Towards sustainable hydrocarbon fuels with biomass fast pyrolysis oil and electrocatalytic upgrading†
Chun Ho
Lam
ae,
Sabyasachi
Das
 be,
Nichole C.
Erickson
c,
Cale D.
Hyzer
c,
Mahlet
Garedew
c,
James E.
Anderson
d,
Timothy J.
Wallington
d,
Michael A.
Tamor
d,
James E.
Jackson
ae and
Christopher M.
Saffron‡
*bce
be,
Nichole C.
Erickson
c,
Cale D.
Hyzer
c,
Mahlet
Garedew
c,
James E.
Anderson
d,
Timothy J.
Wallington
d,
Michael A.
Tamor
d,
James E.
Jackson
ae and
Christopher M.
Saffron‡
*bce
aDepartment of Chemistry, Michigan State University, East Lansing, MI 48824, USA
bDepartment of Chemical Engineering and Materials Science, East Lansing, MI 48824, USA. E-mail: saffronc@msu.edu
cDepartment of Biosystems and Agricultural Engineering, Michigan State University, East Lansing, MI 48824, USA
dResearch and Advanced Engineering, Ford Motor Company, Dearborn, MI 48121, USA
eDOE Great Lakes Bioenergy Research Center, Michigan State University, USA
First published on 6th March 2017
Abstract
The carbon efficiency of bioenergy systems is of critical importance in discussions pertaining to biomass availability for the displacement of petroleum. Classical carbohydrate fermentations to make simple alcohols are carbon inefficient as they discard 1/3 of biomass holocellulose as CO2. Biomass' lignin is typically burned for heat and power instead of liquid fuel, discarding another sizeable fraction of the biomass carbon. Carbon is the backbone element in hydrocarbon fuels and these losses limit full utilization of the carbon captured by photosynthesis. The DOE Billion-ton Study Update optimistically projects enough biomass carbon to cover 2/3 of the estimated fuel usage in the transportation sector by 2030. Fast pyrolysis combined with electrocatalytic energy upgrading using renewable electricity offers a more carbon-retentive pathway for biomass to renewable fuels. This fast pyrolysis-based sequence offers the added benefit of fixing atmospheric carbon in the form of biochar, which provides a mechanism for long-term carbon storage. An associated challenge is that the liquid “bio-oil” from biomass fast pyrolysis contains functional groups like carboxylic acids, carbonyls, and oxygenated aromatics. Their presence hinders the storage and transportation of bio-oil. We propose a potential solution with localized electrocatalytic hydrogenation as an immediate measure to stabilize bio-oil via oxygen removal and carbonyl saturation. Electrocatalytically stabilized bio-oil can be stored and/or transported to centralized refineries for further upgrading. Compared to microbial bioconversion, the strategy proposed here enables significantly higher yields of renewable hydrocarbon fuels and offers a large-scale mechanism for chemical storage of renewable but intermittently generated electrical energy as transportation fuel.
1. Introduction
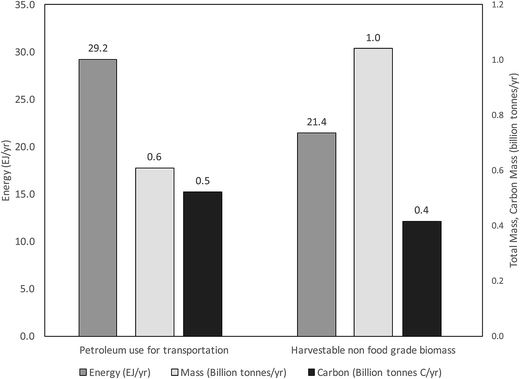
It is now widely recognized that drastic reductions in CO2 and other greenhouse gas (GHG) emissions from human activities will be necessary to avert serious environmental, economic and social dislocations due to climate change. Reduction in CO2 emissions from transportation is significantly more challenging than from stationary applications simply because mobile vehicles must carry their energy supplies with them. This places a large premium on specific and volumetric energy density, where liquid hydrocarbons reign. For example, gasoline carries almost 47 times more energy per unit mass (44.4 vs. 0.95 MJ kg−1 on a higher heating value (HHV) basis) than the most advanced commercially available lithium battery,1 and approximately five times that of liquid hydrogen when containers and insulation are included.2 These characteristics, along with compatibility with existing infrastructure and vehicles, make a renewable liquid hydrocarbon fuel highly desirable.U.S. annual energy consumption for transportation is estimated to be approximately 29 EJ for the year 2015.3 With a transportation energy consumption growth rate of <0.1% per year for the years 2012–2040,4 this value is unlikely to change much until 2030. Considering a specific energy of 48 MJ kg−1 (HHV), this amounts to about 0.6 billion tonnes of petroleum. The projected U.S. annual harvestable biomass by 2030 is estimated optimistically to be 1.04 billion dry tonnes,5 carrying only 21 EJ of energy (see ESI†), which would still not satisfy the energy demand for transportation (Fig. 1). Assuming simple empirical formulae for hydrocarbon fuel (CH2; carbon = 86% of mass 14) and biomass (CH2O; carbon = 40% of mass 30), it can be seen that the carbon content in the biomass feedstock falls short of that needed to meet the hydrocarbon fuel needs of U.S. transportation (Fig. 1).
In this regard, it must be noted that typical processes for biofuel production can only utilize a fraction of the biomass carbon. This carbon loss is illustrated by considering cellulosic ethanol production. Though biological and thermochemical processes to convert biomass to ethanol have seen impressive advances in recent years,6 their intrinsic stoichiometry loses at least 1/3 of the starch/sugar carbon as CO2, while concentrating the feedstock's energy content into a smaller fraction of the starting mass. Most of these biofuel strategies are also powered via combustion of part of the biomass itself, discarding additional CO2. However, the carbon quantity supplied annually from biomass is already below that consumed in fuels. The key limiting factor must then be recognized as carbon, rather than the energy content of the feedstock. Biomass to fuel conversion should thus aim to maximize carbon retention. This makes a fuel process that combines biomass and renewable electricity particularly attractive.
Considering biomass carbon as a limited resource, biomass-to-biofuel conversion strategies that augment energy content and minimize carbon loss are imperative. One example consistent with this approach is fast pyrolysis followed by electrocatalytic hydrogenation (ECH). Biomass can be liquefied through fast pyrolysis, a thermal process characterized by rapid heating in the absence of oxygen, to produce bio-oil.7,8 The bio-oil composition is complex and varies with feedstock. It typically contains a great variety of lower carbon number organic compounds such as aldehydes, ketones, carboxylic acids, aromatics, and about 20 wt% water. As a result of these reactive oxygenated moieties, bio-oil has a low energy content (comparable to the starting biomass), and is vulnerable to polymerization, increasing viscosity, even under ambient temperature storage conditions. Hydrodeoxygenation (HDO) of bio-oil, as used for petroleum upgrading, is of great interest as a strategy to achieve deoxygenation and stabilization of bio-oil. Such a practice could potentially take advantage of the existing petroleum infrastructure.8–20 However, transportation of biomass to a distant centralized location for pyrolysis and upgrading would be costly and energy inefficient on a large scale. Further, the aforementioned instability of untreated bio-oil makes such transport problematic. So far, two common strategies, organic solvent dilution and low temperature storage, have been proposed to improve the bio-oil storage life.21 Neither of these approaches is economically applicable on a large scale. Bio-oil pipelines would require acid-resistant coatings to prevent corrosion of metal surfaces, but these would not address bio-oil stability and thus would only be viable for on-site or short-distance transport.22 Ground transportation of untreated bio-oil would require costly stainless steel containers and refrigeration systems. One alternative is to build a small-scale hydrogenation reactor at the pyrolysis site that would stabilize bio-oil for transportation. However, the costs of small-scale H2 production are undesirably high and the need for flammable/explosive gas handling poses safety concerns. With a simpler and safer alternative, we propose electrocatalytic hydrogenation (ECH) as a mild upgrading approach that could be implemented practically as an immediate stabilization strategy local to the biomass pyrolysis sites. These regional biomass processing depots,23 with facilities for pyrolyzing biomass and stabilizing the resultant bio-oil via ECH will henceforth be referred to as Biomass Upgrading Depots (BUDs). A detailed description of what is entailed by ECH can be found in the ESI.†
Any strategy for direct biomass-to-fuels conversion is fundamentally a solar energy and carbon capture scheme. Plants are naturally evolved as atmospheric CO2 collectors and do so with very low fixed and operating cost. However, while excellent at fixing carbon, plants are relatively inefficient at capturing the sun's energy and storing it in chemical form, with no evolutionary driving force to store more energy than needed for growth and reproduction. Most plants in nature have photosynthetic efficiencies of approximately 1–2%; with 98–99% of the incident solar energy lost to optimal wavelength mismatch, respiration, reflection and transmission.24,25 Furthermore, biomass has only about one third of the specific energy of liquid hydrocarbon fuels; therefore, unmodified plant matter is energetically unsuitable as transportation fuel. Human technologies can capture solar energy much more efficiently than plants, but they do so in the form of electricity which is difficult to store. Commercially available lower-end photovoltaic cells, mostly made of single crystalline silicon, have an energy efficiency of 12–15%.26 In other words, even the oldest form of photovoltaic device on the market still captures solar energy at least ten times more efficiently than plants. Wind electricity is also increasingly cost effective.27 It is a national goal to replace fossil fuels (petroleum oil) with renewable fuels, ideally liquid fuels. Using renewable electricity to raise the energy content of biomass increases carbon utilization, maximizing yields of biomass-derived fuels. In fact, Clausen has demonstrated that fuel yields per unit biomass fed in a thermochemical biorefinery do indeed increase when energy is added via electrolysis.28 However, that strategy uses H2 gas from water electrolysis in conventional hydrodeoxygenation at relatively high temperatures and pressures. In contrast, the direct electrocatalytic reduction scheme (at the BUD) outlined here avoids the use of high pressures or H2 gas and operates at near-ambient conditions.
We argue that for renewable fuel production purposes, ECH is a promising strategy to achieve bio-oil stabilization because (a) it operates at mild conditions; (b) it avoids the storage and use of H2 gas; (c) the chemisorbed hydrogen density and reactivity on the cathode surface can be controlled by current density; and (d) most important of all, ECH reduces H2 consumption during on-site petroleum-style hydro-upgrading.29,30 Furthermore, due to a mismatch between the times of human power demand and the cycles of light and wind availability, renewable electric power generating capacity may go unused at some times; energy upgrading of biomass-derived fragments via ECH thus represents a much-needed time-insensitive method of using and storing alternative energy. Recently, it was demonstrated that the aqueous fraction of bio-oil can be stabilized via ECH.31 The resulting stabilized bio-oil contained significantly greater alcohol content and was resistant to polymerization and aging.32
2. Methods
The ECH scheme discussed in the preceding sections is a mild technique to stabilize bio-oil resulting from fast pyrolysis of biomass feedstocks. The stabilized bio-oil can then be transported to more centralized petroleum refinery complexes for further upgrading. ECH reduction is therefore only one part of the entire biomass conversion process. In this context, it is important to adopt a holistic approach that studies the results of implementing ECH in the overall biomass conversion process.This study models a complete biomass conversion that incorporates ECH for mild upgrading. Henceforth referred to as the Py–ECH process, it starts from the grinding and drying of the raw feedstock and ends with the production of a gasoline-like fuel. The major processes employed are biomass fast pyrolysis (BFP), followed by ECH and hydroprocessing. Detailed descriptions of each of these processes, along with model assumptions, can be found in the ESI.† The values obtained from the developed spreadsheet model along with all necessary sources have also been listed in the ESI.† The Py–ECH approach is then compared to a cellulosic ethanol (CE) process for the biochemical conversion of lignocellulosic feedstock to ethanol in terms of mass, energy and carbon fluxes through each system. The values for these fluxes were presented by NREL in a report published in 2011.33 All necessary values were extracted and then scaled for a fair comparison. Both sets of extracted and scaled values can be found in Tables S13–S17 in the ESI.†
For comparing Py–ECH to CE, corn stover was chosen as the biomass feedstock for each process, starting with the same higher heating value (HHV, 16.498 MJ kg−1 wet weight) and moisture content (20 wt%). The biomass feed rate was fixed at 1.0 billion dry tonnes per year for both systems, which approximates the predicted available biomass in the United States, by the year 2030, that could be produced at approximately $60 per ton.4 All the energy calculations for the Py–ECH process were performed on a higher heating value (HHV) basis. The reported energy values are a summation of the HHVs at the reference state and the sensible and latent heats required to attain the stream conditions, starting from the reference state. The reference state used for the calculations was 25 °C and 1 bar pressure.
2.1. Renewable hydrocarbons by fast pyrolysis, electrocatalysis and hydroprocessing (Py–ECH)
The Py–ECH approach is a thermochemical and electrochemical biomass conversion technique that creates stabilized bio-oil, biochar and hydrogen gas from corn stover. The primary processes involved are fast pyrolysis, electrocatalytic hydrogenation (ECH) in BUDs and a subsequent hydroprocessing step in petroleum refinery complexes. Fast pyrolysis produces the unstable liquid bio-oil product that is subsequently stabilized by the ECH process in the BUD. This stable bio-oil is then transported to the petroleum refinery complexes to be further hydrogenated at high temperature and pressure to yield the upgraded fuel. Both the ECH and the hydroprocessing steps use renewable electricity to generate the H2 for hydrogenation. A detailed description of the entire process, along with all necessary assumptions and calculations can be found in the ESI.†2.2. Cellulosic ethanol (CE) by fermentation
The CE approach considered here follows the process described in detail in NREL's 2011 report.33 According to a classification approach adopted by Cherubini et al., the CE process can be best described as a three-platform (hydrolysate, lignin, and bio-gas) biorefinery for ethanol and electricity from corn stover.34 The major processes involved in the CE approach are pretreatment, hydrolysis, fermentation, distillation, aerobic digestion and anaerobic digestion. The major product is ethanol; electricity, which comes from incinerating the non-fermentable components, is generated as a by-product. In fact, the energy requirement of the entire process is more than satisfied by this generated electricity. The entire design for the CE process has been performed by NREL using Aspen software. Therefore, for our study, all data required for the analyses were extracted from this report. For equal comparison, all the energy, mass and carbon data for the CE process were scaled to accommodate 1 billion tonnes of biomass as is processed by the Py–ECH system.3. Results
The energy analyses for the two renewable fuel production systems are summarized in the Sankey diagrams presented in Fig. 2. Balances for each system component were performed for the Py–ECH system, while overall balances were performed for the CE system. Our analysis shows that the fuel energy produced by the two systems is markedly different, as almost twice as much liquid fuel energy is produced by Py–ECH than by CE using the same biomass input. During CE production, the energy content in the primary fuel stream decreases in every stage, especially during fermentation. In the end, the amount of energy (HHV) available in the product ethanol is 44% of that in the starting biomass. In contrast, the Py–ECH system involves a more modest energy loss through bio-oil production but then regains much of that energy during ECH and hydroprocessing, ultimately yielding hydrocarbon fuel products with approximately 89% of the biomass energy.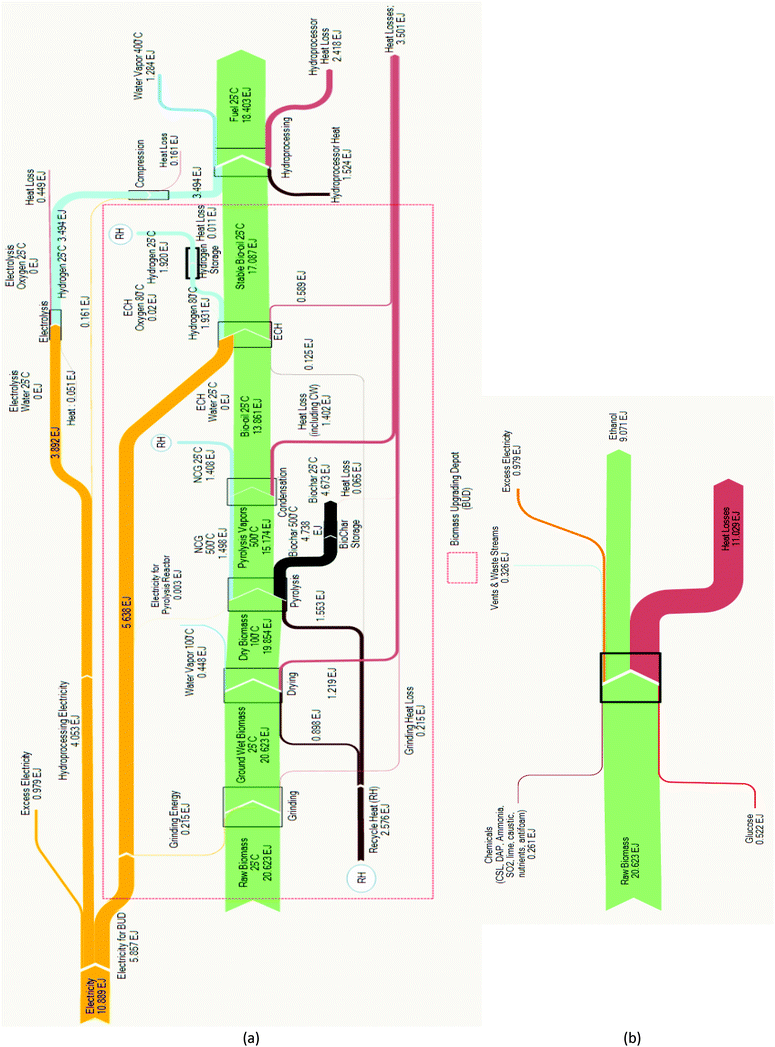 | ||
| Fig. 2 (a) Pyrolysis–electrocatalytic hydrogenation (Py–ECH) energy analysis. Yield: 89%; (b) CE energy analysis. Yield: 44%. | ||
These differences in fuel energy yield are explained by differences in electrical power utilization, heat losses, and excess electricity output. The Py–ECH process is a net consumer of electricity, while the CE process generates electricity. In order to neutralize the advantage of excess electricity produced in the CE process, a similar electrical power output of 0.979 EJ was added to the Py–ECH process (as seen in Fig. 2a). This was done by raising the electrical power input requirement of the Py–ECH process by an equivalent amount. The most noticeable difference between the two systems is the 10.9 EJ of electricity required by the Py–ECH system, while no electrical energy is needed by the CE system. As discussed earlier, this electrical energy input actually benefits the Py–ECH system, as renewable electricity is converted into chemical energy that is stored in the resultant fuel. Net heat losses explain most of the difference, with losses from the Py–ECH process being approximately half of the heat loss from the CE process. No overall heat input is required for the CE process (due to heat integration) or at the BUD for the Py–ECH process as sufficient heat can be provided internally by combustion of the co-product biochar, non-condensable gases (NCG), and H2 gas. At the central refinery, the stable bio-oil can be preheated using the heat from the outgoing hot water vapor stream. This approach reduces the heat input required, and subsequently minimizes external fuel combustion required at the central refinery. Additional process modifications to improve fuel energy yield are possible. As seen in Fig. 2a, the combustion energy of the NCG and the H2 streams (together indicated by RH in Fig. 2a) is more than sufficient to support the entire heat requirement of the BUD. In fact, the excess energy, after compensating for the heat requirement, can also be employed to reduce the electricity requirement of the process. When not combusted, the co-product biochar can be used in a number of ways,35 including land application for carbon sequestration. Use of the molecular hydrogen coproduct, generated during ECH, to further hydrogenate bio-oil in depots should also be considered, though the current analysis only utilizes its combustion energy for heat.
The CE approach is also expected to be more sensitive to the biomass type, specifically as this relates to lignin content. Fuel energy yield for woody biomass, which typically contains greater lignin content, would be less than for corn stover because energy from the lignin does not become liquid fuel in the CE approach but does in the Py–ECH approach.
The mass balance analyses reveal that the expected Py–ECH fuel mass yield of 38% (Fig. 3a) is greater than the CE fuel mass yield of only 26% (Fig. 3b). Biochar and NCG formation account for a mass loss of about 30% as this is not converted into liquid fuel. The overall mass yield for Py–ECH is limited by the high oxygen content of biomass, much of which is converted to water during hydroprocessing. In the CE process shown in Fig. 3b, a smaller fraction of the feedstock is converted into ethanol as lignin is burned for heat and power and 1/3 of the carbohydrate carbon is converted into carbon dioxide. A striking characteristic of Fig. 3b, is the significant water requirement in the process. Much of the incoming water can be attributed to process water consumption, mostly lost during cooling water evaporation. The exiting water stream is larger than the incoming stream as water is generated in the combustor, where the lignin is combusted. A more detailed analysis can be found in the ESI.† For Py–ECH, much less water is likely to be used in the process. Water consumption in the BUD can be attributed to the ECH unit, which splits water to create hydronium ions that are then recombined with electrons and incorporated into the bio-oil and generated H2. However, the total water consumption is reduced after combining the hydroprocessing step, as the water vapor generated during hydroprocessing may be condensed for heat integration. The air required in the Py–ECH process for combusting the NCG and H2 for heat integration, along with the subsequent products of combustion have been excluded from the analysis shown in the Sankey diagram. However, including them does not dramatically change the results as shown in Table S12 in the ESI† section, which depicts the mass balance that includes air and stack gases. Generally, the mass balance analyses show that the two systems manage matter in greatly different ways. Py–ECH loses mass as biochar, which can be used to mitigate climate change, while CE loses carbon as CO2, itself a climate gas.
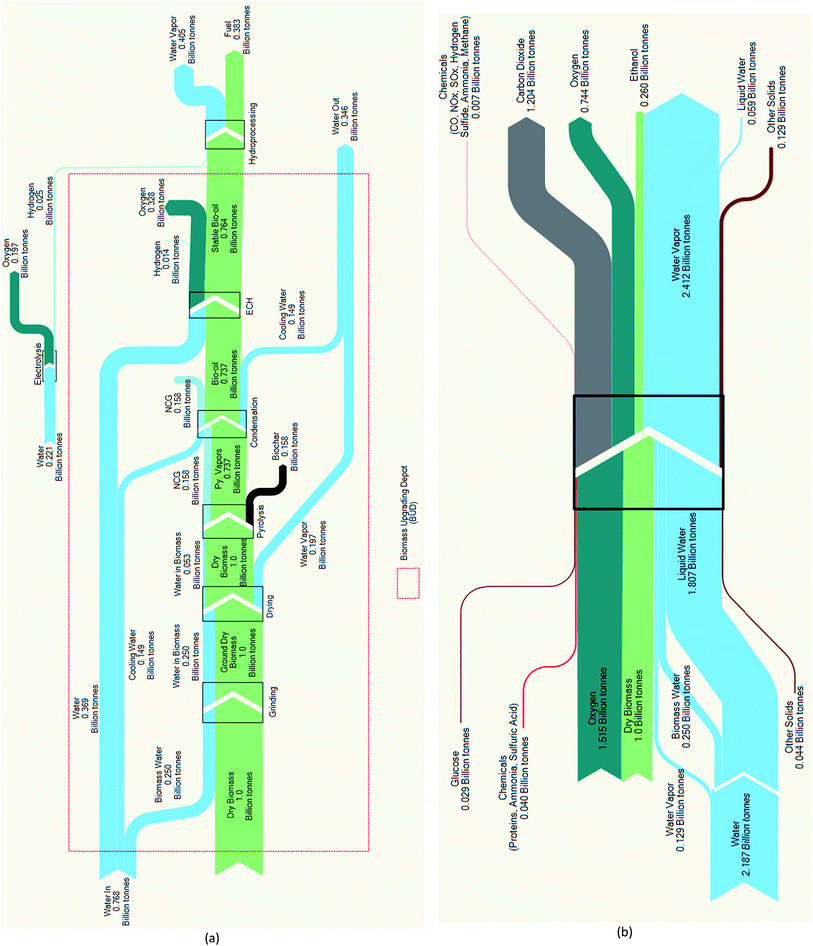 | ||
| Fig. 3 (a) Pyrolysis–electrocatalytic hydrogenation (Py–ECH) mass analysis. Yield: 38%; (b) CE mass analysis. Yield: 26%. | ||
The carbon balance analysis provides additional insight into the fuel product yields discussed previously. In Py–ECH, 63% of the feedstock carbon is retained in the liquid fuel product, while only 30% of feedstock carbon is converted to ethanol by CE as shown in Fig. 4. In fact, for the CE process, most of the carbon is lost as CO2 from the combustion flue gas stack. The retention of carbon in the final fuel is important as the carbon–carbon and carbon–hydrogen bonds are the energy carriers in hydrocarbon fuels.36 In the case of CE, corn stover is considered an optimistic case for high ethanol yield when compared to other likely feedstocks. Though not the composition used in this study, dried corn stover can contain as much as 81.9% of fermentable holocellulose (comprised of cellulose and hemicellulose) and 14.4% of non-fermentable lignin.37 Further, the highest sugar recovery, by acid hydrolysis, has been reported to be 98.4%.38 Regardless, fermentation converts glucose to ethanol at the expense of liberating 1/3 of the carbon as CO2, which constrains the final ethanol yield accordingly. Py–ECH also loses a portion of carbon as non-condensable gases (NCG) and char. However, the NCG (about 12%) released can be utilized for heating. The char, on the other hand, which accounts for about 25% of the biomass carbon, is a useful by-product that may be densified to form a renewable solid fuel or land applied as a soil amendment.39 The liquid bio-oil, with the majority (about 63%) of the carbon, can be stabilized via a localized ECH system. Through ECH, electrical energy is stored in the newly formed chemical bonds in the upgraded bio-oil, increasing its energy density and bridging renewable electricity with liquid fuel. The partial removal of bio-oil's oxygen improves its storage stability and increases its energy density, which enables and improves the efficiency of transporting bio-oil to distant refineries.32 Once there, conventional H2 upgrading of stabilized bio-oil to fuel becomes more economically feasible as a result of the locally available hydrogen and improved stability.
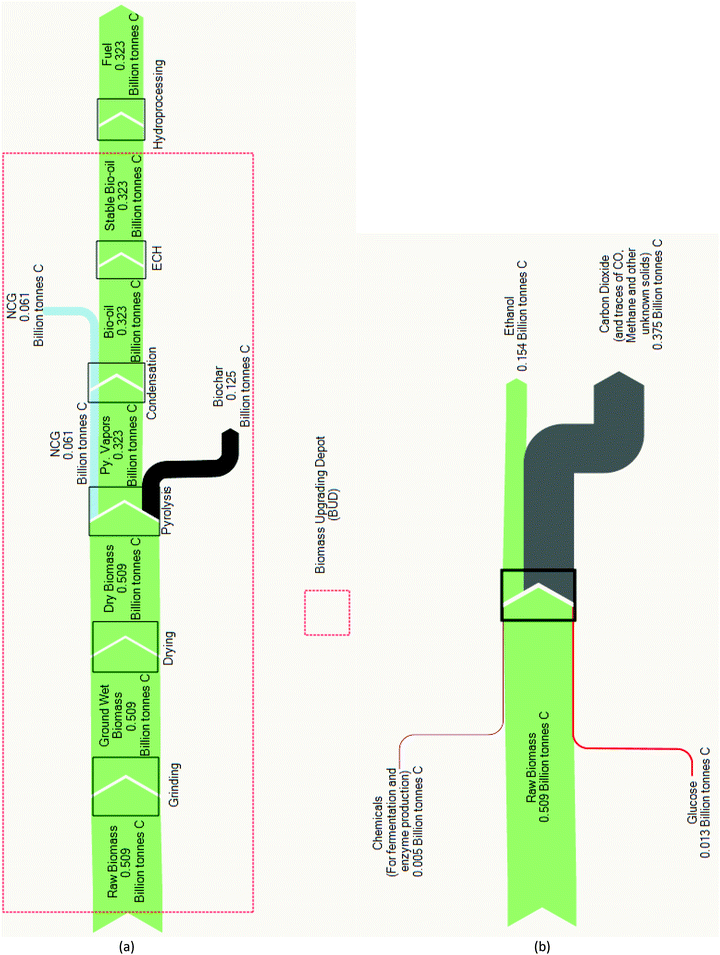 | ||
| Fig. 4 (a) Pyrolysis–electrocatalytic hydrogenation (Py–ECH) carbon analysis. Yield: 63%; (b) cellulosic ethanol (CE) carbon analysis. Yield: 30%. | ||
In summary, Py–ECH is superior to CE in terms of energy yield, carbon yield and mass yield of the final liquid fuel product, as shown in Fig. 5. The difference in energy yield is primarily a result of upgrading bio-oil using renewable electricity and hydrogen that is derived from renewable electricity. Although fast pyrolysis initially discards almost 31% of the biomass energy and 37% of the biomass carbon as biogas and char, the lost energy is mostly replaced as hydrogen incorporated during electrocatalysis and hydroprocessing – a key step of energy upgrading that is not present in the CE scheme. About 75% of the biomass energy, lost during pyrolysis, is recovered after the ECH and hydroprocessing steps. This is because both these processes add electrolytic hydrogen to the pyrolysis bio-oil and the low molecular weight and high specific energy of hydrogen serves as an ideal upgrading ingredient. Further details of the quantitative analysis of hydrogen formation and its incorporation into bio-oil can be found in the ESI† section. Stabilized bio-oil gains little mass while dramatically increasing in energy content and stability relative to raw bio-oil. Fuel production from stabilized bio-oil using hydrodeoxygenation requires less hydrogen than raw bio-oil as significant hydrogenation has already occurred through electrocatalysis. In other words, the stabilized bio-oil, leaving the BUD, is upgraded in energy content, relative to the raw bio-oil. In fact, as seen in Fig. 2a, about 71% of the total upgrading is already achieved by ECH before leaving the depot. Further, if the electricity is derived from renewable sources, then the entire energy content of the fuel would have been derived from renewable sources. Although electrocatalysis has limited ability to deoxygenate bio-oil when compared to traditional hydroprocessing, the energetic and economic value of bio-oil can be augmented substantially under mild conditions by electrocatalysis after pyrolysis in regional biomass upgrading depots.
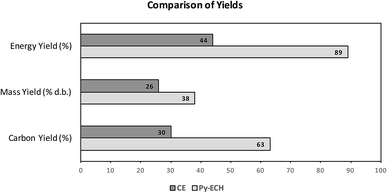 | ||
| Fig. 5 Energy, mass and carbon yield comparison of CE and Py–ECH. The energy yield is significantly higher because of addition of electrical energy. | ||
4. Discussion and outlook
Py–ECH systems appear to be advantaged in terms of energy, mass and carbon balances, though further work is certainly needed to assess the economics and environmental impacts associated with this hydrocarbon fuel strategy. Although the Py–ECH process produces less carbon dioxide than the CE process, a full life-cycle assessment is needed to ascertain its environmental benefits with and without biochar land application. It is noteworthy that climate change potential is significantly reduced when co-product biochar is land applied.40 In addition to reversing the greenhouse effect, biochar has a range of potential applications,35,41 including pollutant remediation42 or stationary power production, although its optimum usage depends on many factors that are location specific.Biomass upgrading depots are integral features of the Py–ECH concept. For commercial adoption, the number, size, and design of these depots should be optimized to balance the logistical advantage of depots with the economies of scale of a large refinery complex. Real geographical landscapes must be considered when deciding where depots should be located in relation to feedstocks and centralized facilities. Therefore, technoeconomic and life-cycle analyses, with system-wide optimization, should be conducted.43 Until direct air-capture of CO2 and subsequent reduction processes are proven economically viable at very large scale, carbon efficient bioenergy systems may prove essential to meeting future demand for low-fossil carbon transportation fuel.
As ECH of bio-oils is a nascent technology, its technology readiness level will need to elevate before it is ready for commercial application. In this regard, bench- and pilot-scale studies of ECH reactor conditions, such as optimization of temperature, catalyst activity, catalyst reusability, current density, residence time, and proton exchange membrane properties must be performed to maximize economic viability.31,32 A similar progression of experimentation is needed to convert post ECH bio-oil into finished hydrocarbons, presumably by catalytic hydroprocessing. Finally, the finished fuel should possess comparable energy content and fuel properties to commercial hydrocarbon fuels (e.g. gasoline, diesel, and jet fuel) and a robust analytical characterization will be needed to determine the quality of such fuels.
5. Conclusions
The incorporation of hydrogen and electricity from renewable sources, such as wind and solar energy, significantly improves bio-oil's potential as a sustainable alternative fuel. In this analysis, carbon retention in the Py–ECH scheme is twice that of producing cellulosic ethanol. The process also consumes significantly less water and thus the distributed Py–ECH process offers many system-level benefits. At the scale of this analysis, synthesis of fuel would use very roughly as much electrical energy as all existing electricity consumers, while the inventory of precursors and fuel products equates to an energy storage system several orders of magnitude larger and cheaper than any that could be assembled from electrochemical batteries. The controllable electrical load of these distributed conversion plants may play an essential role in managing the electric grid with its growing fraction of variable renewable generators. The synergies between carbon efficient use of biomass, familiar liquid hydrocarbon fuel and renewable electricity may be the foundation of a future fossil-carbon-free energy economy.Acknowledgements
This work was funded in part by the Ford Motor Company. Partial funding was provided by the DOE Great Lakes Bioenergy Research Center (DOE Office of Science BER DE-FC02-07ER64494). This project was also supported by the USDA National Institute of Food and Agriculture (Hatch project MICL02289) and Michigan State University AgBioResearch.References
-
G. Thomas, Overview of the Storage Development DOE Hydrogen Program, Sandia National Laboratories, 2000, vol. 9 Search PubMed
.
-
D. R. Dunn, U.S. Energy Information Administration Monthly Energy Review, August 2016 Search PubMed
.
- M. Lynes, International Energy Outlook 2016, Report DOE/EIA-0484, 2016 Search PubMed.
- R. D. Perlack, L. M. Eaton, A. F. Turhollow Jr, M. H. Langholtz, C. C. Brandt, M. E. Downing, R. L. Graham, L. L. Wright, J. M. Kavkewitz and A. M. Shamey, US Billion-ton Update: Biomass Supply for a Bioenergy and Bioproducts Industry, Report DE-AC05–00OR22725, U.S. Department of Energy, 2011 Search PubMed.
- M. Langholtz, B. Stokes and L. Eaton, 2016 Billion-ton Report: Advancing Domestic Resources for a Thriving Bioeconomy, Volume 1: Economic Availability of Feedstock, Report ORNL/TM-2016/160, U.S. Department of Energy, 2016 Search PubMed.
- M. Balat, H. Balat and C. Öz, Prog. Energy Combust. Sci., 2008, 34, 551–573 CrossRef CAS
.
- A. Bridgwater, D. Meier and D. Radlein, Org. Geochem., 1999, 30, 1479–1493 CrossRef CAS
.
- A. V. Bridgwater, Biomass Bioenergy, 2012, 38, 68–94 CrossRef CAS
.
- E. Furimsky, Appl. Catal., A, 2000, 199, 147–190 CrossRef CAS
.
- S. Czernik and A. Bridgwater, Energy Fuels, 2004, 18, 590–598 CrossRef CAS
.
- V. N. Bui, D. Laurenti, P. Afanasiev and C. Geantet, Appl. Catal., B, 2011, 101, 239–245 CrossRef CAS
.
- R. C. Baliban, J. A. Elia and C. A. Floudas, Energy Environ. Sci., 2013, 6, 267–287 CAS
.
- R. Rinaldi and F. Schüth, Energy Environ. Sci., 2009, 2, 610–626 CAS
.
- J. C. Serrano-Ruiz and J. A. Dumesic, Energy Environ. Sci., 2011, 4, 83–99 CAS
.
- C. Zhao, Y. Kou, A. A. Lemonidou, X. Li and J. A. Lercher, Angew. Chem., 2009, 121, 4047–4050 CrossRef
.
- T. Choudhary and C. Phillips, Appl. Catal., A, 2011, 397, 1–12 CrossRef CAS
.
- D. C. Elliott and T. R. Hart, Energy Fuels, 2008, 23, 631–637 CrossRef
.
- J. Wildschut, F. H. Mahfud, R. H. Venderbosch and H. J. Heeres, Ind. Eng. Chem. Res., 2009, 48, 10324–10334 CrossRef CAS
.
- D. A. Ruddy, J. A. Schaidle, J. R. Ferrell III, J. Wang, L. Moens and J. E. Hensley, Green Chem., 2014, 16, 454–490 RSC
.
- M. Saidi, F. Samimi, D. Karimipourfard, T. Nimmanwudipong, B. C. Gates and M. R. Rahimpour, Energy Environ. Sci., 2014, 7, 103–129 CAS
.
-
J. Diebold and I. Thermalchemie, A Review of the Chemical and Physical Mechanisms of the Storage Stability of Fast Pyrolysis Bio-oils, 2000 Search PubMed
.
- T. Pootakham and A. Kumar, Bioresour. Technol., 2010, 101, 7137–7143 CrossRef CAS PubMed
.
- P. L. Eranki, B. D. Bals and B. E. Dale, Biofuels, Bioprod. Biorefin., 2011, 5, 621–630 CrossRef CAS
.
- X.-G. Zhu, S. P. Long and D. R. Ort, Curr. Opin. Biotechnol., 2008, 19, 153–159 CrossRef CAS PubMed
.
- Govindjee and R. Govindjee, What is Photosyntheis?, http://www.life.illinois.edu/govindjee/whatisit.htm, accessed March 26, 2014.
- D. Blaikie, Most Efficient Solar Panels, http://sroeco.com/solar/most-efficient-solar-panels, accessed March 11 2014 Search PubMed.
- E. Lantz, R. Wiser and M. Hand, Report No. NREL/TP-6A20-53510, National Renewable Energy Laboratory, Golden, CO, 2012.
- L. R. Clausen, Energy, 2015, 85, 94–104 CrossRef CAS
.
- Z. Li, M. Garedew, C. H. Lam, J. E. Jackson, D. J. Miller and C. M. Saffron, Green Chem., 2012, 14, 2540–2549 RSC
.
- C. H. Lam, C. B. Lowe, Z. Li, K. N. Longe, J. T. Rayburn, M. A. Caldwell, C. E. Houdek, J. B. Maguire, C. M. Saffron and D. J. Miller, Green Chem., 2015, 17, 601–609 RSC
.
- Z. Li, S. Kelkar, C. H. Lam, K. Luczek, J. E. Jackson, D. J. Miller and C. M. Saffron, Electrochim. Acta, 2012, 64, 87–93 CrossRef CAS
.
- Z. Li, S. Kelkar, L. Raycraft, M. Garedew, J. E. Jackson, D. J. Miller and C. M. Saffron, Green Chem., 2014, 16, 844–852 RSC
.
- D. Humbird, R. Davis, L. Tao, C. Kinchin, D. Hsu, A. Aden, P. Schoen, J. Lukas, B. Olthof and M. Worley, Process Design and Economics for Biochemical Conversion of Lignocellulosic Biomass to Ethanol: Dilute-acid Pretreatment and Enzymatic Hydrolysis of Corn Stover, Report NREL/TP-5100–47764, National Renewable Energy Laboratory (NREL), Golden, CO, 2011 Search PubMed.
- F. Cherubini, G. Jungmeier, M. Wellisch, T. Willke, I. Skiadas, R. Van Ree and E. de Jong, Biofuels, Bioprod. Biorefin., 2009, 3, 534–546 CrossRef CAS
.
- H.-P. Schmidt, Ithaka Journal, 2012, 1, 286–289 Search PubMed
.
- P. McKendry, Bioresour. Technol., 2002, 83, 37–46 CrossRef CAS PubMed
.
- A. Demirbaş, Fuel, 1997, 76, 431–434 CrossRef
.
- Z.-Y. Sun, Y.-Q. Tang, T. Iwanaga, T. Sho and K. Kida, Bioresour. Technol., 2011, 102, 10929–10935 CrossRef CAS PubMed
.
- D. A. Laird, Agron. J., 2008, 100, 178–181 CrossRef
.
- E. Bruun, D. Müller-Stöver, P. Ambus and H. Hauggaard-Nielsen, Eur. J. Soil Sci., 2011, 62, 581–589 CrossRef CAS
.
- J. Lehmann, Nature, 2007, 447, 143–144 CrossRef CAS PubMed
.
- X. Tan, Y. Liu, G. Zeng, X. Wang, X. Hu, Y. Gu and Z. Yang, Chemosphere, 2015, 125, 70–85 CrossRef CAS PubMed
.
- B. D. Bals and B. E. Dale, Bioresour. Technol., 2012, 106, 161–169 CrossRef CAS PubMed
.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6se00080k |
| ‡ Note that all authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2017 |