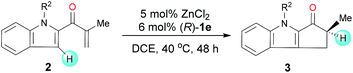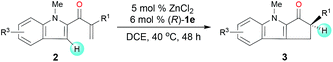Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Enantioselective Nazarov cyclization of indole enones cooperatively catalyzed by Lewis acids and chiral Brønsted acids†
Guo-Peng
Wang
a,
Meng-Qing
Chen
a,
Shou-Fei
Zhu
 *a and
Qi-Lin
Zhou
*a and
Qi-Lin
Zhou
 *ab
*ab
aState Key Laboratory and Institute of Elemento-Organic Chemistry, College of Chemistry Nankai University, Tianjin 300071, China. E-mail: sfzhu@nankai.edu.cn; qlzhou@nankai.edu.cn
bCollaborative Innovation Center of Chemical Science and Engineering (Tianjin), Nankai University, Tianjin 300071, China
First published on 29th August 2017
Abstract
Enantioselective control of the chirality of a tertiary α-carbon in the products of a Nazarov cyclization of enones is challenging because the reaction involves an enantioselective proton transfer process. We herein report the use of cooperative catalysis using Lewis acids and chiral Brønsted acids to control the stereochemistry of the tertiary α-carbon in the products of this reaction. Specifically, with ZnCl2 and a chiral spiro phosphoric acid as catalysts, we realized the first enantioselective construction of cyclopenta[b]indoles with chiral tertiary α-carbons via Nazarov cyclization of indole enone substrates with only one coordinating site. Mechanistic studies revealed that the chiral spiro phosphoric acid acts as a multifunctional catalyst: it co-catalyzes the cyclization of the dienone and enantioselectively catalyzes a proton transfer reaction of the enol intermediate. This new strategy of enantioselective control by means of cooperative catalysis may show utility for other challenging asymmetric cyclization reactions.
Introduction
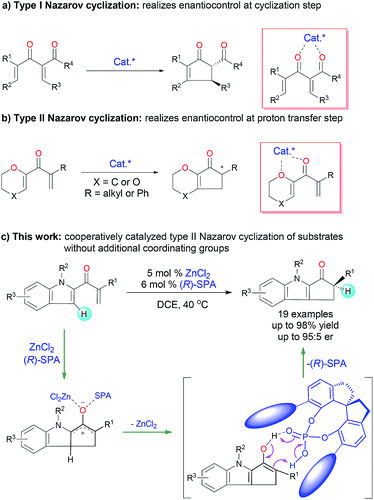
The Nazarov cyclization, a 4π-electrocyclization of cross-conjugated dienones, is one of the most effective methods for constructing functionalized cyclopentenones and has been extensively investigated and widely utilized for the synthesis of cyclopentenoid natural products.1 Catalytic enantioselective Nazarov cyclization is a straightforward method for introducing chiral-center-containing cyclopentenones, which are versatile chiral synthons,2 and this strategy has attracted increasing attention in recent decades.3 The reported asymmetric Nazarov cyclization can be roughly classified into two types on the basis of the enantioselective control step: type I Nazarov cyclization affords products with a chiral center at the β-carbon by means of an enantioselective cyclization step (Fig. 1a), and type II Nazarov cyclization affords products with only one chiral center at the tertiary α-carbon by means of an enantioselective proton transfer step (Fig. 1b). Various chiral Lewis acid4 and Brønsted acid5 catalysts have been reported to afford high enantioselectivity in type I Nazarov cyclization reactions. In contrast, for type II Nazarov cyclization, the stereochemistry-determining step is a proton transfer reaction of an active enol or enolate intermediate generated during electrocyclization, and control of the enantioselectivity is much more difficult.6 To date, only one type II Nazarov cyclization was reported with reasonable enantioselectivity.7 In 2004, Liang and Trauner7a achieved good to excellent enantioselectivity (up to 98.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 enantiomeric ratio [er]) in the asymmetric Nazarov cyclization of α-substituted divinyl ketones by using a Sc-Pybox catalyst (Fig. 1b). These investigators suggested that the catalyst chelates with the substrate in addition to mediating the enantioselective proton transfer reaction of the cyclized intermediate. In 2009, Rueping and Ieawsuwan7b reported the use of a chiral Brønsted acid, typically a chiral N-triflylphosphoramide, to catalyze Nazarov cyclizations of the same substrates as those used by Liang and Trauner with moderate to good enantioselectivities. However, both of these seminal reactions require a dienone substrate with an additional coordinating group (an ether group) for high enantioselectivity. To our knowledge, enantioselective type II Nazarov cyclization of substrates without an additional coordinating group has not been realized, and strategies to address this challenge are highly desirable. In our recent studies of transition-metal-catalyzed enantioselective carbene insertion into heteroatom–hydrogen bonds,8 we developed a method for cooperative catalysis using achiral dirhodium complexes and chiral spiro phosphoric acids (SPAs) to accomplish asymmetric heteroatom–hydrogen bond insertion reactions with excellent enantioselectivity.9 Mechanistic studies revealed that the SPAs catalyze a proton transfer reaction of an ylide, enol, or enolate intermediate by acting as chiral proton transfer shuttles.9b,10 Inspired by these results, we investigated the type II Nazarov cyclization of substrates with only one coordinating group by means of cooperative catalysis with a Lewis acid and a chiral Brønsted acid to control the enantioselectivity of the proton transfer step (Fig. 1c).11 With the combination of ZnCl2 and a SPA, highly enantioselective Nazarov cyclization of indole enones was accomplished.
1.5 enantiomeric ratio [er]) in the asymmetric Nazarov cyclization of α-substituted divinyl ketones by using a Sc-Pybox catalyst (Fig. 1b). These investigators suggested that the catalyst chelates with the substrate in addition to mediating the enantioselective proton transfer reaction of the cyclized intermediate. In 2009, Rueping and Ieawsuwan7b reported the use of a chiral Brønsted acid, typically a chiral N-triflylphosphoramide, to catalyze Nazarov cyclizations of the same substrates as those used by Liang and Trauner with moderate to good enantioselectivities. However, both of these seminal reactions require a dienone substrate with an additional coordinating group (an ether group) for high enantioselectivity. To our knowledge, enantioselective type II Nazarov cyclization of substrates without an additional coordinating group has not been realized, and strategies to address this challenge are highly desirable. In our recent studies of transition-metal-catalyzed enantioselective carbene insertion into heteroatom–hydrogen bonds,8 we developed a method for cooperative catalysis using achiral dirhodium complexes and chiral spiro phosphoric acids (SPAs) to accomplish asymmetric heteroatom–hydrogen bond insertion reactions with excellent enantioselectivity.9 Mechanistic studies revealed that the SPAs catalyze a proton transfer reaction of an ylide, enol, or enolate intermediate by acting as chiral proton transfer shuttles.9b,10 Inspired by these results, we investigated the type II Nazarov cyclization of substrates with only one coordinating group by means of cooperative catalysis with a Lewis acid and a chiral Brønsted acid to control the enantioselectivity of the proton transfer step (Fig. 1c).11 With the combination of ZnCl2 and a SPA, highly enantioselective Nazarov cyclization of indole enones was accomplished.
Results and discussion
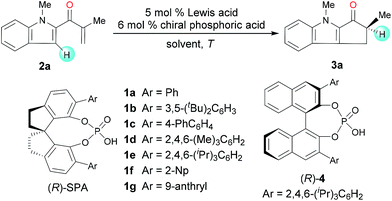
To identify suitable catalysts, we carried out the Nazarov cyclization of 2-methyl-1-(1-methyl-1H-indol-2-yl)prop-2-en-1-one (2a, Table 1). We began by evaluating Sc-Pybox and a chiral N-triflylphosphoramide, which has been previously used in the literature7 to catalyze the type II Nazarov cyclization of substrates with two coordinating groups. However, neither of these catalysts triggered the cyclization reaction of 2a. To our delight though, the combination of FeCl3 (5 mol%) and chiral SPA (R)-1a (6 mol%) promoted the reaction at 25 °C in 1,2-dichloroethane (DCE), affording the desired cyclization product (3a) in 95% yield with 63![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 37 er (entry 1). Various other chiral SPAs were then evaluated (entries 2–7), and (R)-1e, which has 6,6′-di-(2,4,6-tri-isopropyl)phenyl substituents, gave the highest enantioselectivity (85
37 er (entry 1). Various other chiral SPAs were then evaluated (entries 2–7), and (R)-1e, which has 6,6′-di-(2,4,6-tri-isopropyl)phenyl substituents, gave the highest enantioselectivity (85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15 er, entry 5). Chiral phosphoric acid (R)-4, which has a binaphthyl scaffold, showed almost no enantioselectivity (entry 8). Different Lewis acids were then studied with (R)-1e as a co-catalyst. ZnCl2, Zn(OTf)2, AgClO4, Sc(OTf)3, and In(OTf)3 afforded good enantioselectivities (up to 92
15 er, entry 5). Chiral phosphoric acid (R)-4, which has a binaphthyl scaffold, showed almost no enantioselectivity (entry 8). Different Lewis acids were then studied with (R)-1e as a co-catalyst. ZnCl2, Zn(OTf)2, AgClO4, Sc(OTf)3, and In(OTf)3 afforded good enantioselectivities (up to 92![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 er, entries 9–13), although Zn(OTf)2 and AgClO4 showed relatively low yields. Using ZnCl2 and (R)-1e as co-catalysts, we then optimized the reaction conditions. When the reaction was performed at 40 °C, the yield increased to 98%, and the high enantioselectivity was retained (entry 14). Increasing the temperature further (to 60 °C) increased the reaction rate (the reaction was complete after only 20 h) but slightly lowered the enantioselectivity (87
8 er, entries 9–13), although Zn(OTf)2 and AgClO4 showed relatively low yields. Using ZnCl2 and (R)-1e as co-catalysts, we then optimized the reaction conditions. When the reaction was performed at 40 °C, the yield increased to 98%, and the high enantioselectivity was retained (entry 14). Increasing the temperature further (to 60 °C) increased the reaction rate (the reaction was complete after only 20 h) but slightly lowered the enantioselectivity (87![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 13 er, entry 15). The kinetic studies indicate that the reaction achieves 90% conversion within 6 hours, but becomes sluggish thereafter. The use of CHCl3 did not markedly alter the yield or enantioselectivity, whereas the nonpolar solvent cyclohexane lowered both the yield and enantioselectivity (entries 16 and 17).
13 er, entry 15). The kinetic studies indicate that the reaction achieves 90% conversion within 6 hours, but becomes sluggish thereafter. The use of CHCl3 did not markedly alter the yield or enantioselectivity, whereas the nonpolar solvent cyclohexane lowered both the yield and enantioselectivity (entries 16 and 17).
| Entry | Lewis acid | Phosphoric acid | T (°C) | Time (h) | Yieldb (%) | erc |
|---|---|---|---|---|---|---|
a Reaction conditions: Lewis acid/phosphoric acid/2 = 0.01![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.012 0.012![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.2 (mmol) in 3 mL DCE, 40 °C, 48 h.
b Isolated yield.
c Determined by HPLC using a Chiralpak AD-3 or Chiralcel OD-3 column.
d 3 mL CHCl3 used as solvent.
e 3 mL cyclohexane used as solvent.
f 75% conversion of 2a. NA = not analysed. 0.2 (mmol) in 3 mL DCE, 40 °C, 48 h.
b Isolated yield.
c Determined by HPLC using a Chiralpak AD-3 or Chiralcel OD-3 column.
d 3 mL CHCl3 used as solvent.
e 3 mL cyclohexane used as solvent.
f 75% conversion of 2a. NA = not analysed.
|
||||||
| 1 | FeCl3 | (R)-1a | 25 | 36 | 95 | 63![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 37 37 |
| 2 | FeCl3 | (R)-1b | 25 | 30 | 83 | 46![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 54 54 |
| 3 | FeCl3 | (R)-1c | 25 | 35 | 95 | 44![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 56 56 |
| 4 | FeCl3 | (R)-1d | 25 | 48 | 95 | 63![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 37 37 |
| 5 | FeCl3 | (R)-1e | 25 | 48 | 98 | 85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15 15 |
| 6 | FeCl3 | (R)-1f | 25 | 48 | 93 | 52![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 48 48 |
| 7 | FeCl3 | (R)-1g | 25 | 36 | 95 | 57![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 43 43 |
| 8 | FeCl3 | (R)-4 | 25 | 36 | 98 | 48![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 52 52 |
| 9 | ZnCl2 | (R)-1e | 25 | 48 | 78 | 91![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 9 |
| 10 | Zn(OTf)2 | (R)-1e | 25 | 48 | 48 | 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10 |
| 11 | AgClO4 | (R)-1e | 25 | 48 | 50 | 92![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 8 |
| 12 | Sc(OTf)3 | (R)-1e | 25 | 48 | 73 | 91![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 9 |
| 13 | In(OTf)3 | (R)-1e | 25 | 24 | 93 | 86![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 14 14 |
| 14 | ZnCl2 | (R)-1e | 40 | 48 | 98 | 91![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 9 |
| 15 | ZnCl2 | (R)-1e | 60 | 20 | 100 | 87![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 13 13 |
| 16d | ZnCl2 | (R)-1e | 40 | 48 | 95 | 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10 |
| 17e | ZnCl2 | (R)-1e | 40 | 48 | 25 | 62![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 38 38 |
| 18f | ZnCl2 | None | 40 | 48 | 65 | NA |
| 19 | None | (R)-1e | 40–60 | 48 | 0 | NA |
| 20 | ZnCl2 | (R)-1e-Na | 40 | 48 | 0 | NA |
We performed a set of control experiments to elucidate the roles of ZnCl2 and the SPA. In the absence of SPA (R)-1e, ZnCl2 independently catalyzed the cyclization (entry 18), but the reaction rate was clearly slower than when both catalysts were used (entry 14). This result indicates that the SPA accelerated the ZnCl2-catalyzed cyclization reaction of 2a. However, no reaction occurred in the presence of the SPA alone at 40 °C or even at 60 °C (entry 19). Moreover, when we used the sodium salt of the SPA, (R)-1e-Na, the Nazarov cyclization did not occur (entry 20). The anionic SPA’s lack of catalytic activity may have been due to the formation of zinc(II) phosphate (R)-1e-Zn, which was inert under the reaction conditions.
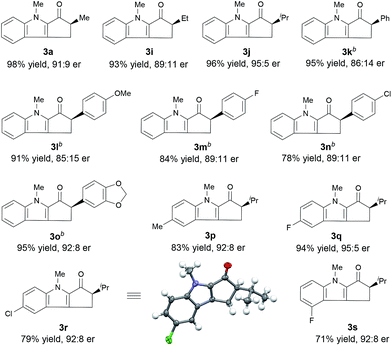
Under the optimal reaction conditions, we evaluated indole enones with various groups (R2) on the N atom of the indole ring (Table 2). Substrates with either an N-alkyl or N-aryl group gave good to excellent yields (76–98%), but substrates with an N-aryl group showed the best enantioselectivities (91![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9–92
9–92![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 er). The presence of an N-aryl group with a strongly electron-withdrawing CF3 group lowered the yield (entry 7).
8 er). The presence of an N-aryl group with a strongly electron-withdrawing CF3 group lowered the yield (entry 7).
| Entry | R2 | Product | Yieldb (%) | erc |
|---|---|---|---|---|
a Reaction conditions: ZnCl2/(R)-1e/2 = 0.01![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.012 0.012![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.2 (mmol) in 3 mL DCE, 40 °C, 48 h.
b Isolated yield.
c Determined by HPLC using a Chiralpak AD-3 or Chiralcel OD-3 column. 0.2 (mmol) in 3 mL DCE, 40 °C, 48 h.
b Isolated yield.
c Determined by HPLC using a Chiralpak AD-3 or Chiralcel OD-3 column.
|
||||
| 1 | Me (2a) | 3a | 98 | 91![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 9 |
| 2 | iPr (2b) | 3b | 98 | 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10 |
| 3 | Allyl (2c) | 3c | 93 | 89![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11 11 |
| 4 | Bn (2d) | 3d | 87 | 88![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12 12 |
| 5 | Ph (2e) | 3e | 90 | 92![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 8 |
| 6 | An (2f) | 3f | 95 | 92![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 8 |
| 7 | 4-CF3C6H4 (2g) | 3g | 76 | 91![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 9 |
| 8 | 2-Np (2h) | 3h | 92 | 92![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 8 |
Next, we determined the effect of the R1 group on the α-carbon of enone substrates 2 (Table 3). When R1 was alkyl, excellent yields and good enantioselectivities were obtained, with a bulky isopropyl group giving the highest enantioselectivity (3j, 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 er). When R1 was aryl, however, Zn(OTf)2 had to be used instead of ZnCl2 to obtain satisfactory enantioselectivities (3k–3o, 85
5 er). When R1 was aryl, however, Zn(OTf)2 had to be used instead of ZnCl2 to obtain satisfactory enantioselectivities (3k–3o, 85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15–92
15–92![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 er). Substrates with a substituent at the 4- or 5-position of the indole ring (3p–3s) underwent the reaction smoothly and gave good yields (71–94%) and high enantioselectivities (92
8 er). Substrates with a substituent at the 4- or 5-position of the indole ring (3p–3s) underwent the reaction smoothly and gave good yields (71–94%) and high enantioselectivities (92![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8–95
8–95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 er). The structure and absolute configuration of (R)-3r were determined using X-ray diffraction of a single crystal.12
5 er). The structure and absolute configuration of (R)-3r were determined using X-ray diffraction of a single crystal.12
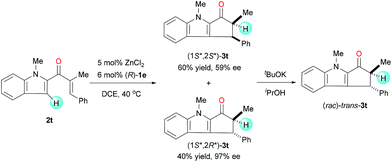
Under the standard reaction conditions, the substrate 2t, having a β-phenyl substitution, smoothly cyclized to give a mixture of diastereoisomers (1S*,2S*)-3t and (1S*,2R*)-3t with 1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr (Scheme 1). The enantioselectivity of (1S*,2R*)-3t is very high (97% ee), though the enantioselectivity of (1S*,2S*)-3t is moderate (59% ee). When the mixture of cyclization products was treated with tBuOK in iPrOH, it transformed to a racemic single diastereoisomer through the epimerization of α-carbons. This experiment clearly implies that the enantio-control on the β-carbon is not achieved during the cyclization step and the enantioselectivity of the reaction is achieved during the protonation step.
1 dr (Scheme 1). The enantioselectivity of (1S*,2R*)-3t is very high (97% ee), though the enantioselectivity of (1S*,2S*)-3t is moderate (59% ee). When the mixture of cyclization products was treated with tBuOK in iPrOH, it transformed to a racemic single diastereoisomer through the epimerization of α-carbons. This experiment clearly implies that the enantio-control on the β-carbon is not achieved during the cyclization step and the enantioselectivity of the reaction is achieved during the protonation step.
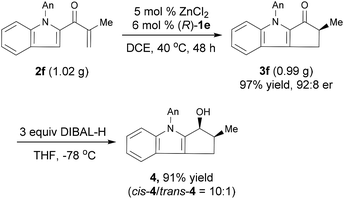
The Nazarov cyclization of 2 could readily be carried out on a gram scale. For example, the reaction of 1.02 g 2f under the standard conditions smoothly produced chiral cyclopentanone[b]indole 3f in 97% yield with 92![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 er (Scheme 2). Reduction of 3f with diisobutylaluminum hydride (DIBAL-H) afforded the corresponding alcohol cis-4 in 91% yield with a high diastereoselectivity (cis/trans = 10
8 er (Scheme 2). Reduction of 3f with diisobutylaluminum hydride (DIBAL-H) afforded the corresponding alcohol cis-4 in 91% yield with a high diastereoselectivity (cis/trans = 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and retained er. The relative configuration of cis-4 was confirmed by a nuclear Overhauser enhancement spectroscopy experiment (see ESI for details†). Note that cyclopenta[b]indoles are common building blocks for indole alkaloids, such as (+)-dasyrachine,13 penitrems A–F,14 15α-hydroxy-14,15-dihydrovindolinine,15 (+)-paxilline,16 and lolitrem B.17 Indeed, a similar non-enantioselective Nazarov cyclization was used in the total synthesis of yuehchukene analogues.18
1) and retained er. The relative configuration of cis-4 was confirmed by a nuclear Overhauser enhancement spectroscopy experiment (see ESI for details†). Note that cyclopenta[b]indoles are common building blocks for indole alkaloids, such as (+)-dasyrachine,13 penitrems A–F,14 15α-hydroxy-14,15-dihydrovindolinine,15 (+)-paxilline,16 and lolitrem B.17 Indeed, a similar non-enantioselective Nazarov cyclization was used in the total synthesis of yuehchukene analogues.18
To obtain more insight into the mechanism, we carried out density functional theory calculations at the B3LYP/def2TZVP-SMD(DCE)//B3LYP/def2SVP level of theory using Gaussian 09 (see ESI for details†). The cyclization of 2a by cooperative catalysis using ZnCl2 and (R)-1e was used as the model reaction (Fig. 2). The calculations indicate that the cyclization starts with the coordination of ZnCl2 to the carbonyl group of the substrate (INT 1). The SPA facilitates 4π-electrocyclization viaTS I to afford intermediate INT II. In the absence of (R)-1e, that is, when ZnCl2 is the sole catalyst, the reaction proceeds via an alternative transition state (TS I′) with a higher (4.5 kcal mol−1) energy barrier. Like the control experiments, the calculation results support our contention that ZnCl2 and (R)-1e cooperatively catalyze the cyclization. The SPA then promotes a proton transfer reaction of INT IIviaTS II to generate the enol intermediate. The formation of TS II has an activation barrier of 12.6 kcal mol−1 in solution, which is not a problem when the reaction is carried out at 25 °C. The calculations also suggest that the Lewis acid ZnCl2 prefers to dissociate from INT II during the proton transfer reaction, because the energy barrier for the formation of TS II′, which contains ZnCl2, is 6.8 kcal mol−1 higher than that for TS II. The enol intermediate can form a complex (CP III-R or CP III-S) with (R)-1e and then undergo [1,3]-proton transfer to give the cyclization product 3a.19 Our calculations indicate that (R)-1e catalyzes the [1,3]-proton transfer via an eight-membered transition state (TS III-R or TS III-S).9bTS III-S is lower in energy than TS III-R by 1.9 kcal mol−1. This result suggests that a product mixture enriched with enantiomer (S)-3a (calcd er = 96![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) should be generated from TS III-S, which is consistent with the experimental result. In the energetically disfavored transition state (TS III-R), the Me group at the α-position of the substrate experiences more steric repulsion from the 2,4,6-triisopropyphenyl group of (R)-1e than the same Me group in the favored transition state (TS III-S). The steric repulsion may be induced by rotation of the indole plane to some extent in the chiral cavity. Other proton transfer processes of the enol intermediate with and without promoters were also calculated, but the energy barriers of all of these other processes were higher than the barrier for when the SPA was used as a catalyst (see Fig. S4†). Our calculations reveal that the SPA first acts as a Brønsted acid catalyst to promote the cyclization and later acts as a chiral proton transfer shuttle to promote an enantioselective proton transfer reaction of the enol intermediate, thus exerting enantioselectivity of the Nazarov cyclization.
4) should be generated from TS III-S, which is consistent with the experimental result. In the energetically disfavored transition state (TS III-R), the Me group at the α-position of the substrate experiences more steric repulsion from the 2,4,6-triisopropyphenyl group of (R)-1e than the same Me group in the favored transition state (TS III-S). The steric repulsion may be induced by rotation of the indole plane to some extent in the chiral cavity. Other proton transfer processes of the enol intermediate with and without promoters were also calculated, but the energy barriers of all of these other processes were higher than the barrier for when the SPA was used as a catalyst (see Fig. S4†). Our calculations reveal that the SPA first acts as a Brønsted acid catalyst to promote the cyclization and later acts as a chiral proton transfer shuttle to promote an enantioselective proton transfer reaction of the enol intermediate, thus exerting enantioselectivity of the Nazarov cyclization.
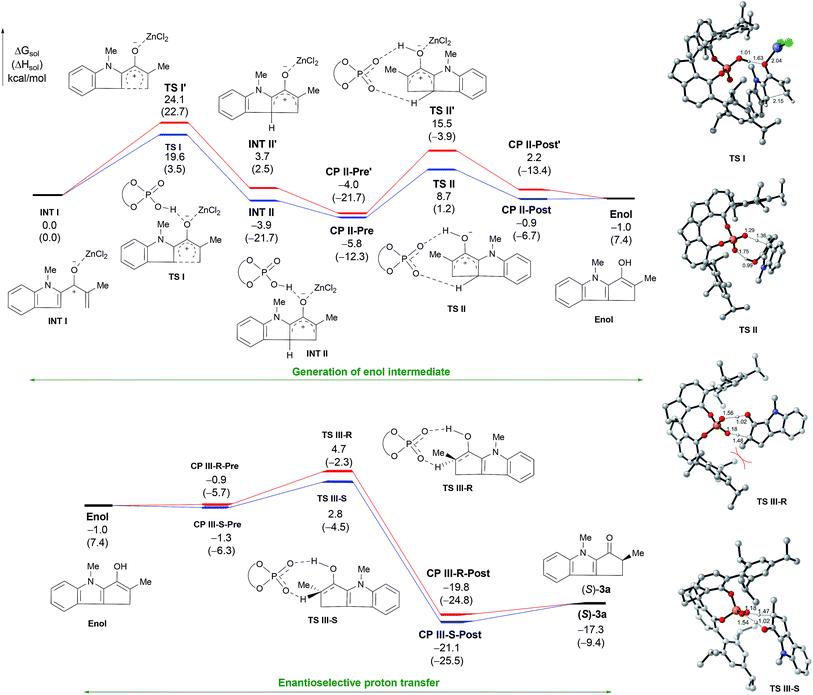 | ||
| Fig. 2 The computed energy surfaces for the Nazarov cyclization of 2a cooperatively catalyzed by ZnCl2 and (R)-1e. | ||
Conclusions
In conclusion, we have accomplished enantioselective Nazarov cyclizations of indole enones with only one coordinating site in high yield and good enantioselectivity by using ZnCl2 as a Lewis acid catalyst and a chiral SPA as a co-catalyst. Proton transfer is the stereochemistry-determining step of the process. The Lewis acid and the SPA promote the cyclization cooperatively. The SPA also promotes a proton transfer reaction of the enol intermediate and exerts enantioselective control over the tertiary α-carbon chiral center generated by the Nazarov cyclization. This new strategy of using cooperative catalysis for enantioselective control has great potential for application to other challenging asymmetric cyclization reactions.Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
We thank the National Natural Science Foundation of China (21625204, 21532003, 21421062, 21290182), the National Basic Research Program of China (2012CB821600), the “111” project (B06005) of the Ministry of Education of China, and the National Program for Support of Top-notch Young Professionals for financial support. We thank Dr Xiao-Song Xue for his constructive discussions on density functional theory calculations.Notes and references
- For a review, see: (a) T. Vaidya, R. Eisenberg and A. J. Frontier, ChemCatChem, 2011, 3, 1531 CrossRef CAS, for recent examples of synthetic applications, see: (b) Y.-B. Shi, B.-C. Yang, S.-J. Cai and S.-H. Gao, Angew. Chem., Int. Ed., 2014, 53, 9539 CrossRef CAS PubMed; (c) A. Shvartsbart and A. B. Smith III, J. Am. Chem. Soc., 2015, 137, 3510 CrossRef CAS PubMed; (d) Z. Zhou and M. A. Tius, Angew. Chem., Int. Ed., 2015, 54, 6037 CrossRef CAS PubMed.
- S. P. Simeonov, J. P. M. Nunes, K. Guerra, V. B. Kurteva and C. A. M. Afonso, Chem. Rev., 2016, 116, 5744 CrossRef CAS PubMed.
- For a review, see: N. Shimada, C. Stewart and M. A. Tius, Tetrahedron, 2011, 67, 5851 CrossRef CAS PubMed.
- For selected examples, see: (a) V. K. Aggarwal and A. J. Belfield, Org. Lett., 2003, 5, 5075 CrossRef CAS PubMed; (b) J. Nie, H.-W. Zhu, H.-F. Cui, M.-Q. Hua and J.-A. Ma, Org. Lett., 2007, 9, 3053 CrossRef CAS PubMed; (c) I. Walz and A. Togni, Chem. Commun., 2008, 4315 RSC; (d) P. Cao, C. Deng, Y.-Y. Zhou, X.-L. Sun, J.-C. Zheng, Z.-W. Xie and Y. Tang, Angew. Chem., Int. Ed., 2010, 49, 4463 CrossRef CAS PubMed; (e) G. E. Hutson, Y. E. Türkmen and V. H. Rawal, J. Am. Chem. Soc., 2013, 135, 4988 CrossRef CAS PubMed; (f) S. Raja, M. Nakajima and M. Rueping, Angew. Chem., Int. Ed., 2015, 54, 2762 CrossRef CAS PubMed; (g) T. Takeda, H. Harada and A. Nishida, Org. Lett., 2015, 17, 5184 CrossRef CAS PubMed.
- For selected examples, see: (a) M. Rueping, W. Ieawsuwan, A. P. Antonchick and B. J. Nachtsheim, Angew. Chem., Int. Ed., 2007, 46, 2097 CrossRef CAS PubMed; (b) A. K. Basak, N. Shimada, W. F. Bow, D. A. Vicic and M. A. Tius, J. Am. Chem. Soc., 2010, 132, 8266 CrossRef CAS PubMed; (c) A. Jolit, P. M. Walleser, G. P. A. Yap and M. A. Tius, Angew. Chem., Int. Ed., 2014, 53, 6180 CrossRef CAS PubMed; (d) B.-M. Yang, P.-J. Cai, Y.-Q. Tu, Z.-Y. Yu, Z.-M. Chen, S.-H. Wang, S.-H. Wang and F.-M. Zhang, J. Am. Chem. Soc., 2015, 137, 8344 CrossRef CAS PubMed.
- J. T. Mohr, A. Y. Hong and B. M. Stoltz, Nat. Chem., 2009, 1, 359 CrossRef CAS PubMed.
- (a) G.-X. Liang and D. Trauner, J. Am. Chem. Soc., 2004, 126, 9544 CrossRef CAS PubMed; (b) M. Rueping and W. Ieawsuwan, Adv. Synth. Catal., 2009, 351, 78 CrossRef CAS.
- S.-F. Zhu and Q.-L. Zhou, Acc. Chem. Res., 2012, 45, 1365 CrossRef CAS PubMed.
- (a) B. Xu, S.-F. Zhu, X.-L. Xie, J.-J. Shen and Q.-L. Zhou, Angew. Chem., Int. Ed., 2011, 50, 11483 CrossRef CAS PubMed; (b) B. Xu, S.-F. Zhu, Z.-C. Zhang, Z.-X. Yu, Y. Ma and Q.-L. Zhou, Chem. Sci., 2014, 5, 1442 RSC.
- (a) X.-C. Wang, X.-S. Song, L.-P. Guo, D. Qu, Z.-Z. Xie, F. Verpoort and J. Cao, Organometallics, 2014, 33, 4042 CrossRef CAS; (b) H. K. Kisan and R. B. Sunoj, Chem. Commun., 2014, 50, 14639 RSC; (c) H. K. Kisan and R. B. Sunoj, J. Org. Chem., 2015, 80, 2192 CrossRef CAS PubMed; (d) H. Liu, J.-X. Duan, D.-Y. Qu, L.-P. Guo and Z.-Z. Xie, Organometallics, 2016, 35, 2003 CrossRef CAS.
- For an earlier example of the use of combined Lewis acids and Brønsted acids to improve the activity of non-enantioselective Nazarov cyclization reactions, see: (a) Z.-G. Xi, L.-H. Zhu, S.-Z. Luo and J.-P. Cheng, J. Org. Chem., 2013, 78, 606 CrossRef CAS PubMed, for selected reviews on cooperative catalysis, see: (b) J. Zhou, Chem.–Asian J., 2010, 5, 422 CrossRef CAS PubMed; (c) C. Zhong and X.-D. Shi, Eur. J. Org. Chem., 2010, 2999 CrossRef CAS; (d) A. E. Allen and D. W. C. MacMillan, Chem. Sci., 2012, 3, 633 RSC; (e) X. Wu, M.-L. Li and L.-Z. Gong, Acta Chim. Sin., 2013, 71, 1091 CrossRef CAS; (f) D.-F. Chen, Z.-Y. Han, X.-L. Zhou and L.-Z. Gong, Acc. Chem. Res., 2014, 47, 2365 CrossRef CAS PubMed.
- CCDC 1539722 contains the supplementary crystallographic data for compound (R)-3r. See ESI for details.†.
- T.-S. Kam, G. Subramaniam and W. Chen, Phytochemistry, 1999, 51, 159 CrossRef CAS.
- S.-S. Gao, X.-M. Li, K. Williams, P. Proksch, N.-Y. Ji and B.-G. Wang, J. Nat. Prod., 2016, 79, 2066 CrossRef CAS PubMed.
- Y.-L. He, W.-M. Chen and X.-Z. Feng, Phytochemistry, 1994, 37, 1055 CrossRef CAS.
- A. B. Smith III, K.-W. Jill, T. L. Leenay, E. G. Nolen and T. Sunazuka, J. Am. Chem. Soc., 1992, 114, 1438 CrossRef.
- G. Philippe, Toxins, 2016, 8, 47 CrossRef PubMed.
- (a) K.-F. Cheng, M.-K. Cheung and Y.-C. Kong, Aust. J. Chem., 1997, 50, 349 CrossRef CAS; (b) K.-F. Cheng, G.-A. Cao, Y.-W. Yu and Y.-C. Kong, Synth. Commun., 1994, 24, 65 CrossRef CAS.
- For other selected DFT calculations of proton transfer of active intermediates, see: (a) Y.-Z. Xia, Y. Liang, Y.-Y. Chen, M. Wang, L. Jiao, F. Huang, S. Liu, Y.-H. Li and Z.-X. Yu, J. Am. Chem. Soc., 2007, 129, 3470 CrossRef CAS PubMed; (b) Y. Liang, H.-L. Zhou and Z.-X. Yu, J. Am. Chem. Soc., 2009, 131, 17783 CrossRef CAS PubMed; (c) N.-K. Fu, L. Zhang, J.-Y. Li, S.-Z. Luo and J.-P. Cheng, Angew. Chem., Int. Ed., 2011, 50, 11451 CrossRef CAS PubMed; (d) X.-S. Xue, X. Li, A. Yu, C. Yang, C. Song and J.-P. Cheng, J. Am. Chem. Soc., 2013, 135, 7462 CrossRef CAS PubMed; (e) J. Monot, P. Brunel, C. E. Kefalidis, N. A. Espinosa-Jalapa, L. Maron, B. Martin-Vaca and D. Bourissou, Chem. Sci., 2016, 7, 2179 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: For CIF data of (R)-3r, experimental procedures, spectral data, and computational study results. CCDC 1539722. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c7sc03183a |
| This journal is © The Royal Society of Chemistry 2017 |