Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Enantioselective N-heterocyclic carbene-catalyzed nucleophilic dearomatization of alkyl pyridiniums†
Darrin M.
Flanigan
and
Tomislav
Rovis‡
 *
*
Department of Chemistry, Colorado State University, Fort Collins, CO 80523, USA
First published on 3rd August 2017
Abstract
NHC-catalyzed nucleophilic dearomatization of alkyl pyridiniums has been achieved to generate 1,4-dihydropyridines with high enantioselectivity. This is a rare example of catalytic, asymmetric addition of a nucleophile to the activated pyridinium that prefers C-4 functionalization leading to the 1,4-dihydropyridine with high selectivity.
Introduction
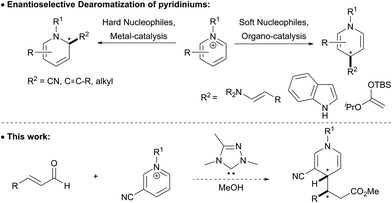
1,4-Dihydropyridines are an important class of pharmaceuticals that are used to treat an assortment of illnesses and have demonstrated antimicrobial, anticancer, antihypertensive, and anticonvulsant activity.1 Aside from being a privileged structure in drug discovery, 1,4-dihydropyridines are also useful intermediates en route to other substituted 6-membered heterocycles, such as pyridines or piperidines.2 Despite the importance of the 1,4-dihydropyridine scaffold, direct and mild methods for their synthesis remains a significant challenge.Historically, the synthesis of 1,4-dihydropyridines has relied on condensation reactions, e.g. the Hantzsch reaction.3 The nucleophilic dearomatization of activated pyridines has emerged as a powerful tool to synthesize a variety of 6-membered nitrogen containing heterocycles.4 However, many of these methods require chiral auxiliaries on the pyridine derivative to direct the approach of the incoming nucleophile, which limits the broad application of this methodology.5 More recently, much effort has been focused on catalytic enantioselective methods that can directly access dihydropyridines stereoselectively.6 Transition metal-catalyzed reactions have been applied to the asymmetric synthesis of dihydropyridines and typically generate 1,2-dihydropyridines (Scheme 1). Anion-binding catalysis has been demonstrated to be an effective catalytic asymmetric strategy for nucleophilic dearomatization of N-Troc pyridiniums with silyl ketene acetals to give primarily 1,2-dihydropyridines.7 This method was extended to coupling indoles with pyridiniums, which gives the 1,4-dihydropyridine with high regioselectivity.8 Secondary amine catalysis has also been employed to synthesize 1,4-dihydropyridines from N-alkyl pyridiniums bearing a nitro group at the 3-position.9 To achieve high diastereo- and enantioselectivity in these reactions, a two-step protocol was developed where the product aldehyde was immediately trapped in situ with Wittig reagents to give the γ-substituted α,β-unsaturated ester in good yield, with high enantio- and diastereoselectivity.
N-Hetereocyclic carbenes (NHCs) are versatile organocatalysts that have been used in a variety of catalytic asymmetric reactions.10 Our group11 and others12 have successfully employed NHC-catalyzed functionalization of enals resulting in carbon–carbon bond formation at the beta position of the aldehyde to give β-functionalized esters. We wondered if the homoenolate intermediate was nucleophilic enough to intercept pyridinium derivatives, and if this nucleophile would selectively add to the 4-position of the pyridinium.
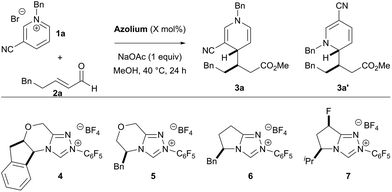
We began optimizing the reaction by screening several chiral catalysts (Table 1). The triazolium scaffolds tested in the reaction demonstrate high tunability of the enantioselectivity, depending on the catalyst structure. However, the regio- and diastereoselectivity was less sensitive to the choice of catalyst. Initially, the product was generated in low yields (entries 1–4), but yields were improved when oxygen was rigorously excluded from the reaction (entries 5 & 6). At this point, we wondered if the reaction could be carried out with lower catalyst loadings. Attempting the reaction with 10 mol% catalyst loading gives the product in 27% yield. To increase the yield of the process, we screened a variety of additives in the reaction and acetic acid (20 mol%) was found to be beneficial to the reactivity, giving the product in 61% yield, without affecting the selectivity of the process.
| Entry | Catalyst | X | Yieldb [%] |
3a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3a′c 3a′c |
drd | eee [%] |
|---|---|---|---|---|---|---|
| a Reaction conducted with 1.5 equiv. of 1a and 1.0 equiv. of 2a. b Combined yield of 3a and 3a′; determined by 1H NMR using 1,3,5-trimethoxybenzene as an internal standard. c Regioisomeric ratio determined by 1H NMR of the unpurified reaction mixture. d Diastereomeric ratio of 3a; determined by 1H NMR of the unpurified reaction mixture. e Enantiomeric excess determined by HPLC using a chiral stationary phase. f Reaction set up in glovebox. g Reaction conducted in presence of 20 mol% acetic acid. | ||||||
| 1 | 4 | 20 | 29 | 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
53 |
| 2 | 5 | 20 | 30 | 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
87 |
| 3 | 6 | 20 | 14 | 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
88 |
| 4 | 7 | 20 | 40 | 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
87 |
| 5f | 7 | 20 | 87 | 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
87 |
| 6f | 7 | 10 | 27 | 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
87 |
| 7 , | 7 | 10 | 61 |
6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
87 |
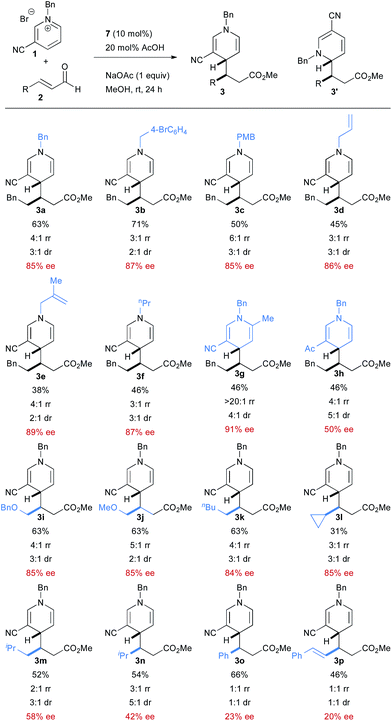
With optimized conditions in hand, we then explored the scope of the reaction. A broad scope of N-alkyl groups are tolerated on the pyridinium (Scheme 2). N-acyl pyridiniums, on the other hand, are not competent substrates. An electron withdrawing group at the 3 position of the pyridinium is required to achieve good reactivity with the cyano group providing the product with the highest ee. Acetyl pyridinium 1h is tolerated, but leads to much lower enantioselectivity, although the diastereoselectivity and regioselectivity are improved compared to the cyano group. 2-Methyl-5-cyano pyridinium is tolerated in the reaction, providing very high regioselectivity. A variety of aliphatic enals are tolerated in the reaction although enantioselectivity suffers with increasing size of the alkyl group. Straight-chain aliphatic enals proceed with the highest enantioselectivity. Interestingly, a cyclopropyl enal is also well tolerated.
Cinnamaldehyde is a competent coupling partner and proceeds with good yield, but with much lower levels of selectivity. The same is true with conjugated enals, which provide the product with good yield, but with no regio- or diastereoselectivity (and nearly racemic product). The reasons for this profound decrease in selectivity are not clear.
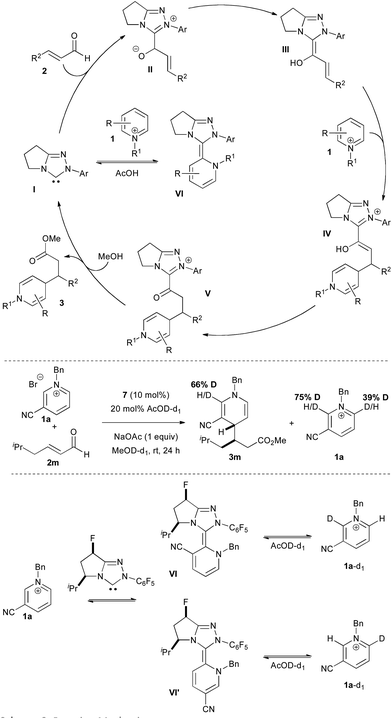
The mechanism of the reaction is illustrated in Scheme 3. The carbene catalyst I adds to the enal to ultimately generate the Breslow intermediate III. Addition to pyridinium 1 forms the enol azolium IV which tautomerizes to keto azolium V. Methanolysis leads to catalyst turnover and product formation.
The extreme sensitivity of this reaction to the presence of oxygen and the need for acetic acid additive for improved yields puzzled us. Most NHC-catalyzed reactions are air and moisture tolerant. Indeed, we have shown that aqueous conditions may be used with α-fluoroenals to deliver the corresponding acids directly.13 We speculated that the NHC catalyst may be adding to the pyridinium salt directly in a consumptive off-cycle path (I to VI, Scheme 3). The presence of acetic acid provides the buffer and acid needed to regenerate the carbene catalyst. We have previously noted such behaviour in the addition of carbene to iminium electrophiles.14,15
Support for this mechanism was gained from an isotope labelling experiment. Subjection of the reaction to AcOD in MeOD resulted in deuterium uptake in the dihydropyridine product as well as deuteration in the unreacted pyridinium starting material (Scheme 3). The latter indicates that both the 2- and 6-positions of the pyridinium are deuterated, likely resulting from carbene addition to form the aza-Breslow intermediates VI and VI'.
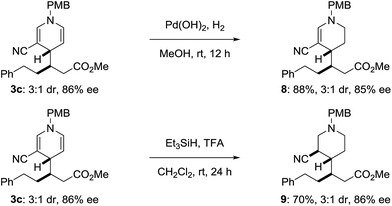
The product dihydropyridines are useful target structures but may also be used toward the synthesis of other heterocyclic scaffolds. Treating dihydropyridine 3c with palladium hydroxide and H2 in methanol generates the tetrahydropyridine 8 (Scheme 4), selectively reducing the more electron rich double bond. The dihydropyridine could also be reduced by treating 3c with excess triethylsilane and trifluoroacetic acid, without eroding the pre-existing stereocenters, delivering piperidine 9 (ref. 16) with three contiguous stereocenters.
Conclusion
In conclusion, we have developed an enantio- and diastereoselective addition of enals to pyridiniums using NHC-catalysis. The 1,4-dihydropyridine products are generated with good regiocontrol over the 1,2-addition product and with high enantioselectivity. Key to the success of this reaction was the addition of catalytic amounts of acetic acid to improve the yield of the reaction, preventing an off-cycle catalyst-pyridinium adduct trap.Acknowledgements
We thank NIGMS (GM72586) for support of this work. Single crystal X-ray diffraction was performed at the Shared Materials Characterization Laboratory at Columbia University. Use of the SMCL was made possible by funding from Columbia University.Notes and references
- (a) T. F. McDonald, S. Pelzer, W. Trautwein and D. J. Pelzer, Physiol. Rev., 1994, 74, 365 CAS; (b) S. Goldmann and J. Stoltefuss, Angew. Chem., Int. Ed., 1991, 30, 1559 CrossRef; (c) M. F. Gordeev, D. V. Patel, B. P. England, S. Jonnalagadda, J. D. Combs and E. M. Gordon, Bioorg. Med. Chem., 1998, 6, 883 CrossRef CAS PubMed; (d) V. A. Burgess, S. G. Davies and R. T. Skerlj, Tetrahedron: Asymmetry, 1991, 2, 299 CrossRef CAS.
- (a) J. Bosch and M. L. Bennasar, Synlett, 1995, 587 CrossRef CAS; (b) D. M. Stout and A. I. Meyers, Chem. Rev., 1982, 82, 223 CrossRef CAS; (c) M. G. P. Buffat, Tetrahedron, 2004, 60, 1701 CrossRef CAS; (d) R. W. Dugger, J. A. Ragan and D. H. B. Ripin, Org. Process Res. Dev., 2005, 9, 253 CrossRef CAS; (e) M. C. Hilton, R. D. Dolewski and A. McNally, J. Am. Chem. Soc., 2016, 138, 13806 CrossRef CAS PubMed; (f) K. Pajuste and A. Plotniece, Chem. Heterocycl. Compd., 2016, 52, 538 CrossRef CAS.
- (a) J. Yu, F. Shi and L.-Z. Gong, Acc. Chem. Res., 2011, 44, 1156 CrossRef CAS PubMed; (b) H. Thu Pham, I. Chataigner and J.-L. Renaud, Curr. Org. Chem., 2012, 16, 1754 CrossRef.
- J. A. Bull, J. J. Mousseau, G. Pelletier and A. B. Charette, Chem. Rev., 2012, 112, 2642 CrossRef CAS PubMed.
- (a) A. I. Meyers, J. Org. Chem., 2005, 70, 6137 CrossRef CAS PubMed; (b) E. Ichikawa, M. Suzuki, K. Yabu, M. Albert, M. Kanai and M. Shibasaki, J. Am. Chem. Soc., 2004, 126, 11808 CrossRef CAS PubMed; (c) P. Mangeney, R. Gosmini, S. Raussou, M. Commercon and A. Alexakis, J. Org. Chem., 1994, 59, 1877 CrossRef CAS.
- (a) Z. Sun, S. Yu, Z. Ding and D. Ma, J. Am. Chem. Soc., 2007, 129, 9300 CrossRef CAS PubMed; (b) M. Á. Fernández-Ibáñez, B. Maciá, M. G. Pizzuti, A. J. Minnaard and B. L. Feringa, Angew. Chem., Int. Ed., 2009, 48, 9339 CrossRef PubMed; (c) N. Christian, S. Aly and K. Belyk, J. Am. Chem. Soc., 2011, 133, 2878 CrossRef CAS PubMed; (d) Z.-P. Yang, Q.-F. Wu and S.-L. You, Angew. Chem., Int. Ed., 2014, 53, 6986 CrossRef CAS PubMed.
- O. G. Mancheño, S. Asmus, M. Zurro and T. Fischer, Angew. Chem., Int. Ed., 2015, 54, 8823 CrossRef PubMed.
- G. Bertuzzi, A. Sinisi, L. Caruana, A. Mazzanti, M. Fochi and L. Bernardi, ACS Catal., 2016, 6, 6473 CrossRef CAS.
- G. Bertuzzi, A. Sinisi, D. Pecorari, L. Caruana, A. Mazzanti, L. Bernardi and M. Fochi, Org. Lett., 2017, 19, 834 CrossRef CAS PubMed.
- (a) D. M. Flanigan, F. Romanov-Michailidis, N. A. White and T. Rovis, Chem. Rev., 2015, 115, 9307 CrossRef CAS PubMed; (b) D. Enders, O. Niemeier and A. Henseler, Chem. Rev., 2007, 107, 5606 CrossRef CAS PubMed; (c) H. U. Vora, P. Wheeler and T. Rovis, Adv. Synth. Catal., 2012, 354, 1617 CrossRef CAS PubMed; (d) M. H. Wang and K. A. Scheidt, Angew. Chem., Int. Ed., 2016, 55, 14912 CrossRef CAS PubMed; (e) C. Zhang, J. F. Hooper and D. W. Lupton, ACS Catal., 2017, 7, 2583 CrossRef CAS; (f) A. Levens and D. W. Lupton, Synlett, 2017, 13, 415 Search PubMed; (g) E. Reyes, U. Uria, L. Carrillo and J. L. Vicario, Synthesis, 2017, 49, 451 CrossRef CAS; (h) R. S. Menon, A. T. Biju and V. Nair, Chem. Soc. Rev., 2015, 44, 5040 RSC; (i) V. Nair, R. S. Menon, A. T. Biju, C. R. Sinu, R. R. Paul, A. Jose and V. Sreekumar, Chem. Soc. Rev., 2011, 40, 5336 RSC.
- (a) D.-F. Chen and T. Rovis, Synthesis, 2017, 49, 293 CAS; (b) N. A. White and T. Rovis, J. Am. Chem. Soc., 2015, 137, 10112 CrossRef CAS PubMed; (c) N. A. White and T. Rovis, J. Am. Chem. Soc., 2014, 136, 14674 CrossRef CAS PubMed; (d) N. A. White, K. E. Ozboya, D. M. Flanigan and T. Rovis, Asian J. Org. Chem., 2014, 3, 442 CrossRef CAS PubMed; (e) N. A. White, D. A. DiRocco and T. Rovis, J. Am. Chem. Soc., 2013, 135, 8504 CrossRef CAS PubMed; (f) P. Wheeler, H. U. Vora and T. Rovis, Chem. Sci., 2013, 4, 1674 RSC; (g) X. Zhao, D. A. DiRocco and T. Rovis, J. Am. Chem. Soc., 2011, 133, 12466 CrossRef CAS PubMed.
- J. A. Bull, J. J. Mousseau, G. Pelletier and A. B. Charette, Chem. Rev., 2012, 112, 2642 CrossRef CAS PubMed.
- H. U. Vora and T. Rovis, J. Am. Chem. Soc., 2010, 132, 2860 CrossRef CAS PubMed.
- D. A. DiRocco, K. M. Oberg and T. Rovis, J. Am. Chem. Soc., 2012, 134, 6143 CrossRef CAS PubMed.
- An experiment involving pyridinium 1a and a stoichiometric amount of NHC precursor revealed the formation of a covalent adduct between the two. Efforts at characterizing this intermediate proved unsuccessful.
- Relative stereochemistry was assigned by single crystal analysis. The coordinates have been deposited (CCDC 1555492†).
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental conditions and compound characterization. CCDC 1555492. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c7sc02648j |
| ‡ Current address: Department of Chemistry, Columbia University, New York, NY, 10027, email: E-mail: tr2504@columbia.edu |
| This journal is © The Royal Society of Chemistry 2017 |