Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Enantioselective copper catalysed, direct functionalisation of allenes via allyl copper intermediates
Alexander P.
Pulis
 *,
Kay
Yeung
*,
Kay
Yeung
 and
David J.
Procter
and
David J.
Procter
 *
*
School of Chemistry, University of Manchester, Oxford Rd, Manchester, M13 9PL, UK. E-mail: alexander.pulis@manchester.ac.uk; david.j.procter@manchester.ac.uk
First published on 8th June 2017
Abstract
The direct functionalisation of allenes under copper catalysis enables efficient access to enantioenriched, densely functionalised molecules. In this review we explore the breadth and depth of a versatile reaction manifold, which involves the element-cupration of allenes to generate allyl copper intermediates that are subsequently coupled with diverse arrays of electrophiles.
1 Introduction
The formation of densely functionalised organic molecules from readily available feedstocks is a critical activity in synthetic science. Such processes are particularly powerful when they operate under mild conditions, utilise readily available inexpensive nontoxic catalysts, and selectively deliver single enantiomers.Allenes, once only an academic curiosity due to their unique geometry, have become versatile building blocks in the synthesis of complex molecules;1 more reactive than alkenes and alkynes, they allow mild and atom efficient transformations, and are ideal substrates for asymmetric catalysis. Possessing 1,2-orthogonal double bonds, the use of allenes is not without challenges since regio- and chemoselective issues can arise and 1,3-disubstituted allenes are chiral entities. Despite this, they have become popular starting materials in multicomponent synthesis since metal-functionalisation of one carbon–carbon double bond often leads to an allyl metal species that can be subsequently reacted further with a variety of coupling partners.2
In recent times, copper catalysis has offered an inexpensive, environmentally friendly alternative to the use of precious metals.3,4 Surprisingly, only recently have allenes and copper catalysts, and the resultant in situ generated versatile allyl copper intermediates, been allied to address the challenges of modern enantioselective synthesis (Scheme 1).5 The general mechanism for the copper catalysed functionalisation of allenes proceeds via initial formation of a copper–element complex 1, for example a copper–boryl complex. Intermediate 1 then allows the direct functionalisation of an allene 2via element-cupration,3a,6 which generally occurs at the least hindered site of the allene to generate an allyl copper7 species 3. Metalotropic rearrangement of 3 can, in some cases, result in the formation of isomerised allyl copper 3′. The resultant functionalised allyl coppers 3 and 3′ subsequently couple with various electrophiles through either the α- or γ-positions to selectively form densely functionalised, enantioenriched products 4 or 4′. Although the regioselectivity in the initial element cupration and subsequent addition to electrophiles adds a further layer of complexity to the challenges of generating highly functionalised molecules in a stereodefined manner from allene feedstocks, remarkably high selectivity has been described. In this sequence, stereochemistry in the products may arise from the original allene carbon skeleton or from a prochiral electrophile, or in some cases from both.
 | ||
| Scheme 1 Copper catalysed functionalisation of allenes allows access to diverse collections of enantioenriched organic frameworks. | ||
Allyl coppers (cf.3) and the analogous propargyl and allenyl coppers utilised in similar enantioselective processes, can also be formed via cupration of related unsaturated carbon frameworks, such as in the works of Hoveyda,8 Buchwald,9 and Shimizu and Kanai10 involving enynes, and Cao and Liao11 employing 1,3-dienes, in addition to processes involving transmetalation.12–15
Allenes have proven versatile starting materials for the in situ generation of allyl copper intermediates (cf.3) by virtue of the breadth of multifunctionalisation reactions reported, and great effort has been invested by research groups worldwide in exploring the potential of this reaction manifold. In this review, we will analyse developments in the enantioselective, copper-catalysed direct functionalisation of allenes that involve allyl copper intermediates, and enable the efficient construction of highly versatile molecular architectures.
2 Copper catalysed enantioselective functionalisation of allenes
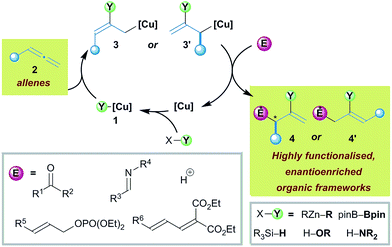
In this section we will detail the diverse array of enantioselective multifunctionalisation reactions of allenes under copper catalysis that proceed through allyl copper intermediates. For each reaction, we will discuss mechanistic features that underpin the key processes, include selected examples from the scope, and where possible, highlight the versatility of the products that are generated.Kanai and Shibasaki reported the copper catalysed functionalisation of allenic esters 5 in a multicomponent coupling with ketones 6 and dialkyl zincs that delivers δ-lactones 8 with excellent enantioselectivity (Scheme 2).16 The reaction proceeds via formation of a chiral-phosphine alkyl copper(I) complex 9 which allows for carbocupration of allenic ester 5 forming allyl copper 10 (Scheme 2B). The reaction of 10 with ketones follows two courses: kinetic α-addition to give aldolate 11, or γ-addition to give 12. Indeed, mixtures of both 11 and 12 are initially formed, but retroaldol in 11 renders the α-addition pathway reversible, and the process selectively delivers the desired lactone product 8. The addition of coordinating additives, such as sulfoxides or HMPA, facilitated the retro-aldol, and was crucial for high yields. The reaction scope was broad and, interestingly, even tolerated the use of α,β-unsaturated ketones (Scheme 2C).
 | ||
| Scheme 2 Kanai and Shibasaki's carbocupration of allenic esters and subsequent coupling to ketones. CuTC = copper thiophene-2-carboxylate; additive = DMSOa, HMPAb, or Ph2SO. | ||
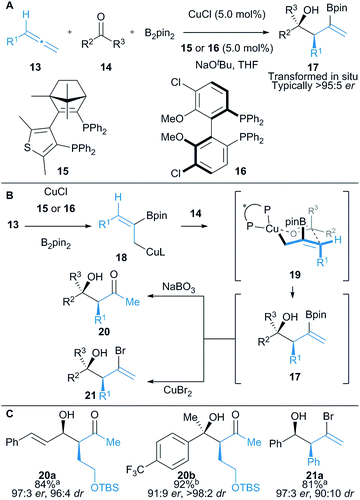
Hoveyda and co-workers described the enantioselective union of aldehydes or ketones (14) with aryl or alkyl substituted allenes (13) and B2pin2 (Scheme 3).17 Borocupration of the allene 13 generates in situ allylcopper 18, which is trapped with an aldehyde or ketone to afford highly functionalised vinyl boronates 17. Syn products are obtained via a proposed 6-membered transition state structure 19, where the substituent on the aldehyde is in a pseudoequatorial position (Scheme 3B). Vinyl boronates 17 were not isolated and were oxidised or brominated in situ to give isolable β-hydroxyketones 20 or alkenyl bromides 21, respectively. Enantioselective addition to ketones allowed access to tertiary alcohols (cf.20b) and interestingly, when α,β-unsaturated ketones were employed (cf.20a), 1,2-allylation was almost exclusively observed despite potential competing boryl–copper and allyl copper 18 conjugate addition pathways.
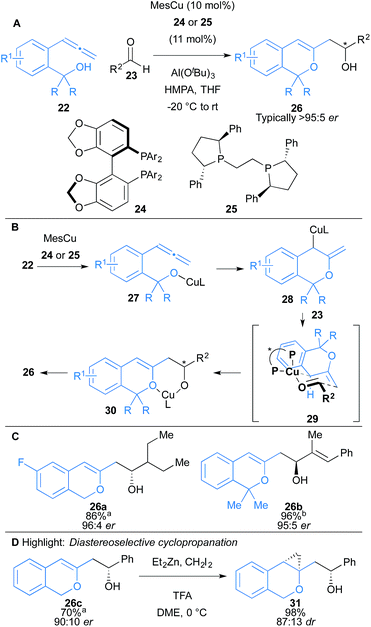
Shimizu, Kanai and co-workers reported an intramolecular oxycupration of allenes 22 (Scheme 4).18 The reaction proceeds through an allylcopper intermediate 28, which then undergoes a subsequent enantioselective addition to aldehydes 23 to afford 1H-isochromene derivatives 26 (Scheme 4B). Ligand 24 was used in the case of allenes 22 containing primary alcohols (R = H) and ligand 25 for tertiary alcohols (R = Me). HMPA was found to enhance enantioselectivity and reduce protonation of the allylcopper intermediate, which led to a major side product in some cases. In more challenging substrates, Al(OtBu)3 was used as a co-catalyst to facilitate liberation of the copper catalyst from intermediate 30. The reaction was compatible with aromatic and aliphatic aldehydes, and gave secondary alcohols 26 with high enantioselectivity (typically >95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 er) (Scheme 4C). Acetophenone was also used, and gave the corresponding tertiary alcohol in 77% yield and 88
5 er) (Scheme 4C). Acetophenone was also used, and gave the corresponding tertiary alcohol in 77% yield and 88![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12 er. The 1H-isochromene skeleton bears a versatile enol ether moiety that could readily undergo diastereoselective cyclopropanation to form a tricyclic scaffold 31 (Scheme 4D).
12 er. The 1H-isochromene skeleton bears a versatile enol ether moiety that could readily undergo diastereoselective cyclopropanation to form a tricyclic scaffold 31 (Scheme 4D).
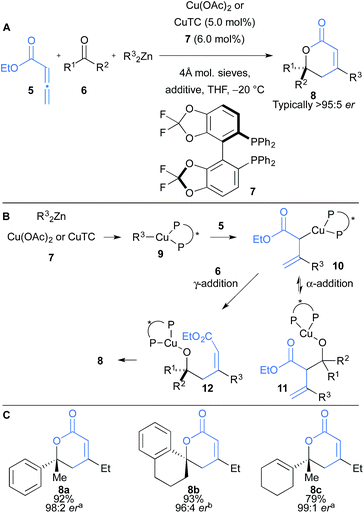
Kanai and co-workers have further developed an intramolecular amido-cupration of allenes 32 and asymmetric addition to ketones and aldehydes (33), to afford substituted indole scaffolds 34 (Scheme 5).19 The one-pot process proceeds with high regio-, chemo- and enantioselectivity with a broad substrate scope including addition to aromatic and aliphatic aldehydes and ketones, where ketones afford products bearing tertiary alcohols (Scheme 5B). In some cases, the additive Mg(OiPr)2 dramatically increased the yield of coupling by decreasing the Brønsted basicity of the allylcopper species, relative to its nucleophilicity, and suppressing the undesired protonation pathway. The synthetic utility of the products was demonstrated using 34c, which was converted to the pharmacophore tetrahydropyranoindole 35 and indoline 36 (Scheme 5C).
 | ||
| Scheme 5 Kanai's intramolecular aminocupration of allenes and subsequent coupling to aldehydes and ketones. aMg(OiPr)2 was used. | ||
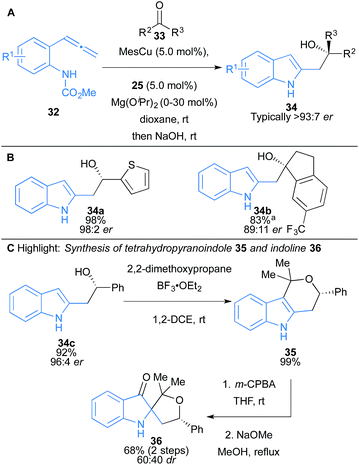
Procter and co-workers reported the enantioselective Cu(I)–NHC catalysed three-component coupling of allenes 37, B2pin2 and aldimines 38 that proceeds with high diastereo- and enantiocontrol and excellent functional group tolerance, to yield homoallylic amines 41 bearing adjacent stereocentres (Scheme 6).20 Regioselective borocupration of allenes leads to in situ formation of an allylcopper intermediate 40. Computational analysis revealed that the allylcopper addition to imine likely proceeds via a chair-like transition state structure 40, where the substituents on the imine are in pseudoaxial positions (Scheme 6A).21 The boronate ester products can be isolated owing to an intramolecular nitrogen–boron interaction. The coupling products 41 could be further functionalised by oxidation to form branched β-amino ketones, and hydrogenated to form secondary alkyl boronates bearing three contiguous stereocentres (Scheme 6C).
 | ||
| Scheme 6 Procter's borocupration of allenes and subsequent coupling to imines. Enantiomeric ratio given for the major diastereomer. | ||
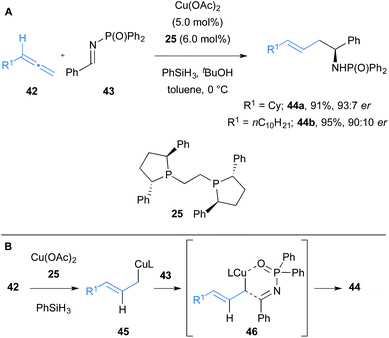
Soon after, Buchwald and co-workers reported a Cu(II)-catalysed regiodivergent and diastereoselective allylation of aldimines 43 to synthesise branched or linear homoallylic amines.22 An enantioselective variant of their linear-selective allylation reaction using N-diphenylphosphinyl protected imines was demonstrated with two examples (Scheme 7). Hydrosilanes were used to form in situ a copper hydride intermediate, which upon hydrocupration of allene 42 and subsequent fast equilibration, afforded the thermodynamically favoured terminal E-allylcopper intermediate 45 (Scheme 7B). The allylcopper species reacts with the imine through the α-position, in contrast to the γ-addition of the allylcopper observed in Procter's work. The transition state structure 46 for the allylation of aldimines 43, supported by DFT studies, was proposed to involve copper coordination to oxygen of the phosphinyl imine, then transfer of the allyl fragment to afford linear products selectively.
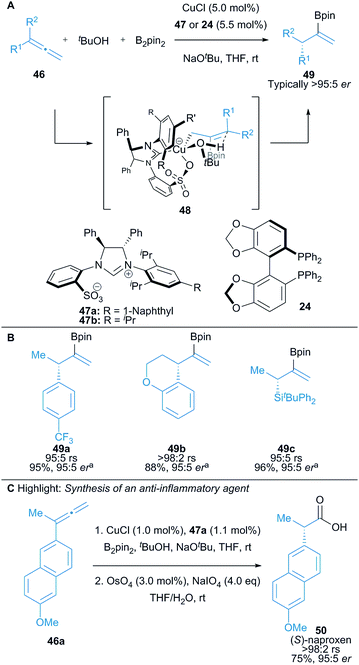
Hoveyda and co-workers demonstrated the use of prochiral 1,1-disubstituted allenes 46 in a Cu(I)-catalysed protoboration reaction affording vinyl boronates 49, with high enantioselectivity achieved using either a chiral NHC precursor 47 or chiral phosphine ligand 24 (Scheme 8).23 The reaction proceeds via γ-protonation of an in situ formed allylcopper intermediate (cf.48) to provide access to enantioenriched vinyl boronates 49 with up to >98% regioselectivity and typically >95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 er in excellent yield (Scheme 8B). 1,1-Disubstituted allenes bearing an alkyl and aryl substituent were used. The use of tert-butanol as the proton source was crucial for the high enantioselectivity of the reaction. The versatility of vinyl boronates was well-demonstrated with representative procedures for the preparation of enantiomerically enriched vinyl bromides, methyl ketones and carboxylic acids. The methodology was also applied in an enantioselective synthesis of the non-steroidal anti-inflammatory agent, (S)-naproxen 50 (Scheme 8C).
5 er in excellent yield (Scheme 8B). 1,1-Disubstituted allenes bearing an alkyl and aryl substituent were used. The use of tert-butanol as the proton source was crucial for the high enantioselectivity of the reaction. The versatility of vinyl boronates was well-demonstrated with representative procedures for the preparation of enantiomerically enriched vinyl bromides, methyl ketones and carboxylic acids. The methodology was also applied in an enantioselective synthesis of the non-steroidal anti-inflammatory agent, (S)-naproxen 50 (Scheme 8C).
 | ||
| Scheme 8 Hoveyda's borocupration of allenes and subsequent protonation of the allyl copper intermediate. rs = regioselectivity (γ/α site selectivity of protonation). ausing 47a. | ||
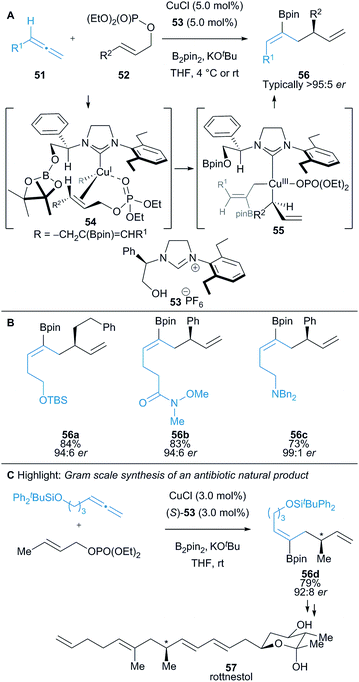
An enantioselective Cu(I)-catalysed borylative allyl–allyl coupling was successfully developed by Hoveyda and co-workers, using an in situ generated allylcopper intermediate (cf.54) and allylic phosphonates 52 to synthesise 1,5-diene motifs 56 (Scheme 9).24,25 Allylic phosphonates can react through a SN2′ or SN2 pathway and can undergo direct borylation to form a nucleophilic allylic boronate, yet despite this, judicious choice of ligand resulted in excellent chemo-, regio-, and enantioselectivity. The proposed reaction pathway is supported by DFT calculations and involves an allylcopper intermediate that coordinates with the allylic phosphate 52 to form a tetrahedral Cu(I) complex 54. Oxidative addition in 54 forms square planar Cu(III) complex 55 which undergoes reductive elimination to afford the desired product 56 (Scheme 9A). Wide functional group tolerance was demonstrated (Scheme 9B) and the methodology applied to the gram-scale synthesis of two natural products, including the antibiotic rottnestol 57 (Scheme 9C).
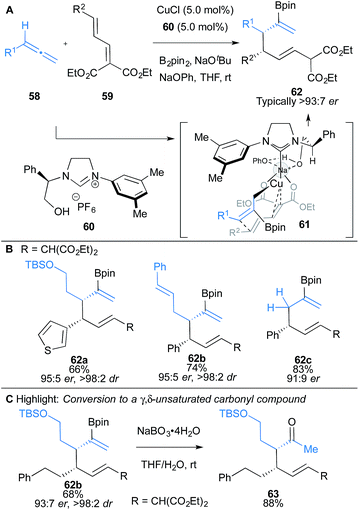
Hoveyda and co-workers have also reported a three-component coupling of allenes 58 with dienoates 59 and B2pin2 leading to highly functionalised 1,5-dienes 62 (Scheme 10).14a Excellent regio-, diastereo- and enantioselectivity were achieved using imidazolinium salt 60 bearing an unprotected hydroxyl group, which was found to be essential for enantioselectivity. DFT studies suggested the hydroxy group provides key ionic interactions and hydrogen bonding in the transition state (Scheme 10A). Substrates bearing heterocycles, alkenyl and alkynyl substituents were compatible (Scheme 10B). The enantiomerically-enriched products 62 allow access to γ,δ-unsaturated ketones 63 with vicinal stereocentres, which are otherwise difficult to access directly (Scheme 10C).
 | ||
| Scheme 10 Hoveyda's borocupration of allenes and subsequent coupling to dienoates. Enantiomeric ratio given for the major diastereomer. | ||
3 Future prospects in copper catalysed allene functionalisation
In addition to the aforementioned enantioselective processes, there have been reports of the non-enantioselective copper-catalysed functionalisation of allenes that enables access to diversely functionalised molecules that are ripe for the development of enantioselective variants. As well as the silylative variants of the aforementioned borylative couplings of aldehydes,26 ketones27 and imines,21 described above, such copper catalysed functionalisations of allenes include protoboration that selectively delivers alkenyl or allylic boronic esters,28 borostannylation that yields β-boryl allyl stannanes,29 hydrocupration followed by branch selective imine allylation,22 carboboration that produces alkenyl boronic esters,30 intramolecular hydroamination for the formation of 3-pyrrolines or 2-alkenylpyrrolidines,31 and conjugate addition type processes of allenoates and their derivatives.32In this section we wish to highlight selected recent advances in non-enantioselective copper catalysed processes that deliver densely functionalised molecules derived from allenes. For example, in 2016, Montgomery reported a rare example of a diastereo- and regioselective copper-catalysed trifunctionalisation of terminal allenes 64 (Scheme 11).33 In this reaction, initial borocupration of allene 64 leads to allyl copper intermediate 67 that is subsequently cyanated with N-cyano-N-phenyl-p-methylbenzenesulfonamide (65) to give intermediate 68. Further borocupration of 68 and protonation provides the trifunctionalised product 66. As with many of the processes presented herein, the method displays impressive functional group tolerance.
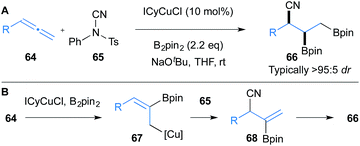 | ||
| Scheme 11 Montgomery's borocupration of allenes and subsequent cyanation. ICyCuCl = chloro[1,3-dicyclohexylimidazol-2-ylidene]copper(I). | ||
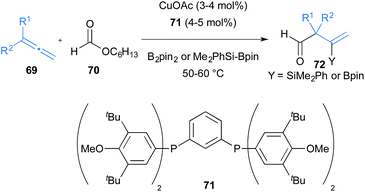
Fujihara, Tsuji and co-workers showed that allenes 69 can be functionalised via boraformylation and silaformylation reactions (Scheme 12).34 The allyl copper generated after initial boro- or silacupration of 1,1-disubstituted allenes 69 with CuOAc and the bulky diphosphine ligand 71 can be trapped with formate ester 70 to deliver β-boryl or β-silyl β,γ-unsaturated aldehydes 72. This reaction is particularly impressive as it has the potential to deliver aldehydes containing α-quaternary carbons.
 | ||
| Scheme 12 Fujihara and Tsuji's boro- and sila-cupration of allenes and subsequent coupling with formates. | ||
Finally, Shimizu and Kanai reported that allenes 73 undergo oxyarylation in the presence of a variety of aryl boronic acids 74 and TEMPO to yield β-arylated allylic alcohol derivatives 75 under copper catalysis (Scheme 13).35 Copper(I) salts were also effective in the transformation but Cu(OTf)2 was optimal. The mechanism is proposed to go through initial transmetallation of copper with aryl boronic acids to form aryl copper 76 (Scheme 13B). Subsequent carbocupration of allene 73 yields allyl copper 77 that undergoes homolysis to form allyl radical 78 and a reduced copper species. TEMPO traps 78 to form product 75, and MnO2 allows for oxidation of copper to reform the catalyst.
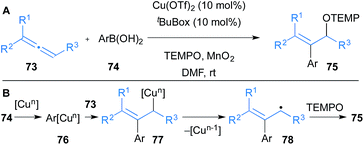 | ||
| Scheme 13 Shimizu and Kanai's carbocupration of allenes and subsequent oxidation. tBuBox = 2,2′-isopropylidenebis[(4S)-4-tert-butyl-2-oxazoline]. | ||
Although these methods are yet to be shaped into enantioselective processes, they yet again show the impressive array of allene functionalisations that are possible under copper catalysis.
4 Conclusions
The use of allenes as feedstocks in conjunction with copper catalysis allows for the efficient construction of diversely functionalised, enantioenriched molecules. The general reaction manifold involves initial element cupration of the allene to generate an allyl copper that undergoes a subsequent coupling event. The key allyl copper species usually reacts through the γ-position with the electrophilic coupling partner, although α-addition is also known. In addition, the catalysis generally operates through Cu(I) species, but the formation of Cu(III), followed by reductive elimination has also been proposed with electrophiles that can oxidatively add to allyl Cu(I) complexes.The above enantioselective transformations only utilise NHC and bisphosphine ligands as chiral inductors. Given the enormous variety of other chiral ligands that might be employed, such as sulfoxides,36 bisoxazolines,37 and even chiral counterions,38 the door is open for further, more diverse copper catalysed functionalisation of allenes.
We anticipate that further, exciting developments will be discovered with the use of enantiomerically pure allenes,39 or chiral racemic allenes, which have not been investigated in this reaction manifold. Enantiomerically pure allenes might be employed in reactions that transfer axial into point chirality with an achiral copper catalyst. In addition, given that the racemisation of allenes with cuprates is precedented,40 resolution processes under copper catalysis can also be envisaged.
Currently, enantioselective processes have been developed that involve boro-, sila-, hydro-, carbo-, amino- and oxy-cupration of allenes to generate allyl coppers that are subsequently coupled with aldehydes, ketones, imines, protons, allyls, and unsaturated carbonyls. And yet, we have only scratched the surface. Given the potential variety of initiating element-cuprations, the plethora of coupling partners available, and the possibility of further in situ transformations, we can look forward to many more diverse copper catalysed processes that result in the enantioselective mono-, di- and even trifunctionalisation of allenes.
Acknowledgements
We thank the University of Manchester (President's Scholarship to K. Y. and Lectureship to A. P. P.), the SCI (Scholarship to K. Y.), and the EPSRC (Studentships to K. Y. and Established Career Fellowship to D. J. P.).Notes and references
- (a) S. Yu and S. Ma, Angew. Chem., Int. Ed., 2012, 51, 3074–3112 CrossRef CAS PubMed; (b) E. Soriano and I. Fernández, Chem. Soc. Rev., 2014, 43, 3041–3105 RSC; (c) J. M. Alonso, M. T. Quirós and M. P. Muñoz, Org. Chem. Front., 2016, 3, 1186–1204 RSC; (d) T. Lechel, F. Pfrengle, H.-U. Reissig and R. Zimmer, ChemCatChem, 2013, 5, 2100–2130 CrossRef CAS; (e) E. Manoni and M. Bandini, Eur. J. Org. Chem., 2016, 3135–3142 CrossRef CAS; (f) R. K. Neff and D. E. Frantz, ACS Catal., 2014, 4, 519–528 CrossRef CAS.
- (a) M. Jeganmohan and C.-H. Cheng, Chem. Commun., 2008, 3101–3117 RSC; (b) G. Eppe, D. Didier and I. Marek, Chem. Rev., 2015, 115, 9175–9206 CrossRef CAS PubMed.
- For recent reviews, see: (a) D. S. Müller and I. Marek, Chem. Soc. Rev., 2016, 45, 4552–4566 RSC; (b) X. Zhu and S. Chiba, Chem. Soc. Rev., 2016, 45, 4504–4523 RSC; (c) M. Shibasaki and M. Kanai, Chem. Rev., 2008, 108, 2853–2873 CrossRef CAS PubMed; (d) S. Thapa, B. Shrestha, S. K. Gurung and R. Giri, Org. Biomol. Chem., 2015, 13, 4816–4827 RSC; (e) C. Maaliki, E. Thiery and J. Thibonnet, Eur. J. Org. Chem., 2017, 209–228 CrossRef CAS; (f) N. Yoshikai and E. Nakamura, Chem. Rev., 2012, 112, 2339–2372 CrossRef CAS PubMed.
- For selected recent publications, see: (a) R. P. Jumde, F. Lanza, M. J. Veenstra and S. R. Harutyunyan, Science, 2016, 352, 433–437 CrossRef CAS PubMed; (b) Y. Yang, I. B. Perry and S. L. Buchwald, J. Am. Chem. Soc., 2016, 138, 9787–9790 CrossRef CAS PubMed; (c) K. Kubota, Y. Watanabe, K. Hayama and H. Ito, J. Am. Chem. Soc., 2016, 138, 4338–4341 CrossRef CAS PubMed; (d) K. Hojoh, H. Ohmiya and M. Sawamura, J. Am. Chem. Soc., 2017, 139, 2184–2187 CrossRef CAS PubMed.
- Y. Shimizu and M. Kanai, Tetrahedron Lett., 2014, 55, 3727–3737 CrossRef CAS.
- For selected reviews concerning element-cuprations, see: (a) K. Semba, T. Fujihara, J. Terao and Y. Tsuji, Tetrahedron, 2015, 71, 2183–2197 CrossRef CAS; (b) E. C. Neeve, S. J. Geier, I. A. I. Mkhalid, S. A. Westcott and T. B. Marder, Chem. Rev., 2016, 116, 9091–9161 CrossRef CAS PubMed; (c) M. Oestreich, E. Hartmann and M. Mewald, Chem. Rev., 2013, 113, 402–441 CrossRef CAS PubMed; (d) H. Yoshida, ACS Catal., 2016, 6, 1799–1811 CrossRef CAS; (e) Z. Galeštoková and R. Šebesta, Eur. J. Org. Chem., 2012, 6688–6695 CrossRef.
- E. R. Bartholomew, S. H. Bertz, S. Cope, M. Murphy and C. A. Ogle, J. Am. Chem. Soc., 2008, 130, 11244–11245 CrossRef CAS PubMed.
- F. Meng, F. Haeffner and A. H. Hoveyda, J. Am. Chem. Soc., 2014, 136, 11304–11307 CrossRef CAS PubMed.
- Y. Yang, I. B. Perry, G. Lu, P. Liu and S. L. Buchwald, Science, 2016, 353, 144–150 CrossRef CAS PubMed.
- X.-F. Wei, X.-W. Xie, Y. Shimizu and M. Kanai, J. Am. Chem. Soc., 2017, 139, 4647–4650 CrossRef CAS PubMed.
- L. Jiang, P. Cao, M. Wang, B. Chen, B. Wang and J. Liao, Angew. Chem., Int. Ed., 2016, 55, 13854–13858 CrossRef CAS PubMed.
- For recent examples involving allyl boranes, see: (a) Y. Yasuda, H. Ohmiya and M. Sawamura, Angew. Chem., Int. Ed., 2016, 55, 10816–10820 CrossRef CAS PubMed; (b) E. M. Vieira, M. L. Snapper and A. H. Hoveyda, J. Am. Chem. Soc., 2011, 133, 3332–3335 CrossRef CAS PubMed.
- For recent examples involving propargylic boranes, see: (a) N. W. Mszar, F. Haeffner and A. H. Hoveyda, J. Am. Chem. Soc., 2014, 136, 3362–3365 CrossRef CAS PubMed; (b) Y. Shi, B. Jung, S. Torker and A. H. Hoveyda, J. Am. Chem. Soc., 2015, 137, 8948–8964 CrossRef CAS PubMed; (c) D. R. Fandrick, C. A. Hart, I. S. Okafor, M. A. Mercadante, S. Sanyal, J. T. Masters, M. Sarvestani, K. R. Fandrick, J. L. Stockdill, N. Grinberg, N. Gonnella, H. Lee and C. H. Senanayake, Org. Lett., 2016, 18, 6192–6195 CrossRef CAS PubMed.
- For recent examples involving allenyl boranes, see: (a) F. Meng, X. Li, S. Torker, Y. Shi, X. Shen and A. H. Hoveyda, Nature, 2016, 537, 387–393 CrossRef CAS PubMed; (b) B. Jung and A. H. Hoveyda, J. Am. Chem. Soc., 2012, 134, 1490–1493 CrossRef CAS PubMed; (c) E. M. Vieira, F. Haeffner, M. L. Snapper and A. H. Hoveyda, Angew. Chem., Int. Ed., 2012, 51, 6618–6621 CrossRef CAS PubMed; (d) X.-F. Wei, Y. Shimizu and M. Kanai, ACS Cent. Sci., 2016, 2, 21–26 CrossRef CAS PubMed.
- For other recent examples, see: (a) V. Hornillos, M. Pérez, M. Fañanás-Mastral and B. L. Feringa, J. Am. Chem. Soc., 2013, 135, 2140–2143 CrossRef CAS PubMed; (b) Y. Shi and A. H. Hoveyda, Angew. Chem., Int. Ed., 2016, 55, 3455–3458 CrossRef CAS PubMed; (c) R. Wada, T. Shibuguchi, S. Makino, K. Oisaki, M. Kanai and M. Shibasaki, J. Am. Chem. Soc., 2006, 128, 7687–7691 CrossRef CAS PubMed; (d) D. Ferraris, B. Young, C. Cox, T. Dudding, W. J. Drury III, L. Ryzhkov, A. E. Taggi and T. Lectka, J. Am. Chem. Soc., 2002, 124, 67–77 CrossRef CAS PubMed; (e) X. Fang, M. Johannsen, S. Yao, N. Gathergood, R. G. Hazell and K. A. Jørgensen, J. Org. Chem., 1999, 64, 4844–4849 CrossRef CAS PubMed.
- K. Oisaki, D. Zhao, M. Kanai and M. Shibasaki, J. Am. Chem. Soc., 2007, 129, 7439–7443 CrossRef CAS PubMed.
- F. Meng, H. Jang, B. Jung and A. H. Hoveyda, Angew. Chem., Int. Ed., 2013, 52, 5046–5051 CrossRef CAS PubMed.
- J. Kawai, P. K. Chikkade, Y. Shimizu and M. Kanai, Angew. Chem., Int. Ed., 2013, 52, 7177–7180 CrossRef CAS PubMed.
- P. K. Chikkade, Y. Shimizu and M. Kanai, Chem. Sci., 2014, 5, 1585–1590 RSC.
- K. Yeung, R. E. Ruscoe, J. Rae, A. P. Pulis and D. J. Procter, Angew. Chem., Int. Ed., 2016, 55, 11912–11916 CrossRef CAS PubMed.
- J. Rae, K. Yeung, J. J. W. McDouall and D. J. Procter, Angew. Chem., Int. Ed., 2016, 55, 1102–1107 CrossRef CAS PubMed.
- R. Y. Liu, Y. Yang and S. L. Buchwald, Angew. Chem., Int. Ed., 2016, 55, 14077–14080 CrossRef CAS PubMed.
- H. Jang, B. Jung and A. H. Hoveyda, Org. Lett., 2014, 16, 4658–4661 CrossRef CAS PubMed.
- F. Meng, K. P. McGrath and A. H. Hoveyda, Nature, 2014, 513, 367–374 CrossRef CAS PubMed.
- K. Semba, N. Bessho, T. Fujihara, J. Terao and Y. Tsuji, Angew. Chem., Int. Ed., 2014, 53, 9007–9011 CrossRef CAS PubMed.
- J. Rae, Y. C. Hu and D. J. Procter, Chem.–Eur. J., 2014, 20, 13143–13145 CrossRef CAS PubMed.
- Y. Tani, T. Yamaguchi, T. Fujihara, J. Terao and Y. Tsuji, Chem. Lett., 2015, 44, 271–273 CrossRef CAS.
- (a) F. Meng, B. Jung, F. Haeffner and A. H. Hoveyda, Org. Lett., 2013, 15, 1414–1417 CrossRef CAS PubMed; (b) W. Yuana and S. Ma, Adv. Synth. Catal., 2012, 354, 1867–1872 CrossRef; (c) W. Yuan, L. Song and S. Ma, Angew. Chem., Int. Ed., 2016, 55, 3140–3143 CrossRef CAS PubMed; (d) K. Semba, M. Shinomiya, T. Fujihara, J. Terao and Y. Tsuji, Chem.–Eur. J., 2013, 19, 7125–7132 CrossRef CAS PubMed.
- Y. Takemoto, H. Yoshida and K. Yakaki, Synthesis, 2014, 46, 3024–3032 CrossRef CAS.
- (a) Y. Zhou, W. You, K. B. Smith and M. K. Brown, Angew. Chem., Int. Ed., 2014, 53, 3475–3479 CrossRef CAS PubMed; (b) I. Kageyuki, I. Osaka, K. Takaki and H. Yoshida, Org. Lett., 2017, 19, 830–833 CrossRef CAS PubMed.
- A. Tsuhako, D. Oikawa, K. Sakai and S. Okamoto, Tetrahedron Lett., 2008, 49, 6529–6532 CrossRef CAS.
- (a) W. Yuan, X. Zhang, Y. Yu and S. Ma, Chem.–Eur. J., 2013, 19, 7193–7202 CrossRef CAS PubMed; (b) S. Pashikanti, J. A. Calderone, M. K. Nguyen, C. D. Sibley and W. L. Santos, Org. Lett., 2016, 18, 2443–2446 CrossRef CAS PubMed; (c) S. B. Thorpe, X. Guo and W. L. Santos, Chem. Commun., 2011, 47, 424–426 RSC; (d) S. Kim and P. H. Lee, J. Org. Chem., 2012, 77, 215–220 CrossRef CAS PubMed.
- W. Zhao and J. Montgomery, J. Am. Chem. Soc., 2016, 138, 9763–9766 CrossRef CAS PubMed.
- T. Fujihara, A. Sawada, T. Yamaguchi, Y. Tani, J. Terao and Y. Tsuji, Angew. Chem., Int. Ed., 2017, 56, 1539–1543 CrossRef CAS PubMed.
- T. Itoh, Y. Shimizu and M. Kanai, Org. Lett., 2014, 16, 2736–2739 CrossRef CAS PubMed.
- B. M. Trost and M. Rao, Angew. Chem., Int. Ed., 2015, 54, 5026–5043 CrossRef CAS PubMed.
- G. Desimoni, G. Faita and K. A. Jørgensen, Chem. Rev., 2011, 111, PR284–PR437 CrossRef CAS PubMed.
- M. Mahlau and B. List, Angew. Chem., Int. Ed., 2013, 52, 518–533 CrossRef CAS PubMed.
- (a) R. K. Neff and D. E. Frantz, Tetrahedron, 2015, 71, 7–18 CrossRef CAS; (b) J. Yea and S. Ma, Org. Chem. Front., 2014, 1, 1210–1224 RSC.
- A. Claesson and L.-I. Olsson, J. Chem. Soc., Chem. Commun., 1979, 524–525 RSC.
| This journal is © The Royal Society of Chemistry 2017 |