Selective H2O2 conversion to hydroxyl radicals in the electron-rich area of hydroxylated C-g-C3N4/CuCo–Al2O3†
Abstract
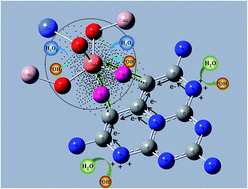
The Fenton reaction has been explored extensively in different fields due to the generation of hydroxyl radicals (˙OH). However, the reaction requires strongly acidic conditions and excessive consumption of H2O2, which impede its widespread application. Here, we report that twice the amount of ˙OH radicals as that of the reacted H2O2 was formed by the reaction of H2O2 with a type of nano-composite OH–CCN/CuCo–Al2O3 under nearly neutral conditions. The nano-composite was achieved by the co-incorporation of Cu and Co into γ-Al2O3, and the sequential complex formation of the σ-type Cu–O–C linker between the surface Cu and the hydroxyl group of the tri-s-triazine ring in C-g-C3N4. From electron paramagnetic resonance spectra and density functional theory simulations, it was verified that an electron-rich area around Cu occurred due to the Cu–π interaction and the different electronegativities of Cu with Co and Al in OH–CCN/CuCo–Al2O3, which reacted with H2O2, resulting in two electron transfer processes. One process is from the electron-rich Cu center to H2O2 to form ˙OH, and the other is from H2O to the N atom of OH–CCN to produce ˙OH. The catalyst shows extremely high activity for the degradation of various refractory organic pollutants in water under mild conditions with a high utilization efficiency of H2O2 (∼90%). Our findings confirm that the construction of an electron-rich area is essential for overcoming the limitations of the classical Fenton process for environmental remediation and other applications.
- This article is part of the themed collection: 2017 Journal of Materials Chemistry A HOT Papers


 Please wait while we load your content...
Please wait while we load your content...