Open Access Article
Open Access ArticleBijels formed by direct mixing
Dongyu
Cai
ab,
Paul S.
Clegg
 *a,
Tao
Li
*a,
Tao
Li
 c,
Katherine A.
Rumble
a and
Joe W.
Tavacoli
a
c,
Katherine A.
Rumble
a and
Joe W.
Tavacoli
a
aSchool of Physics and Astronomy, University of Edinburgh, James Clerk Maxwell Building, Peter Guthrie Tait Road, Edinburgh, EH9 3FD, UK. E-mail: paul.clegg@ed.ac.uk
bInstitute of Advanced Materials, Nanjing Tech University, No. 5 Xinmofan Road, Nanjing, 21009, P. R. China
cBeijing National Laboratory for Condensed Matter Physics and Key Laboratory of Soft Matter Physics, Institute of Physics, Chinese Academy of Sciences, Beijing 100190, China
First published on 3rd July 2017
Abstract
By combining interfacial nanoparticles and molecular surfactants together with immiscible liquids of high viscosity, we develop an alternative strategy for creating bicontinuous interfacially jammed emulsion gels (bijels). These bijels are prepared from common ingredients which are widely used in industry: glycerol, silicone oil, silica nanoparticles together with cetyltrimethylammonium bromide (CTAB) surfactant. We tune the sample composition and develop a multi-step mixing protocol to achieve a tortuous arrangement of liquid domains. We show that the nanoparticle location changes from one of the phases to the interface during mixing. The changes in both the microscopic and macroscopic sample configuration after a waiting time of months were assessed. In order for the structure to have long-term stability we find that the densities of the two phases must be similar which we achieved by filling one of the phases with nanoparticle-stabilised droplets of the other. This work paves the way to the production of bijels using fully immiscible liquids and hence their exploitation in many application areas.
Bijels or bicontinuous interfacially jammed emulsion gels are particle-stabilised emulsions with two inter-penetrating continuous phases.1,2 The morphology is attractive due to the large interfacial area which is contained in a small volume. Additionally, the experimentalist can control the size of the continuous channels via the nanoparticle concentration and the size of the pores between the channels via the nanoparticle size;3 hence, it is possible to fine-tune the structure as a platform for efficient mass transport. The list of potential bijel applications includes use as a template for catalysts, electrodes for batteries and fuel cells, hierarchically porous materials, scaffolds for tissue engineering and cross-flow microreactors.4–8
Initially, bijels were experimentally realised using partially miscible liquids which, when quenched, can phase separate via spinodal decomposition forming a bicontinuous arrangement of fluid domains.9 As phase separation begins the particles are swept onto the interface, with time the fluid domains coarsen and the amount of interfacial area decreases. Consequently, the coverage of particles on the interface progressively increases until eventually the particles become jammed together.4 This method only produces bicontinuous structures if the composition of liquids is such that the phase separation occurs via spinodal decomposition rather than nucleation. Hence this approach forces us to use partially miscible liquids which phase separate into two fluid domains with similar volumes.3,9–14 Further constraints originate from the boiling and melting points of the liquids and their relative densities. While there are a range of partially miscible systems which meet these criteria, many of them include a component which is either toxic or explosive, which limits the wide deployment of these materials. A related approach involves solvent transfer-induced phase separation (STRIPS). This uses a ternary mixture containing two immiscible liquids and a third solvent which in certain proportions renders the two liquids miscible. The removal of this solvent into a bath causes the two immiscible liquids to phase separate via spinodal decomposition forming a bicontinuous structure.13
It is also necessary to use particles which are neutrally wetting so no preferred curvature is imposed on the interface.15 In the first protocol the wetting characteristics of the silica particles were tuned by varying silica particle drying time.9 Another method for tuning the particle wetting is the modification of the silica surface chemistry, and hence the hydrophobicity, by reacting the particles with hexamethyl disilazane (HMDS).3,10 The wetting of graphene oxide sheets, which have also been used to form bicontinuous structures, can be tuned by modifying the carbon to oxygen ratio.11 These methods of particle tuning are often time-consuming and the overall process of making a bijel requires a high level of attention to experimental detail. Thus, there have been some attempts to simplify the process. E.g. by circumventing the arduous particle tuning step by using mixtures of commercially available particles with different hydrophobicities.12
A bicontinuous structure can, additionally, be formed by mixing polymer blends using chaotic advection.16 The starting point is a layered polymer blend which is then stretched and folded repeatedly. The layered domains become so thin that holes begin to form which leads to interconnectivity between the layers and a bicontinuous structure.16 Occasionally, these blends do not rely on stabilisers, such as particles, for formation and stability.17,18 Nonetheless, particles can be incorporated into the polymer blend, not only to improve the stability of the structure, but also to confer new properties to the blend such as electrical conductance, photo-sensitivity and catalytic behaviour.19,20 For example, two immiscible polymers with a bicontinuous morphology and particles of carbon black at the interface form a conducting polymer. This system retains the mechanical properties of the polymer blend because fewer carbon black particles are necessary to obtain conductance when their percolation is constrained by the liquid interface.19,21 Bicontinuous polymer blends have also been created using clay platelets to stabilise the structure. The clay platelets act as a base for the polymers to graft onto creating favorable interactions between each polymer and the grafted clay platelets leading to segregation at the interface and removing the need for particle modification.22
Combinations of particles and surfactants have been explored for the stabilisation of emulsions.23,24 Ionic surfactant molecules can adsorb onto the surface of a charged particle modifying its wetting properties.25,26 A disadvantage to using surfactants is the decrease in the interfacial tension which also determines the free energy of detachment of particles. This lower interfacial tension does, however, allow more interface to be created during the mixing process.27 Most commonly, stabilisers for emulsions,25,26,28 foams29 and non-equilibrium droplets formed by arrested coalescence events30 have been created by adsorption of surfactants onto particles before the interface is created. Alternatively, the particles and surfactants can be introduced at the interface by having one in each phase.23,31
Stabilisation via a combination of particles and surfactants, has recently been applied to the fabrication of bicontinuous structures. Cui et al. claim to have made a bicontinuous structure by stirring a water droplet in a silicone oil mixture containing nanoparticles and surfactants but there was a large discrepancy in the size of the different phases and the microscopic structure was not verified.31 Subsequently, the combination of nanoparticles and surfactants was used to stabilise bijel fibers made by the STRIPS method described above. This method still, however, relies on phase separation via spinodal decomposition and is limited by phase diagrams and phase transition kinetics.13 Hence the creation of stable bicontinuous fluid domains in the laboratory using low molecular weight liquids faces serious obstacles especially using mixing alone. Here we overcome these obstacles via a new method for the fabrication of a particle-stabilised bicontinuous structure using a multi-step mixing process.
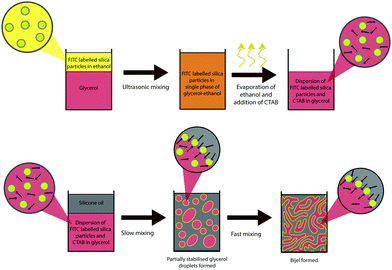
In our optimised protocol, silica nanoparticles† with a radius of 14 nm, labeled with FITC and dispersed in ethanol at a known mass fraction are first weighed into a vial in order to have 0.03 g of silica nanoparticles. 2.97 g of Nile Red labeled glycerol (viscosity 0.64 ± 0.02 Pa s) is then added to the vial to make a 1 (wt%) silica nanoparticles in glycerol solution. This mixture is sonicated using an ultrasonic probe (Sonics Vibracell VCX500) at an amplitude of 20% for 5 seconds on, 5 seconds off for a total of 5 minutes. Before and after this step the probe is cleaned by running two short cycles with ethanol. The ethanol in the silica nanoparticles in glycerol solution is then evaporated off by drying overnight in a 50 °C oven. The relevant mass of CTAB is weighed out into a new vial and for the majority of experiments this is in the mass ratio of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 (nanoparticles
4 (nanoparticles![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CTAB). Next 0.6 g of the silica nanoparticles in glycerol solution is added into the vial. The sample is stirred at 200 rpm for 5 minutes using a magnetic stirrer bar and a magnetic stirrer plate (IKA RCT basic). Next 0.5 g of a 1
CTAB). Next 0.6 g of the silica nanoparticles in glycerol solution is added into the vial. The sample is stirred at 200 rpm for 5 minutes using a magnetic stirrer bar and a magnetic stirrer plate (IKA RCT basic). Next 0.5 g of a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (by mass) mixture of silicone oils (10
1 (by mass) mixture of silicone oils (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cSt and 50 cSt) which is prepared by vortex mixing is added to the vial (viscosity 1.28 ± 0.01 Pa s; glycerol/silicone oil interfacial tension 26.4 ± 0.2 mN m−1). The mixture is then mixed slowly at 200 rpm for 1 minute (IKA RCT basic magnetic stirrer plate) and then mixed quickly for another five minutes (at level 2 on the Stuart stir CB161 magnetic stirrer plate); the time between mixing steps has been kept at or below two minutes. Fig. 1 shows a diagram of this experimental protocol. The samples are then viewed on a Zeiss LSM 700 laser scanning microscope using the 488 nm laser for the FITC labeled nanoparticles, the 555 nm laser for the Nile Red in the glycerol and filters as appropriate.
000 cSt and 50 cSt) which is prepared by vortex mixing is added to the vial (viscosity 1.28 ± 0.01 Pa s; glycerol/silicone oil interfacial tension 26.4 ± 0.2 mN m−1). The mixture is then mixed slowly at 200 rpm for 1 minute (IKA RCT basic magnetic stirrer plate) and then mixed quickly for another five minutes (at level 2 on the Stuart stir CB161 magnetic stirrer plate); the time between mixing steps has been kept at or below two minutes. Fig. 1 shows a diagram of this experimental protocol. The samples are then viewed on a Zeiss LSM 700 laser scanning microscope using the 488 nm laser for the FITC labeled nanoparticles, the 555 nm laser for the Nile Red in the glycerol and filters as appropriate.
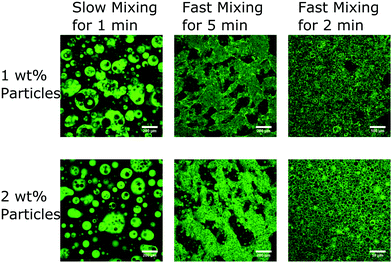
Possibly the most important step is the mixing protocol: a magnetic stirrer bar is used to sequentially give a slow mixing step followed by a fast mixing step with an optional rest period in between. Fig. 2 shows the structure at each stage of the mixing protocol made using the parameters outlined while varying the nanoparticle concentration. In the slow mixing step droplets of glycerol are formed which contain some droplets of silicone oil and excess nanoparticles. After the fast mixing step a non-equilibrium or bicontinuous structure is formed and the nanoparticles are mostly at the interface. If the structure is mixed further this bicontinuous structure is destroyed (see Fig. 2). The CTAB concentration is an important parameter because this affects the nanoparticle properties at the interface as discussed above.23,25,26 Nevertheless, in many experiments it was noted that there is a large window of CTAB concentrations (approximately between 10 mM and 80 mM in glycerol) for which a bicontinuous structure can form. In addition, the volume fraction of nanoparticles required optimisation because there needs to be enough nanoparticles to stabilise a large interface but not so many that the initial droplets are fully covered.34 Despite this the choice of nanoparticles and surfactant is somewhat flexible which is demonstrated by the different choices made in ref. 13 and 31 and the two different sizes of nanoparticles used here (see later). By contrast, the volume of the oil phase is important because being close to equal volumes increases the likelihood of producing a bicontinuous structure. Likewise, the viscosity of the silicone oil was controlled by combining two silicone oils with different viscosities. The value of this parameter influences the likelihood of non-equilibrium structures forming. In addition, the value of the viscosity also influences the composition at which bicontinuity or droplet inversion occurs under shear.35
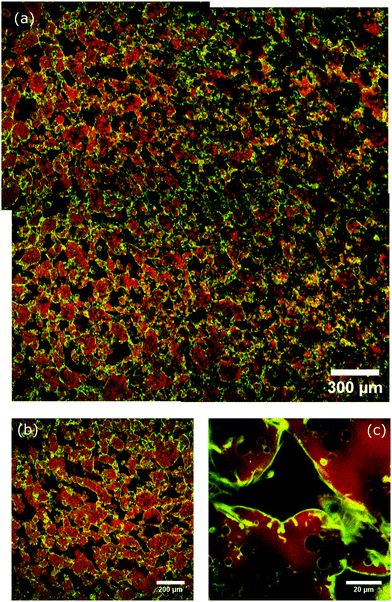
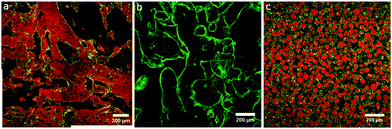
A larger field of view image of an example bicontinuous structure made using our method was created by taking four confocal images which overlap and aligning them afterwards in order to observe the variation of the structure on a larger scale (see Fig. 3a). The structure does vary across the larger area with both large and small glycerol domains present which are interconnected creating a bicontinuous structure. Often a slight orientation to the ordering of the domains is observed which could be due to the shear occurring in one stirring direction. The nanoparticles are mostly on the interface indicating that the structure is particle-stabilised.
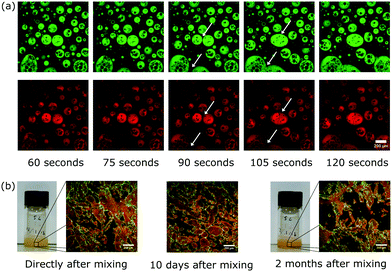
Our two-step mixing approach is curious. The glycerol droplets formed after the slow mixing step do not appear to be particle-stabilised (see Fig. 2 and 4a). These droplets contain both excess silica nanoparticles and silicone oil droplets. That the sample can be left to rest between the mixing steps indicates that whether or not the occasional coalescence events occur at this point is not fundamental to the formation of the bicontinuous structure. If there is, say, a two minute resting period some of the initial droplets start to slowly coalesce but due to the high viscosity of the liquids and the surfactant stabilisation they remain in non-equilibrium shapes (see Fig. 4a). It is important to consider when the particles become trapped at the interface; we note that there is no obvious change in nanoparticle location observed in Fig. 4a. Subsequently, the fast mixing step then creates more interface by shearing the droplets which leads to non-equilibrium and bicontinuous structures. It is only after the fast mixing step that the majority of nanoparticles are observed to be on the interface (compare Fig. 4a with Fig. 3). We emphasise that, neither slow mixing alone nor fast mixing alone, for the full period, result in the formation of this long-lived bicontinuous structure. Slow mixing alone leads to a short-lived tortuous structure, while fast mixing alone yields particle-stabilised silicone oil droplets.
Particle trapping at interfaces in polymer blends is a valuable comparison.36–38 We speculate that the population of droplets created in our first slow mixing step have a very low area fraction of particles on the interface. Such droplets are likely to have an enhanced tendency towards particle bridge formation37 which might be an important step towards the formation of joined up tortuous domains. Indeed, close inspection of Fig. 3 reveals relics of particle bridges. It is possible that our bicontinuous structure forms via a sequence of steps which parallel the changes in behaviour of polymer blends with increasing particle concentration as described by Nagarkar and Velankar.37
Using our method we can create structures which have long-term stability. After ten days the bicontinuous structure was observed to remain intact and even after 2 months a non-equilibrium structure remained (see Fig. 4b). In addition, no macroscopic phase separation was observed during this two month period (see Fig. 4b). Without the tortuous domains separation occurs much more rapidly which underlines the value of this morphology.
A key challenge we face when trying to build-in long-term stability is the large density mis-match between silicone oil and glycerol. We have overcome this by populating the glycerol phase with silicone oil droplets during the slow mixing step (see Fig. 2 and 4a). These silicone oil droplets become particle-stabilised and are also observed in the final bicontinuous structure (see Fig. 3b and c). They are the reason for the high stability in the structures made by this method: the densities of the two phases have been made more similar with the addition of particle-stabilised droplets of silicone oil in the glycerol phase (see Fig. 3b and c). In some of our earlier attempts, these droplets were scarcely present, resulting in beautiful samples which retained their morphology for only about a day (see Fig. 5).
We now demonstrate the subtle style of failure mode which is encountered when this approach is modified. For example, an alternative recipe involves using a 33% less silica nanoparticles and a silicone oil mixture in the ratio 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 for 10
9 for 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cSt
000 cSt![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 cSt. Furthermore the ethanol evaporation step is avoided. The mixing method first involves hand shaking followed by shearing on a rotor-stator at 3000 rpm (5140 s−1). The structure of this sample is shown in Fig. 5a and has two continuous phases which is initially encouraging. Unfortunately this structure has rather large domains and is somewhat unstable with the whole system separating into two distinct layers within a day or two. A similar protocol was also used to make comparable structures which were stabilised by larger nanoparticles (65 nm radius, see Fig. 5b) indicating that to some degree the specific details of the nanoparticles are not important. The structure shown here appears to be an improvement on the one shown in Fig. 5a even though it was created on a larger scale. Nonetheless, long-term stability remains a problem if the density mis-match is not addressed. Returning to the original protocol, structure formation completely fails if the particles are too hydrophobic. Here we have used commercial HDK H2000 nanoparticles (a gift from Wacker) with a small fraction tagged with a fluorophore; these have only 25% coverage of silanol groups on their surfaces. As seen in Fig. 5c, glycerol droplets form within a silicone oil continuous phase. Perhaps unsurprisingly, the addition of surfactant can only adjust the particle wettability within limits.
50 cSt. Furthermore the ethanol evaporation step is avoided. The mixing method first involves hand shaking followed by shearing on a rotor-stator at 3000 rpm (5140 s−1). The structure of this sample is shown in Fig. 5a and has two continuous phases which is initially encouraging. Unfortunately this structure has rather large domains and is somewhat unstable with the whole system separating into two distinct layers within a day or two. A similar protocol was also used to make comparable structures which were stabilised by larger nanoparticles (65 nm radius, see Fig. 5b) indicating that to some degree the specific details of the nanoparticles are not important. The structure shown here appears to be an improvement on the one shown in Fig. 5a even though it was created on a larger scale. Nonetheless, long-term stability remains a problem if the density mis-match is not addressed. Returning to the original protocol, structure formation completely fails if the particles are too hydrophobic. Here we have used commercial HDK H2000 nanoparticles (a gift from Wacker) with a small fraction tagged with a fluorophore; these have only 25% coverage of silanol groups on their surfaces. As seen in Fig. 5c, glycerol droplets form within a silicone oil continuous phase. Perhaps unsurprisingly, the addition of surfactant can only adjust the particle wettability within limits.
Equally dramatic failures can be induced by sequentially removing components. For example, without the silica nanoparticles the mixture made using our optimal procedure does not create a bicontinuous structure; instead silicone oil droplets form. The emulsion is unstable with layers of excess liquids separating out within three days. Without the CTAB, very large particle-stabilised domains form which are highly non-spherical but lack interconnections.
That our method of forming bicontinuous structures is successful is due, in part, to the high viscosity of the liquids used. This slows down the dynamics of the system and increases the likelihood of the structure becoming jammed in a non-equilibrium shape. As shear is applied, initially droplets of one of the phases are created and if the volume fraction of nanoparticles is too low to stabilise all of the interface these droplets will start to coalesce. The nanoparticle coverage of the interface determines when coalescence events are arrested which can be during the event creating non-spherical droplets or afterwards leading to spherical droplets.34 Both the high viscosity of the liquids and the continued shear applied to the system means that, as the coalescence process takes place, the droplets are distorted and these shapes can become particle-stabilised. It is worth noting that slowing down the coalescence by using high viscosity liquids also slows down the nanoparticle motion within the system. The presence of the surfactant in the mixture slows down the relaxation of the non-equilibrium shapes in the period before the nanoparticles reach the interface by lowering the interfacial tension. The continued shear then breaks up and re-forms the non-equilibrium shapes leading to an interconnected structure.
In conclusion, a method for creating bijels by direct mixing was successfully developed using high viscosity liquids, nanoparticles and a surfactant. The viscosity of the liquids is important presumably because it leads to slow dynamics which increases the likelihood of creating non-equilibrium structures. Another important factor is the surfactant which modifies the wetting properties of the nanoparticles and reduces the interfacial tension. These combined with the nanoparticles and a two-step mixing protocol are all essential to form the bicontinuous structure. The stability of the structure has been improved by making the densities of the two phases more similar by the incorporation of particle-stabilised droplets of silicone oil in the glycerol phase.
This study builds on the work of Cui et al. in creating non-equilibrium shapes in a simple way.31 The method bridges the gap between conventional bijel production and that of particle-stabilised bicontinuous structures using bulk polymers which can also be created simply by mixing.18,22 Importantly, the method presented here bypasses the need for the careful particle modification and phase separation steps which are needed to create a bijel.3,9,15 This is achieved by using nanoparticle surfactant mixtures as suggested by Cui et al. and Haase et al.13,31 It is worth noting that, unlike this study, the work of Haase et al. relies on phase separation mechanisms in order to create the bicontinuous structure.13 Our straightforward method of creating a tortuous structure where, within limits, the choice of nanoparticle does not seem to affect the structure made could have many applications. This includes the creation of a bicontinuous structure which has a large surface area to volume ratio and added functionality such as photo-sensitivity, catalytic activity or conductance.
Acknowledgements
We thank EPSRC for funding under grant number EP/J007404/1 and for a PhD studentship for K. A. R. with the Condensed Matter Centre for Doctoral Training (CM-CDT) under grant number EP/G03673X/1. We also thank P&G for supporting the work of T. L. and the National Natural Science Foundation of China (Grant No. 21503110) for supporting the work of D. C. We are grateful to Andrew Schofield for synthesising the nanoparticles.References
- K. Stratford, R. Adhikari, I. Pagonabarraga, J.-C. Desplat and M. E. Cates, Science, 2005, 309, 2198–2201 CrossRef CAS PubMed.
- M. Reeves, K. Stratford and J. H. J. Thijssen, Soft Matter, 2016, 12, 4082–4092 RSC.
- J. W. Tavacoli, J. H. J. Thijssen, A. B. Schofield and P. S. Clegg, Adv. Funct. Mater., 2011, 21, 2020–2027 CrossRef CAS.
- M. E. Cates and P. S. Clegg, Soft Matter, 2008, 4, 2132–2138 RSC.
- M. N. Lee, J. H. J. Thijssen, J. A. Witt, P. S. Clegg and A. Mohraz, Adv. Funct. Mater., 2013, 23, 417–423 CrossRef CAS.
- M. N. Lee and A. Mohraz, Adv. Mater., 2010, 22, 4836–4841 CrossRef CAS PubMed.
- M. N. Lee, M. A. Santiago-Cordoba, C. E. Hamilton, N. K. Subbaiyan, J. G. Duque and K. A. D. Obrey, J. Phys. Lett., 2014, 5, 809–812 CAS.
- J. A. Witt, D. R. Mumm and A. Mohraz, J. Mater. Chem. A, 2015, 4, 1000–1007 Search PubMed.
- E. M. Herzig, K. A. White, A. B. Schofield, W. C. K. Poon and P. S. Clegg, Nat. Mater., 2007, 6, 966–971 CrossRef CAS PubMed.
- L. Bai, J. W. Fruehwirth, X. Cheng and C. W. Macosko, Soft Matter, 2015, 11, 5282–5293 RSC.
- L. Imperiali, C. Clasen, J. Fransaer, C. Macosko and J. Vermant, Mater. Horiz., 2014, 1, 139–145 RSC.
- D. Cai and P. S. Clegg, Chem. Commun., 2015, 51, 16984–16987 RSC.
- M. F. Haase, K. J. Stebe and D. Lee, Adv. Mater., 2015, 27, 7065–7071 CrossRef CAS PubMed.
- H. Firoozmand, B. S. Murray and E. Dickinson, Langmuir, 2009, 25, 1300–1305 CrossRef CAS PubMed.
- A. Mohraz, Curr. Opin. Colloid Interface Sci., 2016, 25, 89–97 CrossRef CAS.
- A. S. Joshi and D. A. Zumbrunnen, Chem. Eng. Commun., 2006, 193, 765–781 CrossRef CAS.
- R. C. Willemse, Polymer, 1999, 40, 2175–2178 CrossRef CAS.
- H. Aref, Phys. Fluids, 2002, 14, 1315–1325 CrossRef CAS.
- R. I. Danescu and D. A. Zumbrunnen, Conductive Polymers and Plastics, 1999, pp. 77–84 Search PubMed.
- S. Gam, A. Corlu, H.-J. Chung, K. Ohno, M. J. A. Hore and R. J. Composto, Soft Matter, 2011, 7, 7262 RSC.
- F. Gubbels, R. Jérôme, P. Teyssié, E. Vanlathem, R. Deltour, A. Calderone, V. Parenté and J. L. Brédas, Macromolecules, 1994, 27, 1972–1974 CrossRef CAS.
- M. Si, T. Araki, H. Ade, A. L. D. Kilcoyne, R. Fisher, J. C. Sokolov and M. H. Rafailovich, Macromolecules, 2006, 39, 4793–4801 CrossRef CAS.
- A. Toor, T. Feng and T. P. Russell, Eur. Phys. J. E: Soft Matter Biol. Phys., 2016, 39, 57 CrossRef PubMed.
- J. Forth and P. S. Clegg, Langmuir, 2016, 32, 6387–6397 CrossRef CAS PubMed.
- B. P. Binks and J. A. Rodrigues, Langmuir, 2007, 23, 7436–7439 CrossRef CAS PubMed.
- B. P. Binks, J. A. Rodrigues and W. J. Frith, Langmuir, 2007, 23, 3626–3636 CrossRef CAS PubMed.
- H. Katepalli and A. Bose, Langmuir, 2014, 30, 12736–12742 CrossRef CAS PubMed.
- I. Akartuna, A. R. Studart, E. Tervoort, U. T. Gonzenbach and L. J. Gauckler, Langmuir, 2008, 24, 7161–7168 CrossRef CAS PubMed.
- U. T. Gonzenbach, A. R. Studart, E. Tervoort and L. J. Gauckler, Angew. Chem., Int. Ed., 2006, 45, 3526–3530 CrossRef CAS PubMed.
- A. R. Studart, H. C. Shum and D. A. Weitz, J. Phys. Chem. B, 2009, 113, 3914–3919 CrossRef CAS PubMed.
- M. Cui, T. Emrick and T. P. Russell, Science, 2013, 342, 460–463 CrossRef CAS PubMed.
- K. A. White, A. B. Schofield, P. Wormald, J. W. Tavacoli, B. P. Binks and P. S. Clegg, J. Colloid Interface Sci., 2011, 359, 126–135 CrossRef CAS PubMed.
- A. Imhof, M. Megens, J. J. Engelberts, D. T. N. de Lang, R. Sprik and W. L. Vos, J. Phys. Chem. B, 1999, 103, 1408–1415 CrossRef CAS.
- A. B. Pawar, M. Caggioni, R. Ergun, W. Hartel and P. T. Spicer, Soft Matter, 2011, 7, 7710–7716 RSC.
- A. Onuki, Phase Transition Dynamics, Cambridge University Press, Cambridge, 2002 Search PubMed.
- A. Göldel, G. R. Kasaliwal, P. Pötschke and G. Heinrich, Polymer, 2012, 53, 411–421 CrossRef.
- S. Nagarkar and S. S. Velankar, J. Rheol., 2013, 57, 901–926 CrossRef CAS.
- L. Bai, S. He, J. W. Fruehwirth, A. Stein, C. W. Macosko and X. Cheng, J. Rheol., 2017, 61, 575–587 CrossRef.
Footnote |
† Ethanol (puriss), silicone oil (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cSt), silicone oil (50 cSt) and Nile Red (for microscopy) were purchased from Sigma Aldrich. Glycerol (laboratory reagent grade ≥98%) and cetyl-trimethylammonium bromide (CTAB) (Pure) were purchased from Fisher Chemical. All chemicals were used as received. The silica nanoparticles (radius 14 nm and 65 nm) were made via the Stöber method32 and fluorescently labeled with fluorescein isothiocyanate (FITC) dye (isomer I, Sigma Aldrich) as described by Imhof et al.33 Viscosity was measured as a function of shear rate using a cone and plate rheometer. The interfacial tension was measured using drop shape analysis on a Krüss EasyDrop tensiometer. 000 cSt), silicone oil (50 cSt) and Nile Red (for microscopy) were purchased from Sigma Aldrich. Glycerol (laboratory reagent grade ≥98%) and cetyl-trimethylammonium bromide (CTAB) (Pure) were purchased from Fisher Chemical. All chemicals were used as received. The silica nanoparticles (radius 14 nm and 65 nm) were made via the Stöber method32 and fluorescently labeled with fluorescein isothiocyanate (FITC) dye (isomer I, Sigma Aldrich) as described by Imhof et al.33 Viscosity was measured as a function of shear rate using a cone and plate rheometer. The interfacial tension was measured using drop shape analysis on a Krüss EasyDrop tensiometer. |
| This journal is © The Royal Society of Chemistry 2017 |