Open Access Article
Open Access ArticleA mitochondrial-targeted prodrug for NIR imaging guided and synergetic NIR photodynamic-chemo cancer therapy†
Hong-Wen
Liu‡
 ac,
Xiao-Xiao
Hu‡
a,
Ke
Li
a,
Yongchao
Liu
a,
Qiming
Rong
a,
Longmin
Zhu
a,
Lin
Yuan
ac,
Xiao-Xiao
Hu‡
a,
Ke
Li
a,
Yongchao
Liu
a,
Qiming
Rong
a,
Longmin
Zhu
a,
Lin
Yuan
 a,
Feng-Li
Qu
a,
Feng-Li
Qu
 *b,
Xiao-Bing
Zhang
*b,
Xiao-Bing
Zhang
 *a and
Weihong
Tan
*a and
Weihong
Tan
 a
a
aMolecular Science and Biomedicine Laboratory, State Key Laboratory of Chemo/Biosensing and Chemometrics, College of Chemistry and Chemical Engineering, College of Life Sciences, Aptamer Engineering Center of Hunan Province, Hunan University, Changsha, 410082, P. R. China. E-mail: xbzhang@hnu.edu.cn
bThe Key Laboratory of Life-Organic Analysis, College of Chemistry and Chemical Engineering, Qufu Normal University, Qufu, Shandong 273165, P. R. China
cKey Laboratory of Environmentally Friendly Chemistry and Applications of Ministry of Education, College of Chemistry, XiangtanUniversity, Xiangtan 411105, P. R. China
First published on 14th September 2017
Abstract
Nontoxic prodrugs, especially activated by tumor microenvironment, are urgently required for reducing the side effects of cancer therapy. And combination of chemo-photodynamic therapy prodrugs show effectively synergetic therapeutic efficiency, however, this goal has not been achieved in a single molecule. In this work, we developed a mitochondrial-targeted prodrug PNPS for near infrared (NIR) fluorescence imaging guided and synergetic chemo-photodynamic precise cancer therapy for the first time. PNPS contains a NIR photosensitizer (NPS) and an anticancer drug 5′-deoxy-5-fluorouridine (5′-DFUR). These two parts are linked and caged through a bisboronate group, displaying no fluorescence and very low cytotoxicity. In the presence of H2O2, the bisboronate group is broken, resulting in activation of NPS for NIR photodynamic therapy and activation of 5′-DFUR for chemotherapy. The activated NPS can also provide a NIR fluorescence signal for monitoring the release of activated drug. Taking advantage of the high H2O2 concentration in cancer cells, PNPS exhibits higher cytotoxicity to cancer cells than normal cells, resulting in lower side effects. In addition, based on its mitochondrial-targeted ability, PNPS exhibits enhanced chemotherapy efficiency compare to free 5′-DFUR. It also demonstrated a remarkably improved and synergistic chemo-photodynamic therapeutic effect for cancer cells. Moreover, PNPS exhibits excellent tumor microenvironment-activated performance when intravenously injected into tumor-bearing nude mice, as demonstrated by in vivo fluorescence imaging. Thus, PNPS is a promising prodrug for cancer therapy based on its tumor microenvironment-activated drug release, synergistic therapeutic effect and “turn-on” NIR imaging guide.
Introduction
Significant advances in cancer diagnosis and therapy have been made in the past years, but there still remain several barriers for improving effectiveness and avoiding severe side effects.1–5 This highlights the need to develop anticancer agents for effectively and selectively killing tumor cells without affecting normal tissues. Photodynamic therapy (PDT), driven by activating photosensitizers (PSs) to generate reactive oxygen species (ROS), generally singlet oxygen for cancer cell killing, is considered to be a safe, minimally invasive treatment.6,7 Highly selective photosensitizers are still desirable for accurately localizing and activatable prodrug to minimize side effects and realize more efficient therapeutic outcome. Recently, some activatable PSs have been developed for further minimizing the side effects of PDT.8,9 The design strategy is generally based on the concept that the prequenched fluorescence and inhibited phototoxicity of the PS which can be restored once a specific trigger is able to separate the quencher or energy acceptor from the vicinity of the PS.10,11 Moreover, the near infrared (NIR) PSs are desired for PDT, because NIR photons can deeply penetrate the skin and underlying tissue with low damage to the biological samples and minimal background interference.12–14 Therefore, it's very significant to develop activatable NIR PSs. On the other hand, chemotherapy is one of the most important modalities of cancer treatment. 5-Fluorouracil (5-FUra) has been used in the treatment of a variety of neoplastic diseases. 5′-Deoxy-5-fluorouridine (5′-DFUR), a prodrug of 5-FUra, can be converted to 5-FUra by the thymidine phosphorylase, which is more abundant in tumors than in normal tissues except for the liver of humans.15 The combination of PDT and chemotherapy with different therapeutic mechanisms has also been proved effective in improving the therapeutic efficiency,16 which has been achieved mainly via co-encapsulated an anticancer drug and a PS in nanocarriers.17,18 In addition, since the extremely short half-life (<40 ns) and small radius of action (<20 nm) of singlet oxygen (1O2) in biological systems,19 direct delivering of PS to hypersensitive subcellular organelles will greatly enhance the PDT efficiency.20–22 Mitochondria are vital intracellular organelles that play valuable roles in energy production, ROS generation, cellular signalling and regulate apoptosis. Owing to the essential and fatal role of mitochondria, several mitochondrial-targeted anti-cancer drugs have been developed to expect optimal therapeutic efficiency.23,24 Many evidences also indicate that the damage of mitochondria is the main pathway for PDT-treated cell apoptosis.20 Thus, mitochondrion is the ideal subcellular target for cancer therapy.The design of molecular fluorescent probe provides the strategy for developing theranostic prodrugs for targeted and image-guided combination cancer therapy.25 Fluorescent imaging can provide realtime informations about where, when, and how the prodrugs are delivered and activated in vivo.26 Generally, in such theranostic systems, masked anticancer drugs and imaging reagents are conjugated by tumor-sensitive linkers.27 Once in the tumor microenvironments, the tumor-sensitive linkers can be broken by tumor over express species, such as low pH,28 high reactive oxygen species level (including H2O2),29–31 high expressed enzymes,32–34 and high glutathione (GSH) concentration,26,35–38 resulting in the release of the anticancer drugs and the activation of the imaging reagents. However, most of previous theranostic drugs do not realize combination of PDT and chemotherapy in a single molecule, which encouraged us to explore molecular theranostic probes with multi-function, including NIR PDT, chemotherapy, subcellular targeting and NIR fluorescence for real-time monitoring the therapeutic effect.
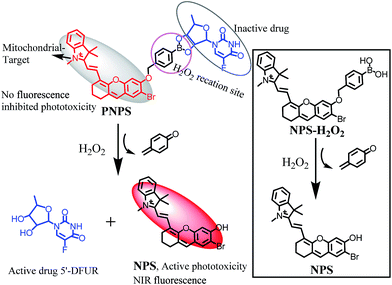
In this work, we developed a mitochondrial-targeted prodrug PNPS for NIR fluorescence imaging guided and synergetic chemo-photodynamic precise cancer therapy for the first time. PNPS is composed of two moieties and a specific recognition linker as shown in Scheme 1. The first moiety is a NIR fluorescent monitor NPS, of which its essential properties of NIR fluorescence and phototoxicity can be countered, yes reversed, by caging its hydroxyl group, act as a prodrug for PDT. Meanwhile, this NPS is preferably localized in mitochondria, due to its lipophilic quaternary ammonium salt structure.39 The second component is an anticancer drug 5′-DFUR. The former two parts are linked and caged through a H2O2-sensitive bisboronate group, displaying no flourescence signal and therapeutic effect. In the presence of H2O2, the bisboronate group is broken, resulting in releasing the free NPS for NIR PDT and free 5′-DFUR for chemotherapy. The activated NPS can also provide a NIR fluorescence signal for monitoring the release of the activated drugs. Taking advantages of the high H2O2 concentration in cancer cells, PNPS exhibited higher cytotoxicity to cancer cells than normal cells, resulting in lower side effects. In addition, based on its mitochondrial-targeted ability, PNPS exhibited enhanced chemotherapy efficiency compare to free 5′-DFUR. Moreover, effective chemo-photodynamic combined therapy effects in cancer cells was observed. The in vitro and in vivo prodrug release was visualized by in situ generated NIR fluorescence. These favorable features of tumor microenvironment-activated ability, effective synergistic thertic effect and NIR fluorescence monitoring of the drug release make PNPS a promising prodrug.
Results and discussion
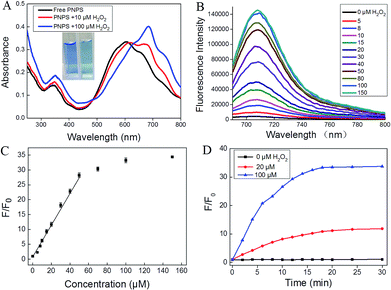
We first developed a novel NIR photosensitizer NPS, which shows the maximum excitation and emission wavelength at 680 nm and 710 nm, respectively. Since activatable photosensitizers share similar activation mechanisms with activatable fluorophores, the inherent fluorescence of NPS is prohibited accompany with inhibited phototoxicity, when the hydroxyl group of NPS is caged. Based on the molecular probe design strategy, we hypothesized to develop a subcellular targeted molecular theranostic prodrug with multi-function, such as fluorescence imaging, PDT, chemotherapy, and real-time monitoring of the therapeutic effect. H2O2 was chosen as the target for its high sensitivity and specificity toward the boronate moiety and intrinsic enhancement of H2O2 levels inside the tumor cell.40 It was easy to obtain prodrug NPS-H2O2 by attaching the H2O2 recognition group (benzoboric acid) to the hydroxyl group of NPS. And then, taking the advantage of the reaction between benzoboric acid and pinacol (5′-DFUR contains pinacol group), we obtained the final theranostic prodrug PNPS. The synthetic route for PNPS was depicted in Fig. S1.† And characterizations of all the new compounds are described in the ESI in detail.†To verify that H2O2 was able to cleave the boronate moiety of prodrug PNPS and consequently activate the NIR fluorophore, a chemical transformation experiment of PNPS was performed in the presence of H2O2 under physiological conditions and monitored by UV-vis and fluorescence spectroscopy. In the UV-vis spectrum as shown in Fig. 1A, PNPS showed a strong absorption peak at λmax = 600 nm. The absorption peak showed a red shift after addition of 100 μM H2O2, accompanied by a distinct color change from blue to blue-green (Fig. 1A insert). As shown in Fig. 1B, PNPS exhibited weak fluorescence centered at 710 nm, and gradual addition of H2O2 up to 0–150 μM to a solution of PNPS induced the increase of fluorescence intensity. The fluorescence intensity at 710 nm increased by 34.3-fold upon reaction with 150 μM H2O2 for 30 min (Fig. 1C). Meanwhile, its intensity increased linearly with the concentration of H2O2 ranging from 5 to 40 μM (Fig. S2†). The catalytic efficiency of H2O2 toward the PNPS was assessed. Fluorescence kinetic curves of H2O2 at varied concentrations (0, 20, 100 μM) reacting with PNPS were depicted in Fig. 1D. As was shown, a higher concentration of H2O2 could induce faster cleavage reaction and therefore result in increased fluorescence intensity. The fluorescence signal of the reaction system could reach a plateau in about 20 min. Meanwhile, in the absence of H2O2, testing the generation of a false positive signal by spontaneous hydrolysis, gave no detectable signal increase, even over 12 h (Fig. S3†), demonstrating that PNPS was stable in the reaction system. These results demonstrated that after reaction with H2O2, PNPS was efficiently activated and the released NPS could provide a turn-on NIR fluorescence signal for drug release and therapeutic efficacy monitoring.
The selectivity of prodrug PNPS for H2O2 over other biologically relevant ROS species, metal ions, amino acids and proteins was evaluated. As shown in Fig. S4A,† no significant changes in fluorescence intensity were observed upon addition of the other ROS species, metal ions, amino acids and proteins to prodrug PNPS. The pH effect on the H2O2-induced fluorescence changes of PNPS was also investigated. As shown in Fig. S4B,†PNPS remained stable and emitted a weak fluorescence within 4.0–9.0 pH range. After treatment by 25 μM of H2O2, the fluorescence at 710 nm was activated and reached saturation in the 7.4–8.0 pH range. The above results indicated that prodrug PNPS can be applied as a H2O2-activated theranostic prodrug under physiological pH condition with high selectivity.
To confirm the fluorescence response mechanism, the reaction products of PNPS with H2O2 were analyzed by HPLC and HRMS. As illustrated in Fig. S5,† pure PNPS showed a unique peak with retention time at about 5.36 min, and compound NPS exhibited a peak at about 6.25 min. After PNPS incubation with H2O2 for 30 min, a new peak at 6.25 min corresponding to compound NPS was clearly observed, and the peak corresponding to PNPS was sharply decreased. Moreover, in the ESI-HRMS (positive ion mode) spectrum of PNPS incubated with H2O2 for 30 min, the peaks of NPS and 5′-DFUR were found at m/z 464.1065 and 269.0462 respectively (Fig. S6†). These results demonstrated that the H2O2 cleaved recognition site, then PNPS was activated and free NPS and 5′-DFUR were released.
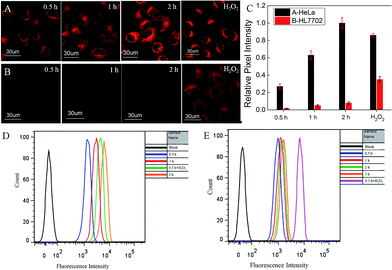
On the basis of the positive results showing H2O2-activated release of active drug and concurrent fluorescence enhancement, PNPS was next tested in cultured cells. Cellular fluorescence imaging of PNPS were investigated in four different cell lines, HeLa, HepG2, HCT116 and HL7702 cells. HeLa, HepG2 and HCT116 cancer cells were pretreated with prodrug PNPS (5 μM) for different time, then fluorescence imaging were carried out. The increased fluorescence intensity of HeLa, HepG2 and HCT116 cells indicated the efficient activation of theranostic PNPS by endogenous H2O2 in the tumor cells without any external inducer (Fig. 2A and S7–S9†). In addition, HeLa cells pretreated with exogenous H2O2 for 0.5 h and then incubated with PNPS for another 0.5 h, the fluorescence intensity dramatically increased. The cellular activation of PNPS were further confirmed by flow cytometry analysis (Fig. 2D). Similar phenomena were also observed in HepG2 cells (Fig. S8†), and the activation performance of PNPS in HepG2 after incubated for 2 h is 10% higher than that for HeLa cells calculated from the results of flow cytometry. These results indicated that the oxidation of boronate moiety of theranostic PNPS generated fluorescence enhancement, depending upon amount of intracellular H2O2, as depicted in Scheme 1. Normal HL-7702 cells were also incubated with prodrug PNPS for an examination of the effect of H2O2 on the bioactivity of PNPS. Very weak fluorescence signals were observed in HL-7702 cells by confocal microscopy after incubating with PNPS for 0.5 h, 1 h or 2 h (Fig. 2B). However, after pretreated with 100 μM H2O2 for 0.5 h, a strong fluorescent enhancement was observed in HL-7702 cells. The flow cytometry analysis results also confirmed that PNPS kept inactivated in HL-7702 cells (Fig. 2E). These results demonstrated that prodrug PNPS showed cancer cells-targeted activated ability.
Furthermore, mitochondrial localization of activated prodrug PNPS was investigated by colocalization experiments using a commercialized mitochondria fluorescent tracker, Mito-tracker Green. As shown in Fig. 3 and S10,† the fluorescence signal that was ascribed to PNPS colocalized well with the Mito-tracker (Pearson's correlation factor is 0.965, 0.943 and 0.958 for HeLa cells, HepG2 cells and HCT116 cells respectively), due to its lipophilic quaternary ammonium salt structure. Further experiments were carried out to demonstrate that most PNPS molecules will locate in mitochondria before reaction with cytoplasm H2O2, with the results showed in Fig S11.† This is mainly because the PNPS molecular can fast diffuse into cells and target mitochondria (within 10 min) before reaction with relatively low concentration cytoplasm H2O2. These results demonstrated the outstanding mitochondrial-targeting ability of PNPS, which may help improve the therapeutic effect.
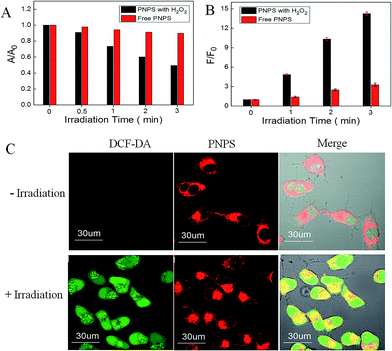
The 1O2 generation of PNPS in buffer solution in the present of H2O2 upon light irradiation was studied using 9, 10-diphenylanthracene (DPHA)41 and fluorescent probe MNAH42 as indicators. DPHA is a 1O2 indicator, whose absorbance decreases upon interaction with 1O2. In the presence of H2O2, when incubating DPHA with PNPS upon white light irradiation, the absorbance of DPHA decreased gradually (Fig. S12A†). Meanwhile, pretreated PNPS with H2O2 and then incubated with fluorescent probe MNAH upon white light irradiation, the fluorescence of MNAH increased gradually (Fig. S13A†). But in the absence of H2O2, the absorbance of DPHA or the fluorescence of MNAH showed limited changes (Fig. S12B and S13B†). And the compared results were shown in the Fig. 4A and Fig. 4B, obvious difference could be observed between those whether pretreated with H2O2. These results demonstrated that PNPS could efficiently generate 1O2 in buffer solution after activated by H2O2, indicated its activable phototoxicity. The 1O2 generation of PNPS in cells upon light irradiation was studied using 2′,7′-dichlorofluorescin diacetate (DCF-DA) as a cell-permeable probe. DCF-DA is nonfluorescent but can be oxidized by 1O2 to yield highly fluorescent 2′,7′-dichlorofluorescein (DCF). As shown in Fig. 4C, strong green fluorescence could only be observed when the cells were treated with PNPS for 2 h followed by light irradiation. The results confirmed the 1O2 generation by activated PNPS in live HeLa cells upon light irradiation.
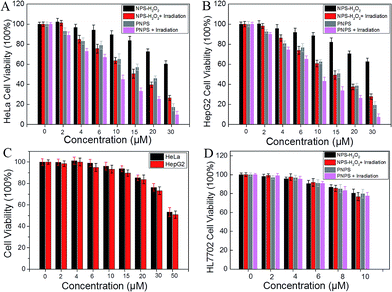
We then investigated the ability of prodrug PNPS to selectively target cancer cells by measuring its cytotoxicity to HeLa, HepG2 and HL-7702 cells. MTS assays were used to study cytotoxicity of PNPS, NPS-H2O2 and 5′-DFUR under dark. After 3 h incubation, NPS-H2O2 and 5′-DFUR exhibited low cytotoxicity even at a high concentration of 15 μM as more than 80% of the tested HeLa and HepG2 cells survived (Fig. 5). On the contrary, PNPS demonstrated much higher dark cytotoxicity with an IC50 value of 16.6 μM and 14.8 μM for HeLa cells and HepG2 cells respectively (Fig. S14 and 15†). Taken together, these results illustrated that PNPS exhibited enhanced cytotoxicity over commercial 5′-DFUR. For PNPS, in addition to the high cytotoxicity under dark conditions, under white light illumination, it could generate 1O2 and exhibited high phototoxicity to cancer cells. Notably, under white light illumination, the IC50 value of PNPS towards HeLa and HepG2 cells was 9.32 μM and 8.15 μM respectively (Fig. S14 and 15†), which was lower than that of PNPS without light irradiation (Fig. 5A and B). And compare to NPS-H2O2, PNPS showed enhanced cytotoxicity to cancer cells, due to its chemo-photodynamic combination therapy. However, both PNPS and NPS-H2O2 showed lower cytotoxicity even at a high concentration of 10 μM towards HL-7702 cells as more than 75% of the tested HL-7702 cells survived (Fig. 5D). As contrast, compound NPS exhibited high phototoxicity to HL-7702 cells (Fig. S16†), with IC50 value of 8.50 μM (Fig. S17†). These results indicated that the prodrugs PNPS and NPS-H2O2 show low cytotoxicity to normal HL-7702 cells, which may mainly because that PNPS and NPS-H2O2 kept inactivated in HL-7702 cells. Collectively, these results demonstrated that the combination chemo-therapy and PDT provides higher anticancer effect compared to the single therapeutic approach alone.
The propidium iodide (PI) staining experiments were further carried out to demonstrate MTS results. PI, a cell impermeable dye, only stains dead cells or late apoptotic cells with damaged membrane. As shown in Fig. S18,†PNPS-treated HeLa cells incubated under the dark was stained whereas nearly all the cells were stained after they were exposed to white light irradiation. Meanwhile, HL-7702 cells were incubated with PNPS much fewer cells were stained even after white light irradiation. However, when HL-7702 cells were first treated with H2O2 and follow with PNPS, most cells were stained after white light irradiation. Collectively, these results indicate that PNPS achieves lower side effects to normal cells.
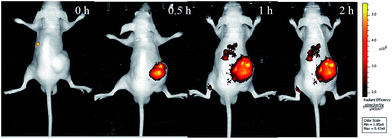
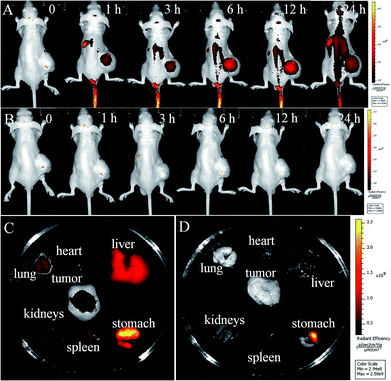
Benefit from its NIR fluorescence, the activation of the prodrug PNPS in tumor-bearing mice could be investigated via in vivo fluorescence imaging. The nude mice bearing HCT116 xenografts were injected in the tumor site with the prodrug PNPS, and in vivo images were obtained at various times. As shown in Fig. 6, NIR fluorescence intensity representing the release of the activated drug, was clearly observed at 0.5 h after the injection of the PNPS, the NIR fluorescence then increased in time-dependent manner. These results indicated that theranostic prodrug PNPS could be effectively activated in the tumor and could be used for real-time fluorescence monitoring of the therapeutic effect in vivo.
We further investigated the activation and bio-distribution of prodrugs PNPS with HCT116 tumor-bearing mice, intravenously injected with PNPS and saline. As shown in Fig. 7A, the obvious fluorescence was seen in the tumor region after treated by prodrug PNPS for 1 h, indicative of the rapid distribution of PNPSvia the blood circulation and the effective activation of PNPS in tumor. And a significant fluorescence enhancement was observed in tumor as time continued before 12 h post injection due to the gradual prodrug activation, and it faded out as time further increased, owing to the excretion of the activated drug. In contrast, negligible signals were obtained in the saline-treated mice (Fig. 7B). And during the distribution process of 12 h, PNPS had the strongest fluorescence, representing the largest amount of released drug, in the tumor. These results indicated prominent tumor-targeting activation ability of PNPS. The tumor-targeting drug release along with fluorescence enhancement could be ascribed to the particularly high H2O2 concentration in cancer cells. Furthermore, in fluorescence images of the internal organs at 25 h post injection after anatomy, an obvious fluorescence was observed in the tumor, liver and stomach of mice treated by prodrug PNPS, and much weaker fluorescence were seen in lung, heart, kidney and spleen (Fig. 7C). Meanwhile, in the control experiments, no obvious fluorescence were observed in the tumor or other internal organs (Fig. 7D). The tumor-targeting ability and the specific drug release in tumor make PNPS a promising prodrug to achieve high efficacy and reduced side effects.
Conclusions
In summary, by conjugating the anticancer drug 5′-DFUR and NIR photosensitizer (NPS) via a bisboronate bond, we developed a novel H2O2-responsive NIR theranostics prodrug PNPS. The phototoxicity and inherent NIR fluorescence of NPS and the cytotoxicity of the drug (5′-DFUR) are quenched by the covalent H2O2-responsive linker. PNPS can be effectively activated by the high concentration of H2O2 in cancer cells or in tumor as monitored by the turn-on NIR fluorescence. PNPS shows enhanced chemotherapy efficiency compare to free 5′-DFUR due to its mitochondrial-targeting ability. Furthermore, it shows remarkably improved and synergistic chemo-photodynamic therapeutic effect for cancer cells. More importantly, PNPS exhibits higher cytotoxicity to cancer cells than that to normal HL-7702 cells, resulting in lower side effects. Therefore, the prodrug PNPS provides a promising platform for specific tumor-activatable drug delivery system, which can be easily monitored by cellular and in vivo NIR fluorescence imaging. This study also suggests that the molecular probe design strategy, which combines the key functions of targeting, release, imaging, and treatment within a single agent, may play an important role in cancer diagnosis and therapy.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grants 21325520, 21521063, 21327009, 21375076, J1210040), the science and technology project of Hunan Province (2016RS2009, 2016WK2002).Notes and references
- A. Umar, B. K. Dunn and P. Greenwald, Nat. Rev. Cancer, 2012, 12, 835–848 CrossRef CAS PubMed.
- P. E. Goss and A. F. Chambers, Nat. Rev. Cancer, 2010, 10, 871–877 CrossRef CAS PubMed.
- X. Li, J. Kim, J. Yoon and X. Chen, Adv. Mater., 2017, 29, 1606857 CrossRef PubMed.
- H. Ju, Sci. China: Chem., 2015, 58, 438 CrossRef CAS.
- W. Pan, H. Yang, T. Zhang, Y. Li, N. Li and B. Tang, Anal. Chem., 2013, 85, 6930–6935 CrossRef CAS PubMed.
- J. P. Celli, B. Q. Spring, I. Rizvi, C. L. Evans, K. S. Samkoe, S. Verma, B. W. Pogue and T. Hasan, Chem. Rev., 2010, 110, 2795 CrossRef CAS PubMed.
- A. P. Castano, P. Mroz and M. R. Hamblin, Nat. Rev. Cancer, 2006, 6, 535–545 CrossRef CAS PubMed.
- X. S. Li, S. Kolemen, J. Yoon and E. U. Akkaya, Adv. Funct. Mater., 2017, 27, 1604053 CrossRef.
- S. Kolemen, M. Isik, G. M. Kim, D. Kim, H. Geng, M. Buyuktemiz, T. Karatas, X. F. Zhang, Y. Dede, J. Yoon and E. U. Akkaya, Angew. Chem., Int. Ed., 2015, 54, 5340–5344 CrossRef CAS PubMed.
- J. F. Lovell, T. W. B. Liu, J. Chen and G. Zheng, Chem. Rev., 2010, 110, 2839–2857 CrossRef CAS PubMed.
- R. Musiol, M. Serda and J. Polanski, Curr. Pharm. Des., 2011, 17, 3548–3559 CrossRef CAS PubMed.
- Z. Guo, S. Park, J. Yoon and I. Shin, Chem. Soc. Rev., 2014, 43, 16–29 RSC.
- X. Chen, D. Lee, S. Yu, G. Kim, S. Lee, Y. Cho, H. Jeong, K. T. Nam and J. Yoon, Biomaterials, 2017, 122, 130–140 CrossRef CAS PubMed.
- N. Li, Z. Yu, W. Pan, Y. Han, T. Zhang and B. Tang, Adv. Funct. Mater., 2013, 23, 2255–2262 CrossRef CAS.
- H. Eda, K. Fujimoto, S.-i. Watanabe, M. Ura, A. Hino, Y. Tanaka, K. Wada and H. Ishitsuka, Cancer Chemother. Pharmacol., 1993, 32, 333–338 CrossRef CAS PubMed.
- M. Olivo, R. Bhuvaneswari, S. S. Lucky, N. Dendukuri and P. Soo-Ping Thong, Pharmaceuticals, 2010, 3, 1507–1529 CrossRef PubMed.
- W. Zhang, J. Shen, H. Su, G. Mu, J.-H. Sun, C.-P. Tan, X.-J. Liang, L.-N. Ji and Z.-W. Mao, ACS Appl. Mater. Interfaces, 2016, 8, 13332–13340 CAS.
- Y. Zhang, F. Huang, C. Ren, L. Yang, J. Liu, Z. Cheng, L. Chu and J. Liu, ACS Appl. Mater. Interfaces, 2017, 9, 13016–13028 CAS.
- P. R. Ogilby, Chem. Soc. Rev., 2010, 39, 3181–3209 RSC.
- R. Hilf, J. Bioenerg. Biomembr., 2007, 39, 85–89 CrossRef CAS PubMed.
- L. Rajendran, H. J. Knolker and K. Simons, Nat. Rev. Drug Discovery, 2010, 9, 29–42 CrossRef CAS PubMed.
- Z. Yu, W. Pan, N. Li and B. Tang, Chem. Sci., 2016, 7, 4237–4244 RSC.
- S. Fulda, L. Galluzzi and G. Kroemer, Nat. Rev. Drug Discovery, 2010, 9, 447–464 CrossRef CAS PubMed.
- Z. Yu, Q. Sun, W. Pan, N. Li and B. Tang, ACS Nano, 2015, 9, 11064–11074 CrossRef CAS PubMed.
- C.-J. Zhang, Q. Hu, G. Feng, R. Zhang, Y. Yuan, X. Lu and B. Liu, Chem. Sci., 2015, 6, 4580–4586 RSC.
- M. Z. Ye, X. H. Wang, J. B. Tang, Z. Q. Guo, Y. Q. Shen, H. Tian and W. H. Zhu, Chem. Sci., 2016, 7, 4958–4965 RSC.
- M. Gao, F. Yu, C. Lv, J. Choo and L. Chen, Chem. Soc. Rev., 2017, 46, 2237–2271 RSC.
- J.-Z. Du, X.-J. Du, C.-Q. Mao and J. Wang, J. Am. Chem. Soc., 2011, 133, 17560–17563 CrossRef CAS PubMed.
- Y. Y. Yuan, C. J. Zhang, S. D. Xu and B. Liu, Chem. Sci., 2016, 7, 1862–1866 RSC.
- E.-J. Kim, S. Bhuniya, H. Lee, H. M. Kim, C. Cheong, S. Maiti, K. S. Hong and J. S. Kim, J. Am. Chem. Soc., 2014, 136, 13888–13894 CrossRef CAS PubMed.
- R. Kumar, J. Han, H.-J. Lim, W. X. Ren, J.-Y. Lim, J.-H. Kim and J. S. Kim, J. Am. Chem. Soc., 2014, 136, 17836–17843 CrossRef CAS PubMed.
- Y. Ichikawa, M. Kamiya, F. Obata, M. Miura, T. Terai, T. Komatsu, T. Ueno, K. Hanaoka, T. Nagano and Y. Urano, Angew. Chem., Int. Ed., 2014, 53, 6772–6775 CrossRef CAS PubMed.
- Y. Y. Yuan, C. J. Zhang, M. Gao, R. Y. Zhang, B. Z. Tang and B. Liu, Angew. Chem., Int. Ed., 2015, 54, 1780–1786 CrossRef CAS PubMed.
- G. Zheng, J. Chen, K. Stefflova, M. Jarvi, H. Li and B. C. Wilson, Proc. Natl. Acad. Sci. U. S. A., 2007, 104, 8989–8994 CrossRef CAS PubMed.
- F. P. Kong, Z. Y. Liang, D. R. Luan, X. J. Liu, K. H. Xu and B. Tang, Anal. Chem., 2016, 88, 6450–6456 CrossRef CAS PubMed.
- X. Wu, X. Sun, Z. Guo, J. Tang, Y. Shen, T. D. James, H. Tian and W. Zhu, J. Am. Chem. Soc., 2014, 136, 3579–3588 CrossRef CAS PubMed.
- S. Bhuniya, S. Maiti, E. J. Kim, H. Lee, J. L. Sessler, K. S. Hong and J. S. Kim, Angew. Chem., Int. Ed., 2014, 53, 4469–4474 CrossRef CAS PubMed.
- S. Santra, C. Kaittanis, O. J. Santiesteban and J. M. Perez, J. Am. Chem. Soc., 2011, 133, 16680–16688 CrossRef CAS PubMed.
- L. Yuan, W. Lin, S. Zhao, W. Gao, B. Chen, L. He and S. Zhu, J. Am. Chem. Soc., 2012, 134, 13510–13523 CrossRef CAS PubMed.
- E. W. Miller and C. J. Chang, Curr. Opin. Chem. Biol., 2007, 11, 620–625 CrossRef CAS PubMed.
- Y. Yuan, C.-J. Zhang, S. Xu and B. Liu, Chem. Sci., 2016, 7, 1862–1866 RSC.
- H.-W. Liu, S. Xu, P. Wang, X.-X. Hu, J. Zhang, L. Yuan, X.-B. Zhang and W. Tan, Chem. Commun., 2016, 52, 12330–12333 RSC.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7sc03454g |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2017 |

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
: