Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Cu/Mn bimetallic catalysis enables carbonylative Suzuki–Miyaura coupling with unactivated alkyl electrophiles†
Dominic R.
Pye
,
Li-Jie
Cheng
and
Neal P.
Mankad
 *
*
Department of Chemistry, University of Illinois at Chicago, 845 W. Taylor St., Chicago, IL 60607, USA. E-mail: npm@uic.edu
First published on 31st March 2017
Abstract
A bimetallic system consisting of Cu-carbene and Mn-carbonyl co-catalysts was employed for carbonylative C–C coupling of arylboronic esters with alkyl halides, allowing for the convergent synthesis of ketones. The system operates under mild conditions and exhibits complementary reactivity to Pd catalysis. The method is compatible with a wide range of arylboronic ester nucleophiles and proceeds smoothly for both primary and secondary alkyl iodide electrophiles. Preliminary mechanistic experiments corroborate a hypothetical catalytic mechanism consisting of co-dependent cycles wherein the Cu-carbene co-catalyst engages in transmetallation to generate an organocopper nucleophile, while the Mn-carbonyl co-catalyst activates the alkyl halide electrophile by single-electron transfer and then undergoes reversible carbonylation to generate an acylmanganese electrophile. The two cycles then intersect with a heterobimetallic, product-releasing C–C coupling step.
Introduction
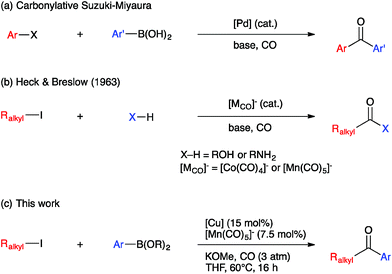
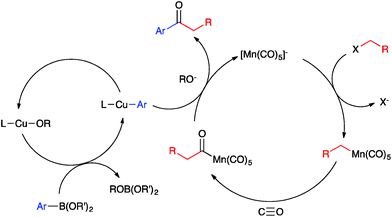
Ketones are ubiquitous functional groups in organic molecules and materials, and furthermore their established reaction chemistry can be used to introduce other heteroatom-containing functional groups at late stages of complex syntheses. A convergent method for constructing ketones from simple building blocks is Pd-catalyzed carbonylative C–C coupling, such as the carbonylative Suzuki–Miyaura, Mizoroki–Heck, and Sonogashira coupling reactions.1–3 These Pd-catalyzed carbonylations have been long established for C(sp2)-hybridized electrophiles, i.e. aryl and vinyl halides (Fig. 1a). The use of C(sp3)-hybridized electrophiles, i.e. alkyl halides, has been reported for specialized cases lacking β-hydrogens,4–9 but cases involving alkylpalladium intermediates susceptible to β-hydride elimination have been more challenging to solve.10–12 Ryu recently reported a Pd-catalyzed method that solves this problem in a general way but requires irradiation with a Xe lamp to generate alkyl radicals that undergo carbonylation at high pressure (45 atm).13 Non-carbonylative Pd-catalyzed reactions to generate alkyl-substituted ketones involve arylcarboxylic acid derivatives as electrophiles14 and include the Liebeskind–Srogl15 and Fukuyama16 coupling reactions. Both carbonylative17,18 and non-carbonylative19 Ni-catalyzed reductive coupling reactions to generate alkyl-substituted ketones also have been reported.Base metal carbonylate complexes are efficient catalysts for heterocarbonylation reactions of alkyl halides. Heck and Breslow first reported the use of Na[Co(CO)4] as a catalyst for the formation of esters from alkyl halides, CO, and alcohols over 50 years ago.20 Since then, the formation of esters and amides via carbonylation of alkyl halides in the presence of an appropriate nucleophile, i.e. an alcohol or amine, has been studied using both Co- and Mn-carbonyl pre-catalysts (Fig. 1b).21,22 Coates has extended this chemistry to include epoxide carbonylation using a bifunctional catalysis approach.23,24 There has not, however, been a suitable carbon nucleophile identified to participate in carbonylative C–C coupling chemistry to form ketones within such systems. Many carbon nucleophiles, such as Grignard reagents, that would be sufficiently nucleophilic to participate in the desired catalytic C–C coupling processes would also react with the ketone products, thus destroying the target molecules. Organocopper nucleophiles are used extensively for 1,4-addition to α,β-unsaturated ketones due to their resistance towards 1,2-addition to carbonyl groups.25 Thus, we hypothesized that a heterobimetallic system consisting of organocopper intermediates in combination with Co or Mn carbonylates would enable carbonylative C–C coupling with alkyl halides to generate ketones, without then consuming the resulting ketone products (Fig. 1c). Our hypothetical mechanism for a carbonylative Suzuki–Miyaura reaction, then, consists of a Heck–Breslow cycle for alkyl halide carbonylation combined with a Cu cycle that generates catalytic quantities of an arylcopper species from a mild arylboronic ester nucleophile (Scheme 1). The two co-dependent cycles intersect with a heterobimetallic C–C coupling step between an arylcopper nucleophile and a metal-acyl electrophile. This mechanistic paradigm represents a new frontier in bimetallic catalysis for C–C coupling,26 which otherwise typically involves catalytic generation of an organometallic nucleophile that undergoes transmetallation with a catalytically generated Pd(II) electrophile,27–34 thus still requiring use of Pd catalysis and its inherent limitations.
Results and discussion
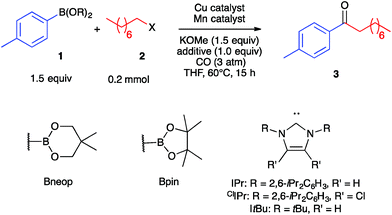
As a starting point, we investigated the reaction of 4-tolylboronic acid neopentyl glycol ester (1a) and 1-iodooctane (2a) under an atmosphere of carbon monoxide. Initial experimentation indicated that the desired ketone (3) formed in 40% yield in THF solvent at 60 °C in the presence of KOMe base (1.5 equiv.) and catalytic amounts (10 mol%) of (IPr)CuCl and Na[Mn(CO)5] (Table 1, Entry 1). Lower yields were obtained with bulkier alkoxide bases or with tricyclohexylphosphine in place of the IPr carbene. The same result was obtained by using catalytic (IPr)Cu–Mn(CO)5, which is expected to assemble upon mixing the Cu and Mn co-catalysts,35 and so optimization was continued using separate pre-catalysts rather than with Cu/Mn-bonded heterobimetallic complexes. Substituting IPr for ClIPr gave a slight increase in yield of 3 to 45%, and performing the reaction under modestly higher CO pressure (3 atm) raised the yield to 59% (Entries 2–3). A screen of numerous copper-carbene co-catalysts at this pressure (see ESI†) identified a number that gave yields in the 70–75% range, and so we continued with commercially available and easily synthesized (IPr)CuCl (Entry 4). Altering the pre-catalyst loadings to 15% (IPr)CuCl and 7.5% Na[Mn(CO)5] was found empirically to increase yield of 3 to 88% (Entry 5). No catalysis was observed when omitting either the Cu co-catalyst or the Mn co-catalyst from the reaction (Entries 6–7), thus verifying the need for bimetallic catalysis. With regard to the nucleophilic coupling partner, efficient reactivity also was observed using the pinacol ester (1b) rather than 1a, while poor reactivity was observed with the unprotected boronic acid (1c) (Entries 8–9).| Entry | B(OR)2 | X | Cu catalyst | Mn catalyst | Yield of 3 (%) |
|---|---|---|---|---|---|
| a p CO = 1 atm. b Yield determined by NMR analysis (1,3,5-trimethoxybenzene internal standard). c Yield determined by GC analysis (decane internal standard). d Additive = Bu4N+I−. | |||||
| 1a | Bneop (1a) | I (2a) | 10% (IPr)CuCl | 10% Na[Mn(CO)5] | 40b |
| 2a | Bneop (1a) | I (2a) | 10% (ClIPr)CuCl | 10% Na[Mn(CO)5] | 45b |
| 3 | Bneop (1a) | I (2a) | 10% (ClIPr)CuCl | 10% Na[Mn(CO)5] | 59b |
| 4 | Bneop (1a) | I (2a) | 10% (IPr)CuCl | 10% Na[Mn(CO)5] | 73b |
| 5 | Bneop (1a) | I (2a) | 15% (IPr)CuCl | 7.5% Na[Mn(CO)5] | 88b (70)c |
| 6 | Bneop (1a) | I (2a) | 15% (IPr)CuCl | None | 5b |
| 7 | Bneop (1a) | I (2a) | None | 7.5% Na[Mn(CO)5] | 0b |
| 8 | Bpin (1b) | I (2a) | 15% (IPr)CuCl | 7.5% Na[Mn(CO)5] | 69c |
| 9 | B(OH)2 (1c) | I (2a) | 15% (IPr)CuCl | 7.5% Na[Mn(CO)5] | 42c |
| 10 | Bneop (1a) | Br (2b) | 15% (IPr)CuCl | 7.5% Na[Mn(CO)5] | 13c |
| 11d | Bneop (1a) | Br (2b) | 15% (IPr)CuCl | 7.5% Na[Mn(CO)5] | 66c |
| 12 | Bneop (1a) | OTs (2c) | 15% (IPr)CuCl | 7.5% Na[Mn(CO)5] | 0c |
| 13d | Bneop (1a) | OTs (2c) | 15% (IPr)CuCl | 7.5% Na[Mn(CO)5] | 74c |
| 14 | Bneop (1a) | I (2a) | 15% (IPr)CuCl | 3.8% Mn2(CO)10 | 56c |
Ketone 3 did not form in high yield when 1-bromooctane (2b) or 1-octyltosylate (2c) were used in place of 2a, but reactivity was restored when these reactions were run in the presence of stoichiometric tetrabutylammonium iodide, presumably via the in situ formation of 2a (Entries 10–13). Lastly, ketone 3 was formed with only slightly compromised yield when using 0.5 Mn2(CO)10 in place of Na[Mn(CO)5] (Entry 14). This result is noteworthy for the use of (IPr)CuCl and Mn2(CO)10 co-catalysts, both of which are commercially available and stable to air and moisture. We verified that no non-carbonylative Suzuki–Miyaura (alkylated arene), Heck–Breslow (ester), or Williamson (ether) products were formed in these reactions. Rather, the mass balance consists of unidentified decomposition products.
In order to investigate functional group compatibility relevant to the synthesis of complex and functionally dense ketone products, we used the carbonylative formation of 3 to conduct a Glorius robustness screen.36 The formation of 3 was found to be robust towards a remarkable range of remote functional groups (Fig. S1†). Strongly electrophilic groups such as aldehydes, esters, and nitriles are tolerated, demonstrating the judicious choice of organocopper nucleophiles in this system. Nucleophilic groups such as a sulfur heterocycle and an unprotected primary amine also were tolerated. Intriguingly, an aryl bromide additive did not have measureable impact on the formation of 3 and was inert under the reaction conditions. This observation is particularly noteworthy, as typical Pd catalysts would readily activate C(sp2)–Br bonds. Only mild poisoning was observed with protic additives such as alcohols and terminal alkynes. Pyridine and N-methyl benzamide were the only additives definitively not tolerated in this robustness screen because they inhibited the reaction or were unstable under the conditions, respectively. Collectively, these results demonstrate that the Cu/Mn-catalyzed carbonylative coupling reaction is ideal both for late-stage functionalization and for the synthesis of ketones bearing latent synthetic handles useful for further elaboration, with only a small number of exceptions. It is noteworthy that many of the functional groups tolerated here would be incompatible with traditional Friedel–Crafts acylation conditions.
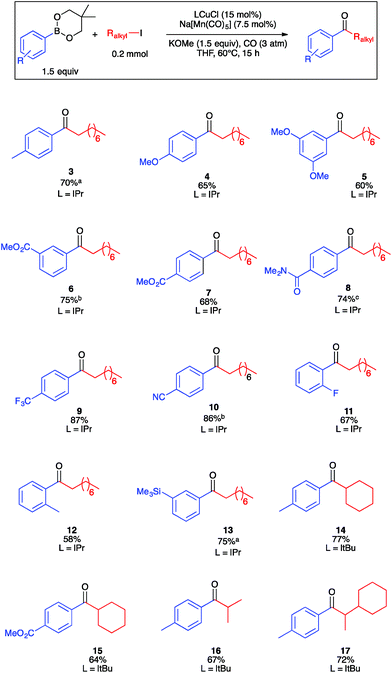
Guided by the results of the robustness screen, we next conducted substrate scope studies to examine steric and electron constraints on the coupling mechanism (Fig. 2). Electron-rich, electron-poor, and sterically encumbered arylboronic esters underwent carbonylative coupling with 2a efficiently to generate ketones 3–13 in moderate to high yields. It is noteworthy that several of these ketones represent aromatic regioisomers that would be unavailable by Friedel–Crafts acylation. A secondary alkyl iodide, iodocyclohexane, underwent carbonylative coupling with 1a in only 10% yield under the optimized conditions. We reasoned that formation of cyclohexyl-substituted ketone 14 may be slow due to the relevant secondary manganese-acyl intermediate being more sterically hindered, and therefore less electrophilic, than the corresponding primary intermediate derived from 2a. Using the smaller and more electron-donating ItBu in place of IPr provided 14 in excellent yield. Under these conditions, ketone 15, which is a synthetic precursor to a glucagon receptor modulator marketed by Pfizer,37 also was synthesized in high yield. Acyclic secondary alkyl iodides also reacted smoothly, allowing for the isolation of ketones 16 and 17. The formation of chiral 17 opens the opportunity for future development of asymmetric catalysis for the enantioselective formation of ketones with α-stereocenters, a possibility that would be challenging for Pd-catalyzed carbonylation. Ketones derived from benzyl or allyl electrophiles were not observed, presumably due to their instability under the basic reaction conditions. Another limitation of the method is that ketones derived from vinyl- or alkynylboronic esters were not observed, presumably because they are susceptible to conjugate addition under the reaction conditions. Lastly, various heteroarylboronic ester nucleophiles that we examined did not undergo productive carbonylative coupling with 2a.
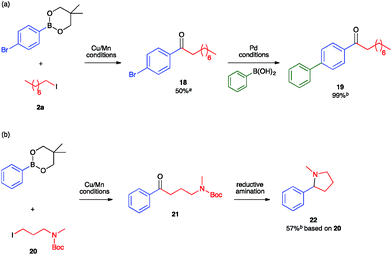
To leverage the assets of our method, we sought both to synthesize ketones bearing synthetic handles for further elaboration and to construct ketones that can serve as synthons for privileged structures relevant to drug molecules. First, to demonstrate the orthogonality of Cu/Mn catalysis and Pd catalysis, we conducted the Cu/Mn-catalyzed carbonylative coupling reaction with an arylboronic ester nucleophile containing an aryl-bromide linkage that would be unstable under Pd-catalyzed conditions (Fig. 3a). Isolation of the resulting ketone 18 proceeded smoothly without activation of the C(sp2)–Br position. Further elaboration at that position was then demonstrated through its use as the electrophilic component of a traditional Pd-catalyzed Suzuki–Miyaura coupling reaction, which provided the 4-biphenyl ketone 19 quantitatively. Second, to demonstrate utility towards the synthesis of drug molecules, we conducted the Cu/Mn-catalyzed carbonylative coupling with 3-aminoalkyl iodide 20, which provided the 3-aminoalkyl ketone 21 (Fig. 3b). Subjecting a crude sample of 21 to standard N-Boc deprotection and reductive amination conditions provided the 2-arylpyrrolidine product 22 in 57% yield from 20. The C(sp3)-C(sp2) linkage α to the nitrogen in 22 was installed by Cu/Mn-catalyzed C–C coupling. Pyrrolidines and related C(sp3)-rich heterocycles are known to be privileged core structures in drug molecule candidates, and the installation of aromatic substituents in the position α to the heteroatom in such structures by coupling methods is a longstanding challenge actively being pursued by several research groups.38–42 Here, the construction of such a motif is enabled by the unique reactivity of the Cu/Mn-catalyzed coupling reaction towards C(sp3)-hybridized substrates.
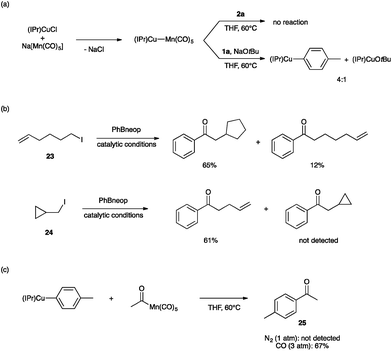
Lastly, we sought to probe the viability of our hypothetical mechanism through stoichiometric reactivity studies. First, because (IPr)Cu–Mn(CO)5 is expected to assemble upon mixing (IPr)CuCl and Na[Mn(CO)5],35 we sought to establish its role in the bimetallic catalysis (Fig. 4a). The metal–metal bonded complex (IPr)Cu–Mn(CO)5 was found to be unreactive towards 2a in THF at 60 °C. On the other hand, exposing this metal–metal bonded complex to NaOtBu and 1a under the same conditions led to a mixture of (IPr)CuOtBu, (IPr)Cu(tol) (tol = p-tolyl), and presumably Na[Mn(CO)5]. Based on these observations, we propose that the metal–metal bonded complex (IPr)Cu–Mn(CO)5 is not an on-cycle catalytic intermediate capable of activating any of the coupling partners directly, but rather it is unstable under the basic conditions of catalysis towards formation of (IPr)CuOMe and the “unmasked” K[Mn(CO)5]. Alkylation of [Mn(CO)5]− by iodoalkanes is well known20 and is thought to proceed by a single-electron transfer mechanism.21,22 Consistent with this proposal, we observed radical cyclization/ring-opening behavior with the radical clock iodoalkanes 23 and 24 (Fig. 4b). Lastly, the ketone-generating, bimetallic C–C coupling step between the arylcopper and acylmanganese intermediates has no precedent in the literature. In order to establish feasibility of this mechanistically novel C–C coupling step, we examined reactivity between isolated samples of (IPr)Cu(tol) and MeC(O)Mn(CO)5 (Fig. 4c). When the experiment was conducted in THF at 60 °C under N2 atmosphere, no 4-methylacetophenone (25) was observed. However, when the same reaction was conducted under an atmosphere of CO (3 atm), the expected ketone product 25 was formed in 67% yield. These results are consistent with the product-releasing C–C bond formation being viable but having to compete with de-insertion of CO, thus requiring the application of CO pressure. This stoichiometric C–C coupling reaction demonstrates the key role of a novel heterobimetallic C–C bond-forming step in the catalytic generation of ketones.
Conclusions
Bimetallic catalysis with earth-abundant Cu and Mn was leveraged to discover C–C coupling chemistry that complements existing methods with single-site Pd catalysis. Conceptually, the bimetallic scheme is novel in the context of transformations featuring two co-dependent catalytic metals in that it does not utilize Pd. A limitation of Pd catalysis, namely the inability to efficiently carbonylate C(sp3)-hybridized electrophiles, was thus overcome.Acknowledgements
Funding was provided by the NSF (CHE-1362294) and the ACS Green Chemistry Institute (Pharmaceutical Roundtable Grant). N. P. M. is an Alfred P. Sloan Research Fellow. Prof. Justin Mohr provided access to a GC.Notes and references
- A. Brennführer, H. Neumann and M. Beller, Angew. Chem., Int. Ed., 2009, 48, 4114–4133 CrossRef PubMed.
- X.-F. Wu, H. Neumann and M. Beller, Chem. Soc. Rev., 2011, 40, 4986–5009 RSC.
- C. F. J. Barnard, Organometallics, 2008, 27, 5402–5422 CrossRef CAS.
- F. Garrido, S. Raeppel, A. Mann and M. Lautens, Tetrahedron Lett., 2001, 42, 265–266 CrossRef CAS.
- R. F. W. Jackson, D. Turner and M. H. Block, J. Chem. Soc., Chem. Commun., 1995, 2207–2208 RSC.
- M. Medio-Simón, C. Mollar, N. Rodríguez and G. Asensio, Org. Lett., 2005, 7, 4669–4672 CrossRef PubMed.
- J. Sävmarker, J. Lindh and P. Nilsson, Tetrahedron Lett., 2010, 51, 6886–6889 CrossRef.
- Y. Wakita, T. Yasunaga, M. Akita and M. Kojima, J. Organomet. Chem., 1986, 301, C17–C20 CrossRef CAS.
- X.-F. Wu, H. Neumann and M. Beller, Tetrahedron Lett., 2010, 51, 6146–6149 CrossRef CAS.
- T. Ishiyama, N. Miyaura and A. Suzuki, Tetrahedron Lett., 1991, 32, 6923–6926 CrossRef.
- Y. Tamaru, H. Ochiai, Y. Yamada and Z. Yoshida, Tetrahedron Lett., 1983, 24, 3869–3872 CrossRef CAS.
- T. Kondo, Y. Tsuji and Y. Watanabe, J. Organomet. Chem., 1988, 345, 397–403 CrossRef CAS.
- S. Sumino, T. Ui and I. Ryu, Org. Lett., 2013, 15, 3142–3145 CrossRef CAS PubMed.
- L. J. Goossen and K. Ghosh, Angew. Chem., Int. Ed., 2001, 40, 3458–3460 CrossRef CAS PubMed.
- L. S. Liebeskind and J. Srogl, J. Am. Chem. Soc., 2000, 122, 11260–11261 CrossRef CAS.
- H. Tokuyama, S. Yokoshima, T. Yamashita and T. Fukuyama, Tetrahedron Lett., 1998, 39, 3189–3192 CrossRef CAS.
- A. C. Wotal, R. D. Ribson and D. J. Weix, Organometallics, 2014, 33, 5874–5881 CrossRef CAS PubMed.
- M. Oçafrain, M. Devaud, M. Troupel and J. Périchon, J. Chem. Soc., Chem. Commun., 1995, 2331–2332 RSC.
- C. Zhao, X. Jia, X. Wang and H. Gong, J. Am. Chem. Soc., 2014, 136, 17645–17651 CrossRef CAS PubMed.
- R. F. Heck and D. S. Breslow, J. Am. Chem. Soc., 1963, 85, 2779–2782 CrossRef CAS.
- T. Kondo, Y. Sone, Y. Tsuji and Y. Watanabe, J. Organomet. Chem., 1994, 473, 163–173 CrossRef CAS.
- C. M. McMahon, M. S. Renn and E. J. Alexanian, Org. Lett., 2016, 18, 4148–4150 CrossRef CAS PubMed.
- J. A. R. Schmidt, E. B. Lobkovsky and G. W. Coates, J. Am. Chem. Soc., 2005, 127, 11426–11435 CrossRef CAS PubMed.
- M. Mulzer, B. T. Whiting and G. W. Coates, J. Am. Chem. Soc., 2013, 135, 10930–10933 CrossRef CAS PubMed.
- M. S. Kharasch and P. O. Tawney, J. Am. Chem. Soc., 1941, 63, 2308–2316 CrossRef CAS.
- D. R. Pye and N. P. Mankad, Chem. Sci., 2017, 8, 1705–1718 RSC.
- K. Sonogashira, J. Organomet. Chem., 2002, 653, 46–49 CrossRef CAS.
- L. K. G. Ackerman, M. M. Lovell and D. J. Weix, Nature, 2015, 524, 454–457 CrossRef CAS PubMed.
- S. D. Friis, M. T. Pirnot and S. L. Buchwald, J. Am. Chem. Soc., 2016, 138, 8372–8375 CrossRef CAS PubMed.
- P. García-Domínguez and C. Nevado, J. Am. Chem. Soc., 2016, 138, 3266–3269 CrossRef PubMed.
- J. J. Hirner, Y. Shi and S. A. Blum, Acc. Chem. Res., 2011, 44, 603–613 CrossRef CAS PubMed.
- K. Semba and Y. Nakao, J. Am. Chem. Soc., 2014, 136, 7567–7570 CrossRef CAS PubMed.
- Y. Zhou, W. You, K. B. Smith and M. K. Brown, Angew. Chem., Int. Ed., 2014, 53, 3475–3479 CrossRef CAS PubMed.
- M. H. Pérez-Temprano, J. A. Casares and P. Espinet, Chem.–Eur. J., 2012, 18, 1864–1884 CrossRef PubMed.
- S. Banerjee, M. K. Karunananda, S. Bagherzadeh, U. Jayarathne, S. R. Parmelee, G. W. Waldhart and N. P. Mankad, Inorg. Chem., 2014, 53, 11307–11315 CrossRef CAS PubMed.
- K. D. Collins and F. Glorius, Nat. Chem., 2013, 5, 597–601 CrossRef CAS PubMed.
- B. J. Simmons, N. A. Weires, J. E. Dander and N. K. Garg, ACS Catal., 2016, 6, 3176–3179 CrossRef CAS.
- Z. Zuo, D. T. Ahneman, L. Chu, J. A. Terrett, A. G. Doyle and D. W. C. MacMillan, Science, 2014, 345, 437–440 CrossRef CAS PubMed.
- J. E. Spangler, Y. Kobayashi, P. Verma, D.-H. Wang and J.-Q. Yu, J. Am. Chem. Soc., 2015, 137, 11876–11879 CrossRef CAS PubMed.
- J. Cornella, J. T. Edwards, T. Qin, S. Kawamura, J. Wang, C.-M. Pan, R. Gianatassio, M. Schmidt, M. D. Eastgate and P. S. Baran, J. Am. Chem. Soc., 2016, 138, 2174–2177 CrossRef CAS PubMed.
- M. H. Shaw, V. W. Shurtleff, J. A. Terrett, J. D. Cuthbertson and D. W. C. MacMillan, Science, 2016, 352, 1304–1308 CrossRef CAS PubMed.
- K. M. M. Huihui, J. A. Caputo, Z. Melchor, A. M. Olivares, A. M. Spiewak, K. A. Johnson, T. A. DiBenedetto, S. Kim, L. K. G. Ackerman and D. J. Weix, J. Am. Chem. Soc., 2016, 138, 5016–5019 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7sc01170a |
| This journal is © The Royal Society of Chemistry 2017 |

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
: