Qualitative characterization of solid residue from hydrothermal liquefaction of biomass using thermochemolysis and stepwise pyrolysis-gas chromatography-mass spectrometry
René B.
Madsen
,
Mads M.
Jensen
and
Marianne
Glasius
 *
*
Department of Chemistry, iNANO, Aarhus University, Langelandsgade 140, 8000 Aarhus C, Denmark. E-mail: glasius@chem.au.dk
First published on 26th September 2017
Abstract
Hydrothermal liquefaction (HTL) produces solid residue (SR) as a side-product with an organic fraction of char highly dependent on the feedstock. In this work the char from batch HTL of poplar, Spirulina, and their 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture was characterized for the first time using stepwise thermal desorption and pyrolysis-gas-chromatography-mass spectrometry (py-GC-MS) along with thermochemolysis. Three distinct compound fractions were identified in the form of trapped or strongly adsorbed compounds, residual lignin, and repolymerized phenolics. The trapped or adsorbed fraction resembled the compounds in the bio-crude and aqueous phase from both poplar and Spirulina. Residual lignin was only found from poplar while repolymerized phenolics were predominantly observed from poplar through ortho and para-directed polymerization. Multiple aliphatic hydrocarbons and some alkylated pyrroles were observed from Spirulina. Co-liquefaction of biomasses led to a markedly different SR from the individual biomasses with multiple alkylated pyrroles and indoles, both volatile and non-volatile, while repolymerized phenolics diminished due to imine formation. This work demonstrates that potential bio-crude is present in the SR from both poplar and Spirulina while co-liquefaction hinders repolymerization of phenolics but also produces a vast number of volatile and non-volatile pyrroles. The work shows that additional information on the reaction pathways of HTL may be found by the characterization of the SR and provides researchers investigating biomass conversion with a method to evaluate the effects on SR formation.
1 mixture was characterized for the first time using stepwise thermal desorption and pyrolysis-gas-chromatography-mass spectrometry (py-GC-MS) along with thermochemolysis. Three distinct compound fractions were identified in the form of trapped or strongly adsorbed compounds, residual lignin, and repolymerized phenolics. The trapped or adsorbed fraction resembled the compounds in the bio-crude and aqueous phase from both poplar and Spirulina. Residual lignin was only found from poplar while repolymerized phenolics were predominantly observed from poplar through ortho and para-directed polymerization. Multiple aliphatic hydrocarbons and some alkylated pyrroles were observed from Spirulina. Co-liquefaction of biomasses led to a markedly different SR from the individual biomasses with multiple alkylated pyrroles and indoles, both volatile and non-volatile, while repolymerized phenolics diminished due to imine formation. This work demonstrates that potential bio-crude is present in the SR from both poplar and Spirulina while co-liquefaction hinders repolymerization of phenolics but also produces a vast number of volatile and non-volatile pyrroles. The work shows that additional information on the reaction pathways of HTL may be found by the characterization of the SR and provides researchers investigating biomass conversion with a method to evaluate the effects on SR formation.
1. Introduction
Reduction of CO2 emissions is the focus point of attempts to mitigate global climate changes. Combustion of fossil fuels for transport is an important contributor of CO2 and renewable bio-fuels from conversion of biomass may reduce CO2 emissions from a life cycle perspective.1 Many factors influence the overall CO2 emission and economic viability of biomass conversion including cultivation, harvest, transportation, pretreatment, process type, catalyst type, and product separation.2,3 Hydrothermal liquefaction (HTL) is a promising technique for conversion of wet biomass avoiding energy intensive drying (necessary for other techniques such as pyrolysis), which significantly reduces the overall generation of CO2.4 HTL utilizes the unique properties of hot compressed water and is carried out at elevated temperature (250–400 °C) and pressure (100–250 bar) with biomass in aqueous slurry. The aqueous media makes virtually all biomasses amenable to HTL including residues, waste, aquatics, and lignocellulosics. Many of these feedstocks can be grown on non-arable land or be used in wastewater treatment prior to conversion.5–7Microalgae have been the focus of many HTL studies due to the high yields of bio-crude along with ease of processing from the good pumping ability at high pressures and limited char formation.8,9 Lignocellulosics have received increasing attention due to their availability. Many of the studies on lignocellulosics have evaluated char formation and it is generally accepted that addition of alkali salts increases bio-crude yields and reduces solid residue (SR) formation by increasing the pH value.10 Pumping of lignocellulosics has long been established as a major challenge for scaling up the process9 while the char formation represents loss of carbon for which an effective utilization is required. Efforts to increase the pumping ability of lignocellulosics include thermal pretreatment, alkali pretreatment, addition of a slurry stabilizer, and recirculation of bio-crude.11,12 Recently, co-liquefaction with macroalgae and microalgae has been suggested, which additionally can compensate for the cost, seasonal variability, and availability of the feedstocks while microalgae also produce minimum char.13,14 The majority of HTL studies are performed without catalysts while many use alkali salts, as in this study, to decrease char formation. Char is defined as the primary decomposition and secondary polymerization of thermally decomposed biomass whereas coke is due to catalytic polymerization inside catalyst pores.15 Hence, in this study only char was considered as no catalyst was used during HTL. The char from HTL is often overlooked as a by-product although it may be used as a fertilizer.16 Finding additional markets for the SR requires extensive knowledge of the composition, which could also improve the understanding of the reaction pathways of HTL.
Characterizations of gas, aqueous phase (AqP), and bio-crude are common with many suggestions made for reaction networks based on analysis by gas chromatography coupled to mass spectrometry (GC-MS). Despite the efforts to minimize char, analyses of the SR have been limited with focus on elemental analysis, and more specific measurements for its application as an adsorbent.17 Standardized methods for molecular characterization of the SR are challenging due to the low solubility in organic solvents. Characterization of SR from HTL (220–260 °C) of cypress wood in ethanol has previously been reported using a combination of Fourier transform infrared spectroscopy (FT-IR), gel permeation chromatography (GPC), and nuclear magnetic resonance (NMR) identifying that the SR consisted predominantly of decomposed and repolymerized lignin.18 Recently, SR from HTL (220–300 °C) of cypress wood in water was characterized using advanced methods such as liquid 2D NMR and pyrolysis-GC-MS (py-GC-MS).19 Both studies involved prior fractionation including milling and multiple extractions.
The purpose of this work was to comprehensively characterize the SR from HTL of biomass. To the best of our knowledge no detailed studies have been presented on the molecular composition of char present in the SR without extensive fractionation. In this work we present a stepwise thermal desorption and py-GC-MS analysis of unprocessed SR from batch HTL of poplar (lignocellulosic), Spirulina (microalgae) and a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of them. Furthermore, thermochemolysis in the presence of tetramethylammonium hydroxide (TMAH) was carried out to identify additional compounds.
1 mixture of them. Furthermore, thermochemolysis in the presence of tetramethylammonium hydroxide (TMAH) was carried out to identify additional compounds.
2. Materials and methods
2.1 Chemicals and reagents
Potassium carbonate, dichloromethane (HPLC grade), and TMAH (25 wt% in water) were purchased from Sigma-Aldrich. Poplar was supplied by the Department of Agroecology, Aarhus University (Denmark). Poplar was separated into stems and leaves. The stems were chopped into coarse pieces of 5–10 cm and dried overnight at 105 °C. The dried pieces were knife milled and further cyclone milled to a particle size of <100 μm. Spirulina was obtained as a powder from commercial sources and used as received. The ash content and elemental distribution of the feedstocks, on dry and ash free basis, are presented in Table 1 along with the biochemical composition.2.2 Feedstock analysis
Ash content was determined by incineration at 575 °C for 5 h following evaporation of moisture. The elemental distribution was measured using a CHNS Elementar Vario Macro Cube analyzer by Elementar Analysensysteme GmbH, Hanau, Germany. The oxygen content was determined by difference. Total carbohydrate content was determined from the reaction of phenol and sulfuric acid with carbohydrate, followed by colorimetric determination at 420 nm.20 The carbohydrate content of poplar was determined by difference as the lignin content interferes with the method. The total protein content was determined according to the Lowry protein assay, with reduction of Folin's reagent and subsequent colorimetric determination at 720 nm.21 The total lipid content was determined gravimetrically following extraction in 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 chloroform and methanol aided by sonication. The total lignin content was determined with the acetyl bromide method upon extensive washing to remove proteins.22 The biochemical composition of the 1
1 chloroform and methanol aided by sonication. The total lignin content was determined with the acetyl bromide method upon extensive washing to remove proteins.22 The biochemical composition of the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture was calculated as the averages from poplar and Spirulina.
1 mixture was calculated as the averages from poplar and Spirulina.
2.3 Hydrothermal liquefaction
Biomass slurries were prepared by mixing 10 wt% biomass, 2 wt% potassium carbonate, and 88 wt% demineralized water. HTL experiments were performed in 20 ml batch reactors from Swagelok. Experiments were initiated by loading 10 ml of 10 wt% biomass slurry into the reactor. Reactors were sealed and lowered into an Omega Engineering FSB-4 fluidized sand bath preheated to 350 °C. A reaction time of 20 min was applied, after which the reactors were cooled to ambient temperature in a water bath. The reactors were vented and the AqP was decanted into a centrifuge tube. The tube was centrifuged and the AqP was transferred to a preparative glass. The centrifuge tube was extracted with 2 ml of dichloromethane and the content of the reactor was extracted with 4 × 3 ml of dichloromethane, which were combined. Prior to the last extraction the walls and bottom of the reactor were scratched with a spatula. The dichloromethane phase was vacuum filtered, and the residue was washed thoroughly with dichloromethane until the filtrate appeared clear. The organic phase was dried under a stream of nitrogen giving the resulting bio-crude. The filter paper with the SR was dried overnight at 105 °C. The SR was carefully wiped off the filter paper into a sample preparation glass.2.4 Py-GC-MS and thermochemolysis
Analysis was performed using a Gerstel multipurpose sampler coupled to a thermal desorption unit (TDU, Gerstel) with a pyrolysis unit (PYRO, Gerstel) inside. The TDU was connected to a cooled injection system (CIS4, Gerstel) mounted on an Agilent 7890B GC coupled to a quadrupole mass filter MS (Agilent, 5977A). Approximately 100 μg of sample was placed inside a 100 μl vial connected to a transport adaptor. The vial was transferred to the TDU and thermally desorbed at 280 °C. The same sample was then stepwise pyrolyzed at 400, 500, and 600 °C. The TDU initial temperature was 40 °C and increased at 12 °C sec−1 to 280 °C for thermal desorption (TD) and to 300 °C for pyrolysis. For pyrolysis the temperature was rapidly increased to the desired temperature and the TDU temperature was held at 300 °C for 1 min after pyrolysis. The helium desorption flow of the TDU was 50 ml min−1. The transfer line was held at 320 °C and the desorbed analytes were trapped in the CIS on a quartz wool liner at −60 °C using liquid N2. The trapped analytes were released onto the column when heating the CIS to 150 °C at a rate of 16 °C sec−1 and to 300 °C at a rate of 12 °C sec−1. A total split of 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 was applied through the TDU and CIS.
1 was applied through the TDU and CIS.
For thermochemolysis the sample was mixed with 2 μl of TMAH solution before it was pyrolyzed at 500 °C following the settings outlined above.
The GC was equipped with a VF-5ms column (60 m × 0.25 mm × 0.25 μm, 5 m EZ-guard, Agilent) with a 5% phenyl, 95% dimethylpolysiloxane stationary phase. The carrier gas was helium (1 ml min−1). The column oven program started at 50 °C (held for 2 min), increased to 160 °C at a rate of 8 °C min−1, increased to 230 °C at a rate of 5 °C min−1, and finally increased to 320 °C at a rate of 10 °C min−1 (held for 10 min) giving a total run time of 46.75 min. The ion source was maintained at 300 °C and the quadrupole at 180 °C. The scan range was 35–500 m/z. Data acquisition was performed using Agilent Masshunter software.
3. Results
3.1 Poplar
The product separation applied means that volatile compounds should either be soluble in the extraction solvent ending up in the bio-crude or be evaporated when drying the SR. The SR was thermally desorbed to evaluate the presence of volatile compounds showing that it contains many of the small organic compounds typically identified in the bio-crude and AqP from HTL of lignocellulosics including small organic acids, cyclopent-2-enones, and phenolics (Fig. 1, green). Small organic acids in the form of acetic acid, propionic acid, and lactic acid were detected from TD, which have been shown to be highly abundant compounds of the AqP.23 The AqP was decanted in this experiment and some AqP may have been trapped in the bio-crude ending up in the SR after extraction and filtration. Several cyclopent-2-enones and phenolics (phenol, guaiacol, and syringol) that are abundant in the bio-crude from lignocellulosics24 were detected from TD despite extensive washing with dichloromethane and subsequent drying. The presence of especially volatile cyclopent-2-enones could indicate that bio-crude is trapped inside the SR or they are strongly adsorbed to the surface of the SR. The ability to adsorb organic compounds from water has previously been demonstrated for the SR from the HTL of biomass.17 Additionally some indanones and chrom-2-ones were identified along with 1-monopalmitate, which have also been observed in the bio-crude.25 The presence of trapped or adsorbed bio-crude in the SR means that potentially increased yields are possible for batch HTL with improved product separation. The majority of continuous HTL systems take advantage of in-line filtering26 and bio-crude is less likely to end up in the SR, which apart from the solvent extraction could explain some of the difference in yields between batch and continuous HTL.27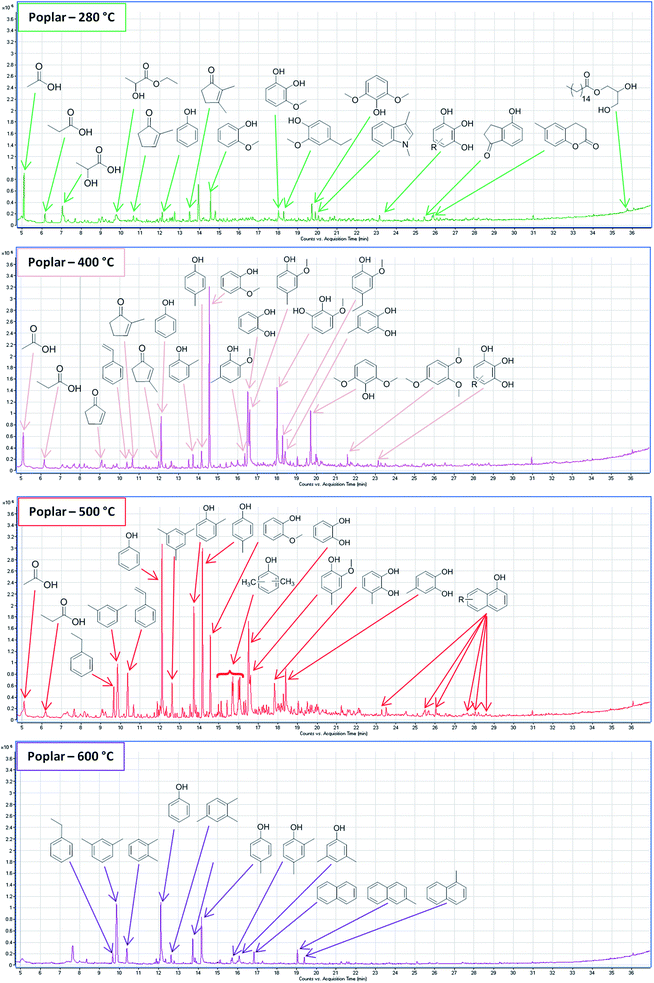 | ||
| Fig. 1 Total ion chromatogram of the solid residue (poplar) with stepwise thermal desorption and py-GC-MS at 280 °C (green), 400 °C (pink), 500 °C (red), and 600 °C (purple). | ||
At 400 °C syringol and alkyl derivatives of phenol, guaiacol, catechol, and syringol dominate the chromatogram indicating that residual and repolymerized lignin forms this part of the SR (Fig. 1, pink). A previous study has shown that pyrolysates of unprocessed lignin are characterized by a higher degree of unsaturated side chains, whereas the liquid product and solid product are a combination of the compounds observed in this study even though markedly different product separations were employed. Generally, it is observed that the alkyl group of the phenolic derivatives is placed in the ortho or para position. The importance of ortho-directed repolymerization in the HTL of lignin has previously been shown28 while para-repolymerization may also occur when the position is not substituted. The complexity of the biomass degradation means that many aldehydes and ketones may be formed from protein, carbohydrates, and lignin. The phenolics may repolymerize under alkaline conditions by the nucleophilic attack of the phenolate ion at the ortho or para position with aldehydes as shown in Fig. 2.
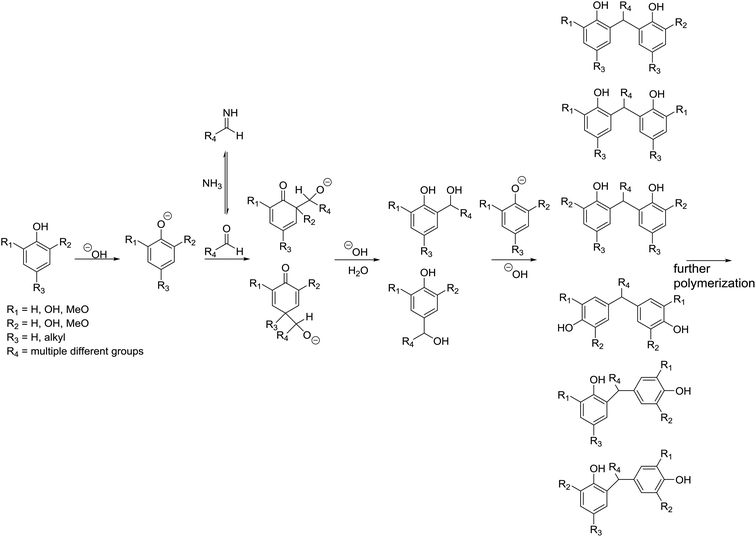 | ||
| Fig. 2 Proposed ortho and para-directed repolymerization of phenolics with aldehyde in the HTL of poplar and proposed change of reaction pathway when mixing poplar with Spirulina. | ||
Another important observation from the pyrolysate at 400 °C is the absence of hydrocinnamic acid, which facilitates the bonding between lignin and carbohydrates. Hence, residual lignin from alkaline HTL may be better described as chemically modified lignin.28 Even with thermochemolysis it was not possible to detect hydrocinnamic acids (see Section 3.3).
Increasing the temperature to 500 °C leads to a shift towards alkylated phenols while derivatives of guaiacol, catechol, and syringol diminish or disappear completely (Fig. 1, red). Hence, this part likely displays the low sterical hindrance of phenols compared to guaiacols, catechols, and syringols leading to more extensive polymerization especially when reacting with aldehydes. The more thermally stable part of the SR may therefore derive from repolymerization of phenol derivatives from lignin breakdown but also from recondensation of small organic acids.24 Additionally this fraction also showed the presence of several methylated naphthols in low abundance whose formation requires further investigation.
Further increasing the temperature to 600 °C shows the presence of predominantly alkylated benzenes, phenolics, and naphthalenes from repolymerized biomass (Fig. 1, purple). Both fractions at 500 °C and 600 °C displayed several aromatic hydrocarbons, which were not considered as they are typical secondary pyrolysis products and may not display the actual composition of the SR.
Based on these results it seems that the SR consists of four fractions; (1) trapped or strongly adsorbed compounds from the AqP and bio-crude, (2) residual lignin and repolymerized phenols, guaiacols, catechols, and syringols, (3) repolymerized phenols from carbohydrate and lignin degradation, (4) repolymerized polyaromatic hydrocarbons and phenols. The majority of the total peak area from the pyrolysates of fractions 2–4 was made up of only 12 different phenolic compounds. This is in contrast to most studies of py-GC-MS of lignin where 30–80 compounds are used to describe the pyrolysates.29,30 High yields of value-added aromatics could potentially be obtained through catalytic pyrolysis of the dry SR from HTL of lignocellulosics and further investigations should be made regarding the potential revenue.
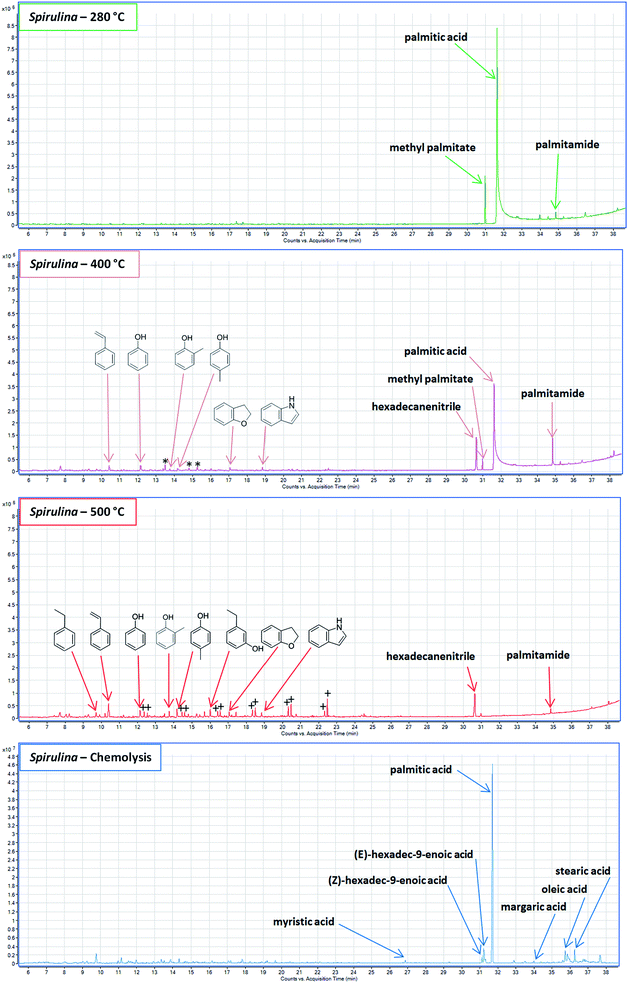
3.2 Spirulina
Generally microalgae produce limited char in HTL due to the absence of lignin and the high protein content. TD of the SR showed almost exclusively palmitic acid, methyl palmitate and palmitamide (Fig. 3, green). Neither small organic acids, cyclic oxygenates, or phenolics were detected in this fraction despite their abundance in especially the AqP.31 Hence, none of the compounds hypothesized to be trapped or adsorbed to the SR from poplar were detected in the case of Spirulina. The lack of volatiles is simply due to the fact that very little char was formed and the SR was mostly made up of ash from Spirulina without adsorption ability. Instead the high ash content means that palmitic acid precipitates to some extent. The almost exclusive precipitation of palmitic acid is in contrast to the fatty acid composition of the bio-crude where the concentration of palmitic acid has previously been reported.25 The bio-crude contained additional fatty acids such as palmitoleic acid, oleic acid, and stearic acid in concentrations that were approximately 10–20% of palmitic acid. The selective precipitation of palmitic acid during batch HTL of microalgae is unlikely to occur for model compounds and during continuous HTL leading to slightly improved bio-crude yields.Increasing the temperature to 400 °C predominantly showed the presence of hexadecanitrile and increasing amounts of palmitamide while the most abundant compound remained palmitic acid (Fig. 3, pink). At 400 °C palmitic acid is likely obtained by the pyrolysis of 1-monopalmitate and 2-monopalmitate, which have previously been observed in bio-crudes of microalgae25 and was also observed in the bio-crude of this sample. Palmitamide may arise from two sources: formation during HTL or as a secondary pyrolysis product. In previous experiments using the same processing conditions we have shown that bio-crude from C. vulgaris contains limited fatty amides while bio-crude of N. gaditana contains numerous fatty amides. The biochemical content of Spirulina is between those of C. vulgaris and N. gaditana. However, we did not observe formation of fatty amides in the bio-crude. Instead the high protein content of Spirulina leads to the formation of ammonium, which may precipitate with anions of the ash potentially reacting with palmitate during pyrolysis.
Another notable difference compared to poplar is the absence of residual lignin and repolymerized guaiacols, catechols, and syringols as lignin is not found in Spirulina. Instead small amounts of phenol pyrolysates, which could stem from repolymerized phenols formed from carbohydrate degradation products24 or potentially aromatic amino acids32 of residual protein, were detected along with a few alkylated pyrroles, benzofuran, and indole.
At 500 °C the same phenol pyrolysates (phenol, o-methylphenol, and p-methylphenol) increased in abundance while a series of aliphatic hydrocarbons (C10–15) appeared (marked by plus signs in Fig. 3, red). In previous work we have argued on the presence of alkylated phenols and catechols, and similar compounds have been found in the pyrolysis of biomass.25,33 Hence, these aliphatics may derive from predominantly long chain alkylated phenolics. The low abundance of repolymerized phenols at both 400 °C and 500 °C could indicate that phenols from carbohydrate degradation products contribute very little to SR formation in general, although it has been shown for model compounds that carbohydrates produce up to 10% SR from HTL.24 Another possibility is that the ammonium produced limits either the formation of phenolics or the repolymerization (further explanation is proposed in Section 3.3). In previous work it has been observed that the bio-crude did not contain less phenol than expected31 meaning that it does not limit the formation of phenol, and ammonia may in fact be a way to minimize repolymerization of phenolics.
Increasing the temperature to 600 °C did not result in anymore pyrolysates. Instead thermochemolysis was carried out to further investigate the presence of fatty acids (Fig. 3, blue).
Thermochemolysis leads to breakage of the labile ester bonds along with methylation of the resulting carboxylic acids while also turning free carboxylic acids into methyl esters. Additional fatty acids were detected compared to TD and they were similar to the ones previously observed in the bio-crude including myristic acid, palmitoleic acid, palmitelaidic acid, oleic acid, and stearic acid.31 Furthermore, the relative abundance of the fatty acids resembled that of the bio-crude. Even though the formation of methyl esters does improve the chromatographic performance, the absence of these fatty acids in the TD analysis shows that only palmitic acid is present as a free fatty acid while the remaining fatty acids are likely present as diglycerides or triglycerides adsorbing to the precipitated palmitic acid.
Compared to SR from poplar it is noteworthy that SR from Spirulina did not contain any small organic acids in either TD, pyrolysis, or thermochemolysis even though they are abundant in the AqP from HTL of microalgae.34 The absence further points towards selective precipitation of palmitic acid and adsorption of predominantly palmitate derivatives to the precipitated palmitic acid.
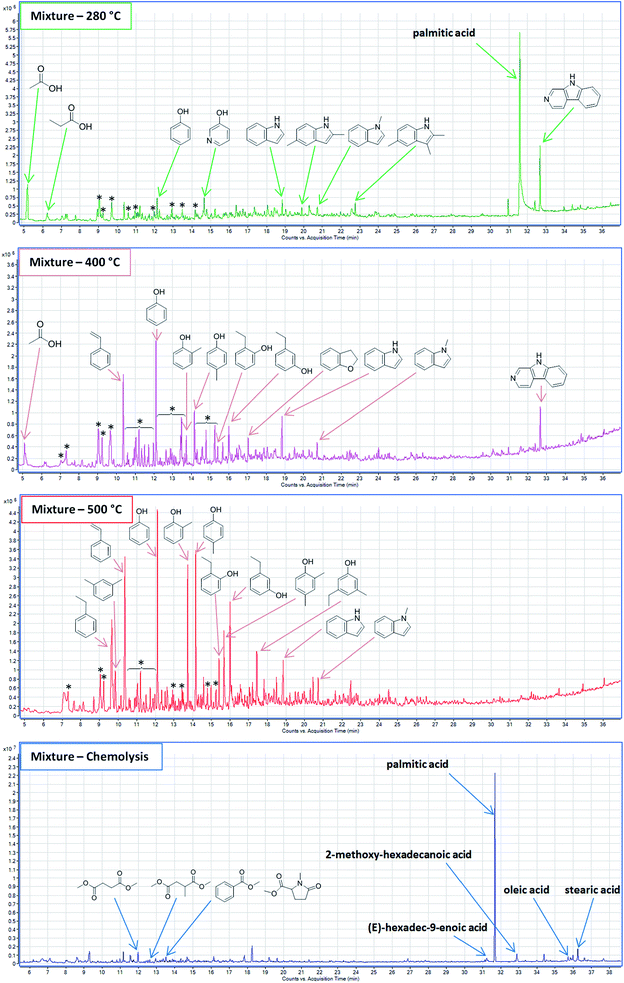
3.3 Co-liquefaction of poplar and Spirulina
Mixing Spirulina and poplar led to a SR that was markedly different from that of either feedstock. TD showed the presence of some small organic acids (acetic acid and propionic acid) at approximately the same abundances as for poplar, while palmitic acid was observed at approximately half the abundance as for Spirulina (Fig. 4, green). Similar to the SR from Spirulina, palmitic acid was found to selectively precipitate as no other fatty acid was observed. Very few of the volatile compounds in the SR from poplar were detected and did not include cyclopent-2-enones and guaiacol. Their absence is mostly explained from the analysis of the bio-crude, which has shown that significantly lower amounts cyclopent-2-enones and guaiacol are formed when mixing poplar and Spirulina due to imine formation.31 At the same time the lowered char formation also limits the adsorption. Instead multiple alkylated pyrroles and indoles were present in the SR along with 3-hydroxypyridine. Pyrroles are typically present in the bio-crude from feedstocks with similar biochemical composition to the mixture but are lost with solvent evaporation.35 This further indicates that bio-crude is either trapped in the SR or the volatile compounds are strongly adsorbed to the surface. Previous experiments have shown that formation of 3-hydroxypyridines and pyrazines is strongly favored in the bio-crude and AqP of this mixture.31 Yet pyrazines were not observed in the SR despite having similar vapor pressure to pyrroles. This shows that the SR of the mixture preferentially adsorbs pyrroles over pyrazines and a selective displacement of nitrogenated compounds occurs where pyrazines almost exclusively appear in the AqP while pyrroles almost exclusively occur in the SR when mixing these biomasses during batch HTL. Hence, to fully study the reaction pathways with batch HTL it is required to characterize at least the bio-crude, AqP, and SR.Pyrolysis at 400 °C showed more than 20 different alkylated pyrroles along with predominantly alkylated phenols and indoles (Fig. 4, pink). The pyrroles and indoles may be formed from the interaction of carbohydrate and protein degradation products as proposed in previous work where the diversity of alkyl side chains is linked to the reaction between amino acids and carbonyls.31 The repolymerization of pyrroles occurs rapidly under acidic conditions or in the presence of oxidants and the diversity of these polymers is increased further by reactivity in either position of the carbon ring depending on sterical hindrance.36Fig. 5 shows the potential polymerization through radicals and the diversification that is possible.
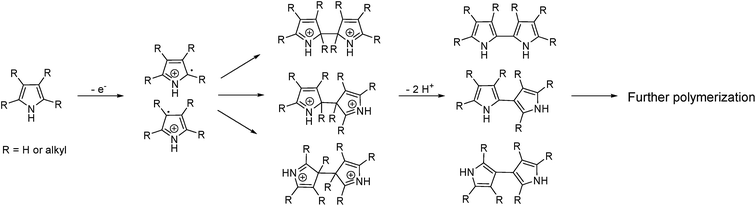 | ||
| Fig. 5 Proposed reaction for polymerization of pyrroles based on radical formation. The R group of the reacting carbon is required to be an H group. | ||
The absence of guaiacols, catechols, and syringols shows that repolymerized lignin is minimized and the presence of Spirulina may assist in this process. This effect is likely the result of several factors including imine formation between ammonia from amino acid deamination and ketones and aldehydes from degradation of carbohydrates and lignin (Fig. 2). These interactions are complex and require further investigation. Furthermore, palmitic acid was not observed in the pyrolysate showing that co-liquefaction may aid in the degradation of glycerides.
Pyrolysis at 500 °C shows substantially fewer and less abundant pyrroles. However, the variety of pyrroles and their presence from TD and in both pyrolysates shows that they react in no specific pattern creating multiple different polymers of alkylated pyrroles. These polymeric pyrroles are more likely to end up in the bio-crude during continuous HTL with in-line filtering increasing the nitrogen content of the heavy oil with potential impact on upgrading that may not be encountered in the bio-crude of batch HTL. In contrast, pyrolysis at 500 °C also showed multiple and abundant alkylated phenols (Fig. 4, red). The diversity of alkylated phenols was even greater than for SR from poplar while guaiacols and catechols were still absent. This further supports our hypothesis that residual lignin is minimized and repolymerization (mainly ortho- and para directed) of only phenols from lignin and carbohydrate products is the main contributor of the char formation from the mixture.
The chromatogram at 600 °C did not show any peaks. Instead thermochemolysis showed a similar group of fatty acids to that of the SR of Spirulina with approximately half of the abundance (Fig. 4, blue). This shows that the precipitation of palmitic acid and adsorption of other fatty acids and glycerides hereto is only dependent on the amount of ash from Spirulina, even though analysis of the concentration of palmitic acid in the bio-crude showed less than expected concentrations.31 A few other small organic acids were observed, which included succinic acid and methylsuccinic acid that are both abundant in the AqP from HTL of carbohydrates and protein.37
4. Conclusion
Detailed characterization of SR from batch HTL of biomass suggests that both volatile and semi-volatile compounds of bio-crude and AqP are trapped or strongly adsorbed to the SR. Residual lignin and repolymerized guaiacol, catechols, and syringols constituted a fraction from poplar, absent from co-liquefaction with Spirulina. Co-liquefaction leads to formation of multiple pyrroles and indoles, both volatile and non-volatile. The presence of pyrroles across all fractions shows the extensive and non-selective polymerization. Co-liquefaction also hindered the formation and subsequent repolymerization of guaiacols, catechols, and syringols through imine formation. The most thermally resistant fraction consisted predominantly of repolymerized phenols in all samples, although the SR from Spirulina also showed aliphatic hydrocarbons, which may derive from phenolics with long aliphatic chains. Generally the repolymerization of phenolics was found to be predominantly ortho- and para directed.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We would like to acknowledge Professor Bo B. Iversen and his research group for providing access to process equipment. This study was funded by Innovation Fund Denmark [Grant No. 1305-00030B] and the Danish Centre for Food and Agriculture, and carried under the auspices of the Aarhus University Centre for Circular Bioeconomy.References
- X. Liu, B. Saydah, P. Eranki, L. M. Colosi, B. G. Mitchell, J. Rhodes and A. F. Clarens, Bioresour. Technol., 2013, 148, 163–171 CrossRef CAS PubMed.
- L. Ou, R. Thilakaratne, R. C. Brown and M. M. Wright, Biomass Bioenergy, 2015, 72, 45–54 CrossRef CAS.
- Y. Zhu, M. J. Biddy, S. B. Jones, D. C. Elliott and A. J. Schmidt, Appl. Energy, 2014, 129, 384–394 CrossRef CAS.
- Y. H. Chan, R. R. Tan, S. Yusup, H. L. Lam and A. T. Quitain, Clean Technol. Environ. Policy, 2016, 18, 1759–1768 CrossRef CAS.
- A. Abdolali, W. S. Guo, H. H. Ngo, S. S. Chen, N. C. Nguyen and K. L. Tung, Bioresour. Technol., 2014, 160, 57–66 CrossRef CAS PubMed.
- W. Chen, Y. Zhang, J. Zhang, C. Yu, L. Schideman, P. Zhang and M. Minarick, Bioresour. Technol., 2014, 152, 130–139 CrossRef CAS PubMed.
- D. Winckelmann, F. Bleeke, B. Thomas, C. Elle and G. Klöck, Int. Aquat. Res., 2015, 7, 221–233 CrossRef.
- C. Jazrawi, P. Biller, A. B. Ross, A. Montoya, T. Maschmeyer and B. S. Haynes, Algal Res., 2013, 2, 268–277 CrossRef.
- D. C. Elliott, P. Biller, A. B. Ross, A. J. Schmidt and S. B. Jones, Bioresour. Technol., 2015, 178, 147–156 CrossRef CAS PubMed.
- S. S. Toor, L. Rosendahl and A. Rudolf, Energy, 2011, 36, 2328–2342 CrossRef CAS.
- E. Lappa, P. R. Christensen, M. Klemmer, J. Becker and B. B. Iversen, Biomass Bioenergy, 2016, 87, 17–25 CrossRef CAS.
- I. M. Sintamarean, I. F. Grigoras, C. U. Jensen, S. S. Toor, T. H. Pedersen and L. Rosendahl, Biomass Convers. Biorefin., 2017 DOI:10.1007/s13399-017-0247-9.
- I. M. Sintamarean, T. H. Pedersen, X. Zhao, A. Kruse and L. Rosendahl, Ind. Eng. Chem. Res., 2017, 56, 4562–4571 CrossRef CAS.
- D. W. F. Brilman, N. Drabik and M. Wadrzyk, Biomass Convers. Biorefin., 2017 DOI:10.1007/s13399-017-0241-2.
- S. Du, J. A. Valla and G. M. Bollas, Green Chem., 2013, 15, 3214–3229 RSC.
- A. R. K. Gollakota, N. Kishore and S. Gu, Renewable Sustainable Energy Rev., 2017 DOI:10.1016/j.rser.2017.05.178.
- L. Leng, X. Yuan, H. Huang, J. Shao, H. Wang, X. Chen and G. Zeng, Appl. Surf. Sci., 2015, 346, 223–231 CrossRef CAS.
- H. Liu and Y. Liu, Bioresour. Technol., 2014, 151, 424–427 CrossRef CAS PubMed.
- H. Liu, M. Ma and X. Xie, Ind. Crops Prod., 2017, 108, 63–71 CrossRef CAS.
- S. M. Gerchakov and P. G. Hatcher, Limnol. Oceanogr., 1972, 17, 938–943 CrossRef CAS.
- J. Waterborge, The Lowry Method for Protein Quantification, Human Press, New Jersey, 2002 Search PubMed.
- F. C. Moreira-Vilar, R. C. Siqueira-Soares, A. Finger-Teixeira, D. M. Oliveira, A. P. Ferro, G. J. Rocha, M. L. L. Ferrarese, W. D. Santos and O. Ferrarese-Filho, PLoS One, 2014 DOI:10.1371/journal.pone.0110000.
- E. Panisko, T. Wietsma, T. Lemmon, K. Albrecht and D. Howe, Biomass Bioenergy, 2015, 74, 162–171 CrossRef CAS.
- T. H. Pedersen and L. Rosendahl, Biomass Bioenergy, 2015, 83, 206–215 CrossRef CAS.
- R. B. Madsen, H. Zhang, P. Biller, A. H. Goldstein and M. Glasius, Energy Fuels, 2017, 31, 4122–4134 CrossRef CAS.
- A. J. Mørup, J. Becker, P. R. Christensen, K. Houlberg, E. Lappa, M. Klemmer, R. B. Madsen, M. Glasius and B. B. Iversen, Ind. Eng. Chem. Res., 2015, 54, 5935–5947 CrossRef.
- P. Biller, R. B. Madsen, M. Klemmer, J. Becker, B. B. Iversen and M. Glasius, Bioresour. Technol., 2016, 220, 190–199 CrossRef CAS PubMed.
- M. M. Jensen, R. B. Madsen, J. Becker, B. B. Iversen and M. Glasius, J. Anal. Appl. Pyrolysis, 2017, 126, 371–379 CrossRef CAS.
- S. Wang, B. Ru, H. Lin, W. Sun and S. Luo, Bioresour. Technol., 2015, 182, 120–127 CrossRef CAS PubMed.
- M. Zhang, F. L. P. Resende and A. Moutsogluo, Fuel, 2014, 116, 358–369 CrossRef CAS.
- R. B. Madsen, R. Z. K. Bernberg, P. Biller, J. Becker, B. B. Iversen and M. Glasius, Sustainable Energy & Fuels, 2017, 1, 789–805 CAS.
- S. Tsuge and H. Matsubara, J. Anal. Appl. Pyrolysis, 1985, 8, 49–64 CrossRef CAS.
- D. Tamburini, I. Bonaduce and M. P. Colombini, J. Anal. Appl. Pyrolysis, 2015, 111, 33–40 CrossRef CAS.
- B. Maddi, E. Panisko, T. Wietsma, T. Lemmon, M. Swita, K. Albrecht and D. Howe, Biomass Bioenergy, 2016, 93, 122–130 CrossRef CAS.
- S. S. Toor, L. Rosendahl, M. P. Nielsen, M. Glasius, A. Rudolf and S. B. Iversen, Biomass Bioenergy, 2012, 36, 327–332 CrossRef CAS.
- Y. T. Tan and K. Ghandi, Synth. Met., 2013, 175, 183–191 CrossRef CAS.
- R. B. Madsen, P. Biller, M. M. Jensen, J. Becker, B. B. Iversen and M. Glasius, Energy Fuels, 2016, 30, 10470–10483 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2017 |