6 heating/cooling cycles for 3 s). Considering the very low carbonization temperature and high porosity, this is a remarkable result keeping in mind the usually fast ignition of the untreated cotton or paper (cellulose). This indicates the efficient and homogeneous mixing of the liquid precursors, leading to water elimination and N-heterocycle formation at comparably low temperatures. This essentially is a reflection of the low mass loss revealed by TGA measurement, which is mainly due to the gases released from cellulose and/or carbon, which otherwise promote the flaming phase, whereas the release of water under the formation of an oxidation stable N-doped carbon causes the observed fire inertness (Fig. SI-4†). Of course, this makes the material suitable as a catalyst support or for high temperature thermal insulation based on the micropore content.
In a further test, the accessibility and functional character of the pore system of carbons was exemplified via dye sorption using methylene blue as a comparably large model sorbent for visualization (Fig. 6b, Video ESI†). For this, carbon was placed in a syringe followed by the addition of the dye solution. As can be observed from the colorless liquid at the syringe tip after squeezing through the carbon sorbent, even the comparably short contact time during the flow allows efficient dye removal. This is attributed to the structural nitrogen content, which increases the sorption strength of the carbon surface and good accessibility of the pore system created by the aqueous salt porogen removal during the synthesis, which allows the preservation of the initial cookie shape.
Materials and methods
The water-free sugars glucose, fructose, galactose, and xylose were obtained from ROTH. Urea, zinc chloride, and cellulose were purchased from Sigma-Aldrich. All materials were used without further purification.Synthesis
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5), a clear and slightly yellow viscous liquid was formed by heating it in an open glass vial using an oil bath at 90–130 °C. This liquid can be cooled down and later heated again. For the synthesis of the final carbon, this liquid is then directly heated in a nitrogen atmosphere, resulting in nitrogen-doped carbon. Heating program: 30 min purging at room temperature, heating rate 2.5 K min−1, final temperature 500 °C and 700 °C, maintain for 1 h.
1.5), a clear and slightly yellow viscous liquid was formed by heating it in an open glass vial using an oil bath at 90–130 °C. This liquid can be cooled down and later heated again. For the synthesis of the final carbon, this liquid is then directly heated in a nitrogen atmosphere, resulting in nitrogen-doped carbon. Heating program: 30 min purging at room temperature, heating rate 2.5 K min−1, final temperature 500 °C and 700 °C, maintain for 1 h.
Characterization
Elemental analysis was performed as combustion analysis using a Vario Micro device. Scanning electron microscopy (SEM) was performed using a Gemini Leo-1550 instrument after sputtering with platinum. Fourier transformed infrared spectra (FT-IR) were obtained using a Varian 1000 FT-IR spectrometer with an attenuated total reflection setup. Nitrogen sorption measurements were accomplished with N2 at 77 K and CO2 at 273 K, 284 K, and 302 K (after degassing the samples at 150 °C under vacuum for 20 h) using a Quantachrome Quadrasorb SI porosimeter. The apparent surface area was calculated from the nitrogen sorption measurements by applying the Brunauer–Emmett–Teller model to the isotherm data points of the adsorption branch for p/p0 < 0.3 (BET area). The pore volume was calculated from the obtained physisorption data using the program QuadraWin (Version 5.11), where the mesopore volume was calculated by subtracting the micropore volume from the total pore volume. The pore size distribution, the cumulative pore volume, and the average pore size were also determined using the program QuadraWin (version 5.11) via the quenched solid functional theory (QSDFT) for slit/cylindrical pores applied in the nitrogen adsorption isotherm.For the fire-retardant experiment, the carbon monolith prepared from glucose–urea–zinc and 39 wt% after a carbonization temperature of 500 °C was repeatedly placed into the flame of a butane/propane gas burner and weighed before and after. For the dye sorption test, the same carbon was milled and used in combination with an aqueous methylene blue solution (10 mg L−1). This carbon was then placed into a syringe equipped with a piece of cellulose filter paper followed by the addition of the dye solution and dropwise squeezing through carbon.
Conclusion
In this study, the synthesis of porous nitrogen-doped carbon materials using processable sugar glasses as a precursor system was presented. It was shown that the addition of urea to several sugars substantially reduces the melting point of the solid mixtures and can even suppress recrystallization. All substances are cheap, safe, and available in large quantities, which allows efficient carbon synthesis. Through the addition of salt and cellulose as a porogen and structural filler, respectively, it was further possible to prepare storable green bodies, which are an important aspect with regard to industrial processes. Carbonization of these intermediates resulted in functional porous carbons in the form of monoliths, i.e. carbon cookies. These materials were then demonstrated to be stable against oxidation and could serve as adsorbents in aqueous media. As the synthesis approach is modularly adjustable, scalable, resource efficient, and compatible with standard industry equipment, we believe that it would allow further progress in fields where high amounts of porous and functional carbons such as energy storage and catalysis are needed.Acknowledgements
The authors would like to thank the Max Planck Society for financial support. Open Access funding provided by the Max Planck Society.References
- K. R. Goldfein and J. L. Slavin, Compr. Rev. Food Sci. Food Saf., 2015, 14, 644 CrossRef.
- J. Giri, W.-J. Li, R. S. Tuan and M. T. Cicerone, Adv. Mater., 2011, 23, 4861 CrossRef CAS PubMed.
- B. Kang, T. Opatz, K. Landfester and F. R. Wurm, Chem. Soc. Rev., 2015, 44, 8301 RSC.
- M. A. Mensink, H. W. Frijlink, K. v. d. V. Maarschalk and W. L. J. Hinrichs, Eur. J. Pharm. Biopharm., 2017, 114, 288 CrossRef CAS.
- M. Z. Saavedra-Leos, A. Grajales-Lagunes, R. González-García, A. Toxqui-Terán, S. A. Pérez-García, M. A. Abud-Archila and M. A. Ruiz-Cabrera, J. Food Sci., 2012, 77, E118 CrossRef CAS.
- A. Simperler, A. Kornherr, R. Chopra, P. A. Bonnet, W. Jones, W. D. Samuel Motherwell and G. Zifferer, J. Phys. Chem. B, 2006, 110, 19678 CrossRef CAS.
- E. J. Miller, J. Am. Chem. Soc., 1924, 46, 1150 CrossRef.
- J. P. Paraknowitsch and A. Thomas, Macromol. Chem. Phys., 2012, 213, 1132 CrossRef CAS.
- D. Carriazo, M. C. Serrano, M. C. Gutierrez, M. L. Ferrer and F. del Monte, Chem. Soc. Rev., 2012, 41, 4996 RSC.
- C. Weinberger, S. Haffer, T. Wagner and M. Tiemann, Eur. J. Inorg. Chem., 2014, 2787 CrossRef CAS.
- X. Zhu, P. C. Hillesheim, S. M. Mahurin, C. Wang, C. Tian, S. Brown, H. Luo, G. M. Veith, K. S. Han, E. W. Hagaman, H. Liu and S. Dai, ChemSusChem, 2012, 5, 1912 CrossRef CAS PubMed.
- N. Fechler, N. P. Zussblatt, R. Rothe, R. Schlögl, M.-G. Willinger, B. F. Chmelka and M. Antonietti, Adv. Mater., 2016, 28, 1328 CrossRef.
- N. López-Salas, M. C. Gutiérrez, C. O. Ania, J. L. G. Fierro, M. L. Ferrer and F. del Monte, J. Mater. Chem. A, 2014, 2, 17387 Search PubMed.
- N. Fechler, T.-P. Fellinger and M. Antonietti, Adv. Mater., 2013, 25, 75 CrossRef CAS PubMed.
- Y. Men, M. Siebenbürger, X. Qiu, M. Antonietti and J. Yuan, J. Mater. Chem. A, 2013, 1, 11887 CAS.
- S. Sandoval, N. Kumar, A. Sundaresan, C. N. R. Rao, A. Fuertes and G. Tobias, Chem.–Eur. J., 2014, 20, 11999 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7ta02052j |
| This journal is © The Royal Society of Chemistry 2017 |