Blending problem-based learning and peer-led team learning, in an open ended ‘home-grown’ pharmaceutical chemistry case study
Clinton G. L.
Veale
 *a,
Rui W. M.
Krause
*a,
Rui W. M.
Krause
 b and
Joyce D.
Sewry
b
b and
Joyce D.
Sewry
b
aFaculty of Pharmacy, Rhodes University, Grahamstown, 6140, South Africa
bDepartment of Chemistry, Rhodes University, Grahamstown, 6140, South Africa. E-mail: C.Veale@ru.ac.za; Tel: +27-46-603-8096
First published on 10th October 2017
Abstract
Pharmaceutical chemistry, medicinal chemistry and the drug discovery process require experienced practitioners to employ reasoned speculation in generating creative ideas, which can be used to evolve promising molecules into drugs. The ever-evolving world of pharmaceutical chemistry requires university curricula that prepare graduates for their role as designers with the capability of applying complex concepts in pharmaceutical chemistry, thereby improving the decision-making process. Common methods of teaching drug discovery, including the linear nature of the traditional case study model, do not provide a realistic picture of the underlying complexity of the process, nor do they equip students with the appropriate tools for personal sense making and abstraction. In this work, we discuss the creation of an open-ended, nonlinear case study for 3rd year pharmaceutical chemistry students, developed from drug discovery research conducted at Rhodes University. Furthermore, we discuss blending problem based learning (PBL) with peer-led team learning (PLTL) in the context of curriculum transformation, underpinned by the theory of semantic waves, to assist students in the early attainment of abstract concepts and answer questions of contextualisation, personal sense making, relatability, relevance and ultimately the skills for lifelong learning.
Introduction
Pharmaceutical chemistry that encompasses drug discovery and medicinal chemistry is a discipline at the intersection of chemistry, pharmacology, and biochemistry (Holbrook and Garneau-Tsodikova, 2017). In their simplest form, medicines are chemicals, and their biological interactions are mediated by basic chemical and physical principles. However, these need to be crafted into discipline specific concepts such as lipophilicity, solubility, metabolic stability, and how these relate to the patient interaction in terms of half-life, dosing intervals, route of administration etc.Recently, Rafferty spoke to problems within the pharmaceutical industry regarding the appropriate training of candidates for drug discovery (Rafferty, 2016). He discussed the traditional preference for candidates trained in organic chemistry, who are retrofitted with skills in medicinal chemistry via ‘on-the-job’ training. It is this lack of in-depth drug discovery and pharmaceutical chemistry training, which he argues leads to poor decision making, that ultimately compounds the high attrition rates of new chemical entities observed in the pharmaceutical industry. He goes on to argue that the ever-evolving world of pharmaceutical chemistry requires graduates who have received formalized training that emphasizes the core principles and practices of drug design, who can rather be retrofitted as organic chemists. Furthermore, he states that globally in academia, many curricula do not prepare graduates for their role as designers (Rafferty, 2016). Importantly, McInally and Macdonald, responding to Rafferty, reported a comprehensive program between the University of Nottingham and Glaxo-Smith Kline, which focusses on teaching appropriate principles of drug discovery, in a method differentiated from traditional chemistry curricula (McInally and Macdonald, 2017). However, we have noted that even within formalized pharmaceutical chemistry curricula, where these core principles are a central pillar, undergraduate students are not necessarily equipped to apply these complex principles outside of the specific context in which they were taught. Therefore, conceptualisation and mastery of these concepts is not adequately achieved to alleviate the issues identified by Rafferty. Furthermore, in the context of large class sizes and limited resources, the model of McInally and Macdonald is not necessarily practical. In this paper, we discuss our approach of blending problem based learning (PBL) with peer-led team learning (PLTL) in the context of curriculum transformation, underpinned by the theory of semantic waves, to answer questions of contextualisation, personal sense making, relatability, relevance and ultimately the skills for lifelong learning, for skills in drug discovery for 3rd year pharmaceutical chemistry students.
Drug discovery education
Conventionally, the concepts of drug discovery are illustrated through a series of examples, where a specific problem is presented, e.g. poor solubility of a lead compound, and possible solution offered, e.g. addition of an ionisable functional group (Fig. 1). Examples such as these can be used for all concepts in drug discovery, and can be further discussed in terms of their potential benefits and drawbacks, e.g. amino groups are susceptible to dealkylation and conjugation, therefore accelerating metabolism.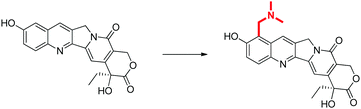 | ||
| Fig. 1 An example of a modification of a molecule in order to improve water solubility (Li et al., 2006). This example is discussed in terms of why the highlighted modification would increase solubility, as well as the impact it may have on other important elements such as metabolism, absorption, dosing etc. However, this is a complex problem and the nature of the example may give an incorrect perception of the time taken in solving this problem. | ||
The desired learning outcome is to understand why these molecular changes were made, and how they relate to overall pharmacological outcomes, rather than knowing a specific transformation. However, when viewing these examples in isolation the assumption is often made that a student will see these examples as demonstrations of a complex principle, which can rationally be applied to any unknown example. This is particularly problematic considering the inherent complexity of the drug discovery process and the sheer volume of variables in any given drug design choice.
Case studies are commonly used as complimentary methods to synthesise and contextualise theoretical content. Generally, case studies discuss the discovery of a medicine, highlighting areas related to drug discovery principles along the way. The nature of this format means that the story follows a linear path, concluding in the development of a medicine such as atorvastatin (Roth, 2002) (Fig. 2).
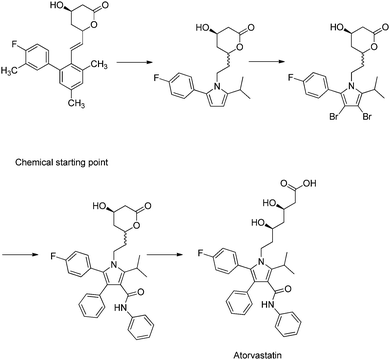 | ||
| Fig. 2 A case study, which describes a linear progression toward a drug, in this case atorvastatin (Roth, 2002). The narrative of the case study implies that atorvastatin was the logical goal, as if it is a perfect correct answer. However, this molecule is the acceptable balance of several complex criteria. | ||
Unfortunately, this gives the incorrect perception to the unknowing observer, that the drug was discovered by an expert practitioner solving a series of problems in isolation, eventually culminating in a new medicine as a final perfect solution. No actual context is given as to the depth of each problem, and how a single chemical modification can alter several important properties, which need to be investigated. In reality, each step in the process of designing new medicines has no correct solution, but rather an acceptable balance of parameters, which are brought about by analysing hundreds of modified compounds.
While the concepts that Rafferty (2016) identified as critical in medicinal chemistry education are discussed, the fundamental skills that address decision making in the discovery of a new drug are not adequately covered. The question we have asked is: can we find an open-ended model for case study, which does not follow the linear model? And further, can this be used as a tool for students to apply theoretical principles broadly to creatively assist in the case study, thereby facilitating deep learning (Biggs, 1999), and higher order cognition? Additionally, can this be used as a tool to motivate large classes in excess of 150 students to engage with course material across the spectrum of their degree and can it motivate some students to enter post graduate study?
Semantic waves in pharmaceutical chemistry
The concept of semantic waves, first described by Maton (2011, 2013) and which has recently been applied in the context of chemistry by Blackie (2014), is the relationship between abstraction and complexity. Chemistry, and by extension pharmaceutical chemistry, is described using information dense symbolism. Furthermore, chemistry is not intuitive, since we cannot directly observe chemistry, only its resultant effects.Semantic gravity is the degree to which context and meaning are related (degree of abstraction), and semantic density relates to the volume of meaning condensed within a symbol (complexity). When placed on overlapping axes, we can assign the relationship into one of four quadrants. In general, as Blackie describes, chemistry occupies the upper right quadrant (Fig. 3), combining weak semantic gravity with strong semantic density. In other words, abstract concepts are described using information dense symbolism. The chemical structure of penicillin, for example, could be considered as having strong semantic density, while how this relates to the other important parameters of what makes a good drug is far more abstract, requiring an assimilated bulk of previous knowledge, hence giving it a low semantic gravity and placing it in the top right quadrant. Blackie (2014) goes on to argue, that to help students progress from the bottom left quadrant into the top right, teaching should progress via both the top left quadrant (initially weaken semantic gravity by introducing abstract concepts) as well as the bottom right quadrant (initially strengthen semantic density by introducing context specific symbolism). This allows knowledge to be transferred between simpler, context dependent meanings, and complex decontextualized meanings.
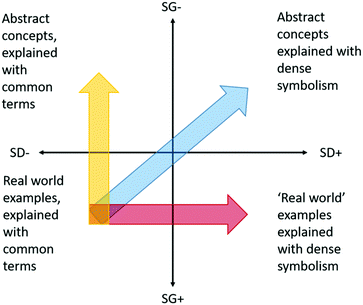 | ||
| Fig. 3 Adaptation of Maton's semantic gravity and density axes (Maton, 2011, 2013). The goal of teaching is to take students from the bottom left quadrant into the upper right. Maton and Blackie argue that this understanding should be achieved going via both the bottom right and top left quadrants, moving back and forth as needed. | ||
Blackie (2014) points out that in chemistry, weakening semantic gravity is more challenging than increasing semantic density, and that tasks such as written tests and exams tend to favour attaining knowledge via the bottom right quadrant, where real world examples can be applied to give the illusion of abstract understanding, such as is the case with Fig. 1 and 2. As Blackie describes, this results in students not remembering abstract concepts, since they were never mastered in the first instance. In contrast, tasks which examine the logic behind a decision require a descriptive answer. This results in an earlier attainment of abstract concepts, thus facilitating movement into the top right quadrant via the top left quadrant, or deep learning. This is somewhat analogous to Biggs’ deep and surface learner concept (Biggs, 1999).
It is likely that assessment of the progression through the upper left quadrant may benefit from a more formative approach to assessment, since it is the logic of choices, rather than the choice itself which is the desired outcome of teaching. Science educators in higher education are becoming increasingly cognisant that for students to become scientifically literate members of society, their learning and understanding must encompass more than the content of a curriculum in order to foster higher order cognitive skills (Kishbaugh et al., 2012) an idea stemming from Bloom's taxonomy (Krathwohl, 2002). The question remains; how can this be applied to the teaching and assessment of pharmaceutical chemistry? Sevian and Talanquer (2014) have argued that conceptualisation is a process of forming abstractions from a sensory experience, which needn’t rely on language, while Taber has called for a reformative shift in chemical education, allowing for modelling and sense making, through active involvement and authentic investigation (Taber, 2015), which has traditionally been lacking in general chemical education (Van Berkel et al., 2000). An approach which incorporates these concepts would arguably assist in the transition into the top right quadrant via the top left.
The fundamental theme of the theories discussed above suggests that facilitation of abstraction, conceptualisation, contextualisation, personal sense making, reflection and ultimately the skills for lifelong learning, requires a space for students to play with, and share ideas, discuss, speculate, and generate solutions to broad problems. This can be provided in addition to traditional methods of assessment such as tests and examination.
Open ended ‘home-grown’ case study
A major discussion point in South African higher education is that of post-apartheid curriculum transformation and decolonisation (Cloete et al., 2004). This is a multifaceted and complicated problem, which questions our approach to pedagogy and knowledge systems (Watson-Verran and Turnbull, 1995) as well as what content we choose to populate our courses. In that regard, we decided to develop a case study based on work conducted at our university.Through post-graduate led investigations, our research group has generated a series of roughly 50 new antimalarial compounds which inhibit malaria growth in infected red blood cells with varying success (Svogie et al., 2016). While the mechanism of action of these compounds is currently unknown, each member of this series was designed to answer specific structure activity relationship (SAR) questions. Albeit on smaller scale, this approach is comparable to the very early stages of the drug discovery pipeline.
As is normally the case with this early stage, we have limited information on this class of compounds. Relevant chemical questions currently being asked include: which molecular regions are critical for activity; are these regions important because of an inherent chemical property or the chemical space it occupies; what can be changed; and most importantly, what impact will modifications have on important drug properties?
To illustrate this, we understand that a chlorine substitution at the C-6 position is less active than a C-5 subsisted product (Fig. 4). We also know that changing the sulfur atom for a nitrogen increases toxicity. These are two examples of many small studies we have conducted to elucidate the SAR.
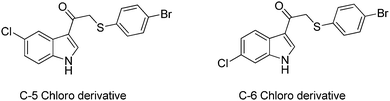 | ||
| Fig. 4 Two analogues in our study. Alteration of the position of the substituted chlorine significantly influenced antimalarial activity (Svogie et al., 2016). This can be unpacked in terms of a variety of related principles, leading to a non-linear case study, with enough depth to assist in student participation and self-sense making. | ||
Problem-based learning and peer-led team learning
Successful scientific programmes rely on teams where individuals, each with their own unique skill sets and viewpoints, debate and negotiate and attempt to solve complex problems through well designed trial and error (Gosser et al., 2010). Similarly, the process of trial and error based molecule design has been applied as a useful approach to teach drug discovery (Meisenheimer et al., 1982), and is, in essence, the philosophy behind PBL. PBL is an increasingly common pedagogical tool which revolves around independent or collaborative problem solving tasks, assisting in developing fundamental understanding of course content through the facilitation of deeper engagement (Ram, 1999; Mc Donnell et al., 2007; Page, 2013). With respect to our proposed case study, we felt that the fact that the work itself is unfinished and ongoing presented an opportunity for students to engage in the case study in a manner which would facilitate sense making and understanding. To this end, students were divided into teams of nine or ten students, each effectively forming a drug discovery panel. Each panel was asked to discuss and reflect on the SAR data as it currently stands. What do we know? What data are missing? From this engagement, the groups were asked to design five new molecules which ask pertinent questions in the optimisation of this class of compounds. In addition, we felt that this process could be enhanced by PLTL.Similarly to PBL, PLTL was originally conceived as a social learning tool for an active educational experience, developing skills in problem solving, communication and leadership, which were designated as critical work place skills (Gosser et al., 1996). This also resulted in improvements in student performance and interest in the course material (Woodward et al., 1993). Research conducted on the use of peer-led team learning has suggested that in contrast to lectures, the reduced hierarchy and approachability of peer-leaders forms a hospitable academic environment, resulting in lowered anxiety, a greater desire for students to express themselves and explore a variety of options even if they may be considered mistakes (Gosser et al., 2010). Accordingly, this provides a space for students to develop and make sense of concepts as well as construct meaning, through collaborative tasks (Bodner, 1986; Driver et al., 1994; Watson, 2001). PLTL can enhance the PBL mediated cognitive progression into the top right quadrant, via the top left, as discussed by Blackie. Peer-leaders not only serve as facilitators of group discussion and debate, they are also viewed as role models, who have succeeded from an intellectual and social viewpoint, within the paradigms of the specific course and the culture of the institution (Wilson and Varma-Nelson, 2016), which is particularly important in the South Africa context.
Therefore, the use of peer-leaders seemed ideal for the drug discovery ‘panel’. Accordingly, each group was assisted by a 4th year student peer-leader, whose role was to facilitate and mediate group discussion that lessens the student – teacher hierarchy. Peer-leaders, were 4th year pharmacy students who had been through the same curriculum as the current third years (with the exception of this case study) only one year previously, and could relate to similar frustrations with the content, workloads, campus life and stress factors associated with success. They could use their own experiences and learning mechanisms to coach the 3rd years into understanding drug discovery. Importantly, the majority of peer-leaders did not join this program out of a simple love of chemistry, but rather a love of education and a desire to be the mentors they wish they had during their degrees.
Methodology
Ethical clearance
The Rhodes University Faculty of Pharmacy ethics committee approved this research. Following ethical clearance, permission to collect data was obtained from the Rhodes University registrar. All experiments complied with relevant laws and guidelines of Rhodes University. Informed consent was obtained for all participants.Work flow
Prior to the start of the course, the 4th year peer-leaders received training regarding the case study, how it relates to their previous course content, what we were aiming to achieve, and specifically, what the role of a peer-leader is. The 3rd years were then systematically taken through the case study as it currently stands, by combining reading the paper (Svogie et al., 2016), with structured lectures highlighting what work has been done, and how the data has subsequently been interpreted. This process was used to slowly build up a hypothetical drug-receptor model, and structured to offer the students clues as to what good initial lines of investigation might be. For example, if we refer back to the position of the chlorine atom (Fig. 4), we can begin unpacking this idea around discipline specific principles, such as hydrophobicity, electron withdrawing nature, electrostatics, atomic radius etc. Students are already exposed to theory, which teaches them that a drug needs to interact with a putative drug receptor in a certain manner to exert an effect. This interaction can be enhanced, or diminished, depending of the position and nature of chemical functionality. In addition, for a drug to interact with said receptor, it needs to be lipophilic enough to penetrate the relevant cell, as well as be hydrophilic enough to dissolve in biological media.So, the question as to why the C-5 chlorine analogue is more active than the C-6 immediately provides a multicomponent problem, which requires students to engage with their theory to make sense of the problem. Covalently bonded chlorine atoms are known to increase lipophilicity, and enhance cellular penetration of organic molecules. A reasonable question would then be: is the increased activity of the molecule due to the chlorine enhanced cellular penetration? However, this needs to be seen in the context of the C-6 chlorinated analogue, which would impart a similar degree of lipophilicity, yet is less active. Therefore, what we know is that the C-5 chlorine improves activity, and that in addition to increased lipophilicity, the position of the substituent is important.
To confirm the importance of lipophilicity, we could mimic the lipophilic nature of chlorine through a bioisosteric methyl group. Group 7 elements are electron withdrawing, and can affect the electronic environment of the overall molecule. Would replacing the chlorine with an electron withdrawing cyano group tell us anything new? Other group 7 elements such as fluorine, bromine and iodine have different atomic radii, and therefore occupy different volumes of chemical space. Would these possibly enhance interactions with the receptor? How would this affect solubility, absorption, metabolism, excretion, and toxicity? If we add lipophilic groups to enhance a certain effect, should we consider modifying other regions of the molecule to balance water solubility? In other words, instead of there being a simple solution, it is a complex, multifaceted problem that needs to be considered from many sides to move forward.
After the initial data interrogation, 3rd year students were allocated two 1.5 h slots with their peer-leaders, in which time they were encouraged to discuss ideas as a panel. Students were grouped according to the alphabetical order of their first names, in order to avoid the more common last name group assignment. During the first slot, their first task was to suggest five new molecules which ask pertinent questions in the optimisation of this class of compounds. To this end, the students were not limited to a specific question, or problem, nor were parameters set for these new molecules, other than that they were required to provide well justified reasons for their modifications, and what they hope to learn from these. They had to inspect the data, identify a potential problem, and ask a relevant research question. Two weeks after their first meeting, they were then asked to share their ideas as a short five-minute presentation where they were expected to provide reasons, underpinned by theory, for their design choices. Furthermore, the class forum was open for questions and feedback from the class. In the second slot, students met in their groups to reflect on their progress and the feedback from the class forum, as well as what they had learned from the other groups’ presentations. They were then required to submit a five-page group report, detailing their final molecules and the underlying choices. Prior to the presentations and report submissions, we were pleased to learn that many of the groups and their peer-leaders had arranged additional meetings to further discuss their projects and ideas.
Learning outcome and assessment
This case study is unique in that there is deliberately no correct answer. Students and peer-leaders would be expected to speculate and engage with the theory and involve themselves in an investigation into the possible way forward for our new class of antimalarial compounds. Furthermore, we aimed at developing critical cross-field outcomes through skills in problem solving, team-work, time management, and oral and written communication (Deborah, 2006; Page, 2013). Instead of a student being able to produce a ‘correct’ answer, a successful student/group would be one who can demonstrate understanding of the process through a well-motivated descriptive answer. If an idea seems unusual to begin with, success could still be achieved if reasonable theory could be applied, i.e. will this question possibly give new insight? Thus, conceptualisation of abstract concepts can be constructed, via an initial weakening of semantic gravity. Third year students (both the presenters and the audience) displayed high levels of enthusiasm during the presentations. They seemed keen on challenging their colleagues, without any noticeable aggression. Overall, we were pleased with the level at which the students engaged with the content. In addition to the use of appropriate terminology, the presentations and reports showed evidence of good engagement with the literature.In one illustrative example, a specific molecular modification was justified, because a similar chemical moiety was present in an anti-malarial natural product they had encountered in their research.
The important element of this example is that in a previous course we had a series of lectures discussing the importance of natural products in drug discovery due to the novel chemical structures inherent in nature. This group combined content engagement, with authentic investigation, to rationally inform their drug design process. This allowed for complex concepts to be aligned and synthesised into a deeper understanding of the process, thus progressing into the top right quadrant. Other examples included groups commenting on how their modifications would alter drug metabolism and half-life, and how this would relate to patient acceptability in terms of dosing. This, amongst numerous examples, indicated to us that the groups had sought to integrate knowledge from various parts of the course, to assist in their personal sense making of pharmaceutical chemistry as a whole, and its place in pharmacy.
Results and discussion
Following the completion of the presentations, consenting students and peer-leaders were asked to complete a questionnaire, designed to gauge student and peer-leader perceptions of the process, including, PBL, PLTL, and a home-grown example.In addition, a semi-structured interview was conducted with the peer-leaders as a group to allow them freedom to comment outside of the structured questions.
Relative difficulty of tasks
Based on the structure of the assignment, we had identified six elements as possible practical barriers to success namely:(a) Working in a group
(b) Design of new molecules
(c) Rationalisation of choices using theory
(d) Presentation of results
(e) Responding to questions
(f) Written report
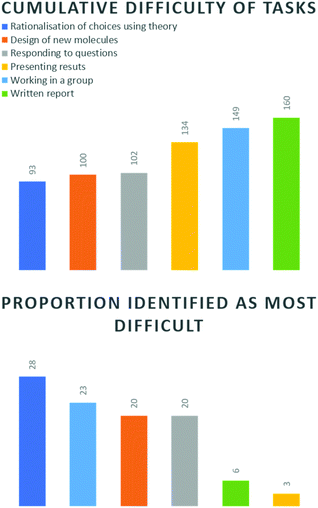
Applying the method of Mc Donnell et al. (2007) students were asked to rank these six fields in order of most to least difficult. The lowest cumulative score indicates the task which was found the most difficult overall (Fig. 5, top). Based on this analysis, rationalisation of choices using theory was considered the most challenging comparative aspect of this project. This was not altogether surprising, since this aspect required the greatest intellectual effort, and was ultimately where the core skill of this task lay.
Designing of new molecules and responding to questions were very close to each other in 2nd and 3rd place. This indicated to us that students identified that the task of designing a molecule and rationalisation and synthesis of knowledge were equally critical to success. Furthermore, once a sound rationalisation had been achieved, they felt more comfortable in being able to defend their work. From experience, students do not typically feel comfortable in being asked on-the-spot questions pertaining to their fundamental understanding of this subject, so we felt this was an encouraging result. Working in a group, presenting results and the written report, were perceived as comparatively less difficult. This might be due to the growing number of reports, both written and oral required at third-year level. However, the ranking of working in a group is worthy of later discussion.
We also inspected what proportion of each task was identified as the most difficult, i.e. percentage of ‘1’ rankings (Fig. 5, bottom). Again, the greatest proportion of students (28%) listed rationalisation of choices as the most difficult element and presenting (3%) and written report (6%) as the least difficult. Working in a group was identified by 23% of students as the most challenging element. This is interesting, since it speaks to the dynamics of a group. Cumulatively, most students did not find group work difficult (Fig. 5, top), however our data indicates that those who did, found it more difficult than designing new molecules. It also highlights the importance of appropriately considering how group work is perceived and how we deal with weight of opinion. Finally, the design of new molecules and responding to questions were both considered the most difficult element by 20% of the class, which correlated with the cumulative result.
Course structure
Students were asked a series of yes/no questions to directly identify their feeling of specific elements of the project. The questions were as follows.(a) Has this process helped develop your understanding of drug discovery?
(b) Was using a ‘home grown’ example beneficial? Briefly explain your choice below
(c) Did Group discussion assist the process of molecule design?
(d) Did you feel you had sufficient time to prepare your presentation and write up?
(e) Was this case study and process relevant to pharmaceutical chemistry 3?
(f) Were the peer-leaders punctual and professional?
(g) Did the peer-leaders facilitate the design process?
(h) Was the experience of the peer-leaders helpful in this process?
These questions were aimed at gauging the perceptions about the project (Fig. 6). Specifically, they look at whether this format of an open-ended home-grown case study and PLTL were considered valuable and relatable. Furthermore, owing to time constraints within the wider degree curriculum, we wanted to determine whether students felt as if they had sufficient time to prepare. 82% of students indicated that this process helped develop their understanding of drug discovery, while only 8% did not. A similar response was noted for the assistance derived from group discussion. One of the main goals of this project was to give students the space to discuss ideas in an unintimidating environment to develop their skills in the area, so this ratification was pleasing. The positive response was somewhat lower with regard to preparation time, with 79% of students feeling that they had sufficient time to prepare their presentations and write up, while 21% felt they needed more time. While in the minority, this was by far the most significant ‘no’ result.
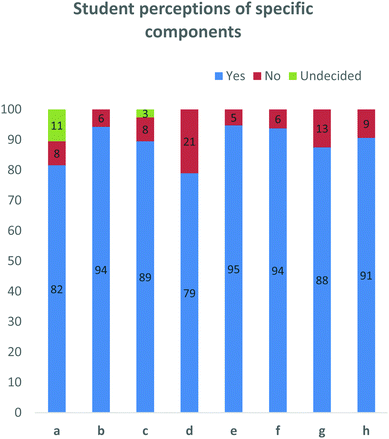 | ||
| Fig. 6 Data obtained from a series of yes/no questions. This data indicates that students, felt the format of this project including the contribution of the peer-leaders was helpful. | ||
This is an interesting problem to consider, since meetings and presentations are by their nature time consuming, which is amplified by their tight schedules, particularly as many groups made extra time for additional meetings. A possible solution is scheduling more official meeting times during lecture slots, which may result in fewer extracurricular meetings.
Students were also asked to rate the involvement of the peer-leaders in this project. 94% of students felt that the peer-leaders were punctual and professional. Furthermore, 88% of students felt that the peer-leaders facilitated the design process, while 91% of students indicated that the experience the peer-leaders had of the course, was helpful to the 3rd years.
Relevance
Students were also asked whether this case study and process was relevant to 3rd year pharmaceutical chemistry as well as whether using a home-grown example was beneficial. Overwhelmingly, 95 and 94% of participants respectively answered ‘yes’ to these questions (Fig. 6), while 100% of peer-leaders answered ‘yes’ to both questions (data not shown).To elaborate this important question, students were asked to explain why a home-grown example was beneficial. Comments from this question were grouped into common themes and from them, 17 comments included elements which linked to its relevance, while four comments spoke to the relatability of the example. It was noteworthy that seven comments spoke of the home-grown example being motivating or inspiring (Fig. 7).
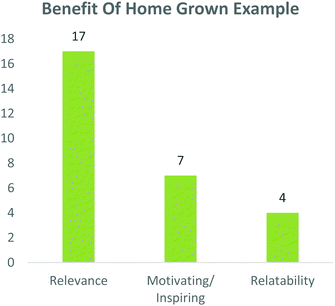 | ||
| Fig. 7 Students indicated that the use of a ‘home grown example’ was relevant, motivating and relatable. | ||
Some direct quotes lifted from the evaluations are as follows:
‘This gave us confidence and motivation as this was done by someone who we meet every day and talk to everyday. It proved that everything is possible’
‘Felt as if we were contributing to the University – pride’
‘Felt involved and that their knowledge was contributing’
‘Easier to relate to than classic examples which seem like a miracle’
The peer-leaders were also asked to comment on their experience of a home-grown example, from which similar trends emerged. Two quotes in particular spoke to the ultimate goal of this project.
‘It put science into an African context’
‘It helped show that people from the same system as us can make breakthroughs in novel thinking’
Higher education in South Africa is facing a shortage of quality individuals willing to enter higher degrees to stock the next generation of academics. A commonly cited reason is that students, simply do not think they can accomplish these feats. If we can manage to convince students that they are capable of quality research, then possibly we could begin to remedy the crisis (Akojee and Nkomo, 2007). It is from that point of view that comments mentioning that appropriate content contextualisation can convince students that they are capable of becoming quality scientists are exciting.
Positives and negatives
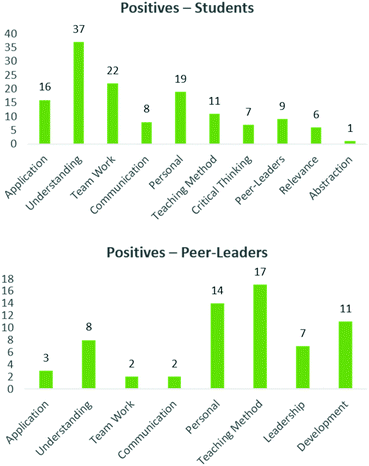
Having gauged the perceptions as to the structure and relevance of the course, both the peer-leaders and students were asked to identify five positives and negatives of their experiences in this programme, which were again grouped into common themes (Mc Donnell et al., 2007). Generally, the comments from positives were more diverse than the negatives, which tended to focus on specific elements, and will be expanded below. With respect to the positive elements, the majority of comments spoke to understanding and sense making (top right quadrant) (Fig. 8, top). Students mentioned how this process had enhanced their understanding of the drug discovery process as well as many complex concepts inherent to the science. Team work and application were two other themes which students identified as major positives from their experiences. Some quotes lifted from the evaluation include:‘I was a little confused about binding sites in the lectures, but after interacting with my peer-leader and group members, and the molecule itself, I found that I actually understood concepts that even the other groups used in their presentations’
‘I have been able to apply my knowledge from lectures (even previous years) and now I remember them much better compared to sitting and just reading notes’
‘Its somewhere we actually applied things we were taught’
Another major positive theme which emerged spoke to how this process affected the participants personally. Here, students identified improved independence and responsibility and finding their love for the subject, as well as a greater awareness of chemistry in the pharmaceutical arena.
‘This experience has helped me in terms of how to study and gather information going forward. I gained a lot of knowledge about chemistry and drug discovery alike. It also helped me realise how much I know about chemistry will help me with my confidence and ultimately my marks.’
‘Please keep doing this, it is a great way to inspire students to pursue their love for chemistry and do further studies and research.’
Additional, less prominent, themes which students identified as positive included skills in communication, the teaching method, skills in critical thinking, the relevance of this project and the positive impact of the peer-leaders.
The peer-leaders identified similar positive themes (application, understanding, communication, teaching method and team work, Fig. 8, bottom) Here they spoke to elimination of parrot fashion learning (i.e. deep learning, Biggs, 1999) enhanced creativity, learning from discussion, a relaxed environment, the facilitation of engagement and holistic knowledge integration. The overwhelming positive experiences that emerged were linked to how this process benefitted them personally, including leadership skills, time management and autonomy and additionally gave them an insight into assessment practices. These are all encouraging aspects, but the personal aspects in particular speak to the motivation of the peer-leaders to be involved in a project such as this, confirming many of the themes identified for PLTL (Wilson and Varma-Nelson, 2016).
‘It helped me gain more insight into the drug discovery process. Getting to explain to other students made me realise there's many ways to approach a problem other than being robotic and sticking to the rules’
‘I gained skills in being able to listen, correct and assist in decision making without necessarily making the decisions.’
It was encouraging to see the positive elements which the students and peer-leaders gleaned out of this project. Generally, they were diverse in nature, with a handful of underlying themes. Importantly, the positives elements tended to reflect the desired outcomes we identified at the outset of this project.
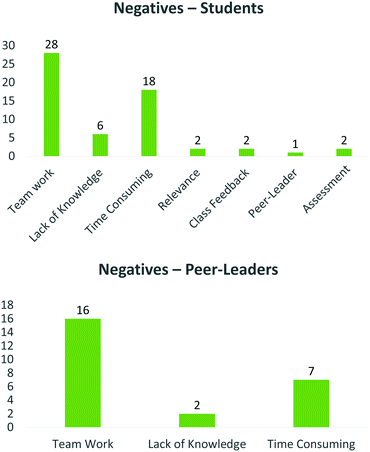
With regard to project negatives, the peer-leaders identified only three themes (Fig. 9, bottom). The most minor (lack of knowledge) spoke mainly of the perception that some students did not prepare adequately for the contact sessions, which may be symptomatic of a very busy curriculum.
This was followed by a greater volume of comments identifying the time commitment as a negative as well as team work, where student participation and attendance was identified as a negative.
With respect to the students, several less prominent themes emerged. These included limited contact with peer-leaders, relevance of the course to students who do not want to enter research, changes to the usual method of assessment, challenges in communicating their results, difficulty in researching and applying knowledge and coming to terms with the content (lack of knowledge) (Fig. 9, top). One such theme was class feedback. In some instances, students felt that class feedback was malicious and unnecessary. So often class discussion and feedback is identified as an important learning tool and we had initially identified class feedback as an important element of the programme, which would assist in molecular design. However, while only two comments mentioned class feedback as a negative aspect, no one identified class feedback as a positive of the process. It seems therefore that small group discussions amongst students in the same team, striving for the same objective is possibly a more effective means of inducing a constructive learning environment than large class forums, which may introduce an element of unnecessary competition. Similar to the peer-leaders, the two dominant themes emerging from the students’ negative comments spoke to team work, and how time consuming the project was.
The fact that time consumption was a negative is not altogether surprising, nor particularly difficult to remedy, either by reducing the workload required, or scheduling more time for each specific exercise. However, the large quantities of negative comments referring to team work is a little more problematic. Layers of this theme mainly included problems with relying on others, as well as problems with non-contributing/unprepared team members, while some students felt that groups were too big and/or would prefer individual projects. While the peer-leaders also identified elements of team work as a negative, the reasons differ between the groups, as the peer-leaders found it difficult to help non-attendees, while the students perceive non-participating members as unfairly benefiting from another member's work. Another important element of this specific result is that team work was identified by numerous students as a positive element of this course. This strongly implies that students perceive the value associated with collaborative group work in the manner intended by assessors, and are happy to share ideas and work co-operatively to achieve success, but are acutely aware of the lack of contribution by some of the team members.
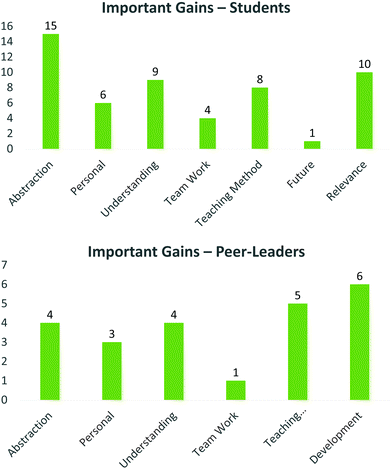
Important gains
Students and peer-leaders were asked to identify specific important personal gains from the PBL, PLTL home grown case study applied here. From the students’ perspective, a wide range of themes emerged, many of which were identified in the positive outcomes, including greater understanding of concepts, relevance (understanding why certain elements are in the course), skills in team work, learning their own capabilities (personal) as well as benefitting from the teaching method (Fig. 10, top). Encouragingly, a small proportion of participants spoke to how this process made them consider a future in research. The overwhelming gains identified by students were the ability to apply previously learnt knowledge to this project, as well as the perceived ability to create abstractions, i.e. deeper understanding of underlying mechanisms of drug discovery, the underlying complexity of the process, and the ability to rationalise their choices, which are all elements of the ‘top right’ quadrant of semantic waves.‘I think this is a very good way of gaining critical thinking and thinking at a higher level. This is what is needed at university to help us stand on our feet when we graduate. We need more work like this’
‘Being able to engage for myself with the material given to us and instead of stating what we have learned in lectures, we could think for ourselves and present our ideas collaboratively. It really was a great experience – better than writing a test!’
‘Awareness of how diverse molecules and pharmacy itself can be’
‘The fact that we are all capable of doing something, even if it may be challenging if we work smart’
‘It made me consider going into research and industry’
‘Anyone can memorise facts, but using it is more beneficial’
‘The drug discovery process isn’t as easy as it seems’
‘It takes a lot of knowledge and time to formulate molecules that could potentially have no effect, but you have to pursue longer and harder as you could make a breakthrough and it will pay off’
Again, the peer-leaders identified similar themes, at different proportions, identifying the insight into teaching and assessment and their personal development as the major gains (Fig. 10, bottom). Important minor comments relating to how this process affected them personally, included improved confidence, as well as identifying that ‘it is ok not to know everything, even as a teacher/leader’. This is related to comments on the teaching method where sharing ideas and ‘playing’ are powerful tools for developing understanding and personal sense making (Taber, 2015).
‘I gained skills in being able to listen, correct and assist in decision making without necessarily making the decisions.’
‘Participating in group discussions with fellow students allows for the development of a better understanding of the subject matter and improves creativity.’
‘It was awesome to get insight as to how the lecturers go about marking our work. It was awesome to be able to help someone else understand something that may seem so arbitrary at first.’
‘As there was no correct answer that the students had to work towards, it allowed more freedom and creativity in their thinking process.’
‘Because I am a really shy person and I have had problems when it comes to speaking in front of a group of people, I have gained so much confidence in this project, which improved as time went on.’
Semi-structured interview
The final analysis of this project involved a semi-structured de-briefing interview with the 12 peer-leaders which was aimed as discussion points from which they could share their personal experiences. Many of the responses confirm the theory already relating to PLTL (Wilson and Varma-Nelson, 2016).(1) Did you feel that your presence facilitated the student's engagement with, participation in and understanding of their task?
All the peer-leaders replied yes to this question.
From their experience, peer-leaders felt as if undergraduates can be intimidated by lecturers and post-graduates. They felt that peer-led groups break down walls and that their presence means students are more likely to open up. In their experience, post-graduate tutors can have an air of arrogance, and since they are not volunteers, they are not necessarily invested in undergraduate education. Post-graduates had been taught a different curriculum or did their undergraduate studies at other universities and therefore cannot relate to what is expected as well as peer-leaders can. Peer-leaders have recently passed pharmaceutical chemistry 3 and can therefore relate experiences. A challenge that the peer-leaders identified is limited authority over former class mates who were repeating the course. Furthermore, they identified student non-attendance as a difficult to manage.
(2) Did your participation and leadership role provide you with deeper insight into the drug discovery pipeline?
All the peer-leaders replied yes to this question.
Peer-leaders mentioned having to revise their work in preparation for this task enhanced their knowledge and reminded them of things they forgot. It also highlighted where they went wrong the previous year and motivated them for possible post-graduate work. They also spoke to the nature of the case study; other case studies required cramming, whereas this required application, creativity and understanding because there was no obvious end point. In addition, a local example was more relatable.
(3) Did this process differ from what you envisioned?
All the peer-leaders replied yes to this question.
Peer-leaders thought this process would be shorter. Furthermore, they thought they would have to do all the talking and do more work to get students up to scratch. However, they were pleasantly surprised by the level of student knowledge/preparation and reading and that students were more interactive than they anticipated.
Peer-leaders found it a challenge to facilitate discussion rather than just give the answers.
(4) Would you have benefitted from this process as a third-year pharmaceutical chemistry student?
All the peer-leaders replied yes to this question.
Peer-leaders feel they did more ‘rote’ learning in their year, and that open-ended study and discussion would have helped their understanding.
They mentioned that cramming for a test or exam is easier than understanding. Since this process takes students out of their comfort zone and forces one to apply the concepts of drug discovery, this process would have helped their long-term learning.
They commented that this process showed them that science is actually a creative space with broader boundaries than they thought and that this process gives a better understanding of the concept of chemical space.
(5) Would you recommend the younger students to become peer-leaders in their 4th year?
All the peer-leaders replied yes to this question.
The peer-leaders mentioned that while it was a lot of work, it is worthwhile if you are interested in chemistry and/or education and want to develop leadership skills and people skills, which would be important in their pharmacy careers. Furthermore, they wanted to be the demonstrators they wish they had/deserved, and felt that their participation was a way of improving the system. Some of the peer-leaders identified themselves as students who had underperformed in previous years, but who were able to succeed after a change in perspective. They wanted to help other students who may have been in the same boat and offer alternative perspective and study techniques.
(6) Other items
Peer-leaders had difficulty with non-participating students, which made them feel uncomfortable and felt they could not force someone to participate.
Peer-leaders mentioned that groups had a range of different personalities, and they had to learn to strike a balance by toning down the ‘loud’ students without dampening their enthusiasm and giving an opportunity for shy/quiet students to share. Peer-leaders acknowledged the importance of group work but were concerned that ‘slackers’ would get good marks because of hard working individuals.
Peer-leaders’ perceptions had been that university would be very structured like school, where knowledge would be structured in neat packages. This project did not conform to this structure and taught concepts rather than facts, which they felt helped understanding, made concepts easier to remember and was more applicable.
Importantly they found the process fun and encouraged that this approach should be applied to other subjects.
Conclusion
The process of drug discovery is one which is littered with pitfalls and failures. It requires experienced practitioners to employ reasoned speculation to generate ideas, which can be used to evolve promising molecules into drugs. These important pharmaceutical chemistry skills are based on sound chemical principles, which need to be applied in a complex biological environment, which requires critical analysis, and the consideration of numerous processes. Here, various questions and problems are considered, and molecules designed to give new insight into what a good drug would require. Furthermore, rather than there being a golden solution, with no associated problems, the properties of a drug would feature a balance of benefits and risks.The traditional method of teaching drug discovery tends to imply that problems with a promising molecule are trivially solved with a single modification, and does not provide a realistic picture of the underlying complexity of the process, nor does it equip students with the appropriate tools for personal sense making and abstraction. This therefore limits the ability of a graduate to apply their understanding to a wide range of problems in drug design. This case study was designed to challenge the traditional pharmaceutical chemistry case study models, by blending problem based learning with peer-led team leading to give students an unintimidating space to discuss ideas and assist in personal sense making. Under the guise of sematic waves, we sought to encourage creative, descriptive solutions to self-identified problems, thus weakening semantic gravity, and assisting in the mastery of the content. The quality of the presentations and the nature of the feedback suggested that this was successfully achieved. Furthermore, we were eager to use a ‘home-grown’ case study model to provide an open-ended problem that was more relatable to our students, and would hopefully inspire their entry into post-graduate study. Study participants recognised the value of team work, and all the groups delivered good projects. However, the majority of negative comments from both the peer-leaders and the third year students were related to non-participating group members. Future iterations of this model will likely require the peer-leaders to provide consistent feedback to the third-year students, regarding their participation, which could be incorporated into personalised assessments. Overall, third year students and peer-leaders responded positively to the project, mentioning how reduced hierarchy, facilitated discussion, engagement, and enhanced creativity, as well as integration of content across the spectrum of their curriculum. Furthermore, the use of a home-grown example was found to be motivating, and that 3rd year and 4th year students can contribute to quality science.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors would like to acknowledge the contribution of the peer-leaders to this project. Thank you to Graham Parker for proof-reading, and Dr Sue Southwood for guidance.References
- Akojee S. and Nkomo M., (2007), Access and quality in South African higher education: the twin challenges of transformation, S. Afr. J. High. Educ., 21(3), 385–399.
- Biggs J., (1999), What the student does: teaching for enhanced learning, High. Educ. Res. Dev., 18(1), 57–75.
- Blackie M. A. L., (2014), Creating semantic waves: using Legitimation Code Theory as a tool to aid the teaching of chemistry, Chem. Educ. Res. Pract., 15, 462–469.
- Bodner G. M., (1986), Constructivism: a theory of knowledge, J. Chem. Educ., 63(10), 873–878.
- Cloete N., Maassen P., Fehnel R., Moja T., Perold H. and T. G. (ed.), (2004), Transformation in higher education: global pressures and local realities in South Africa, Dordrecht: Kluwer Academic Publishing.
- Deborah J., (2006), Concept analysis of cricital cross-filed outcomes in the context of private service providers within further education and training (FET), PhD thesis, University of Pretoria.
- Driver R., Asoko H., Leach J., Scott P. and Mortimer E., (1994), Constructing scientific knowledge in the classroom, Educ. Res., 23(7), 5–12.
- Gosser D., Roth V., Gafney L., Kampmeier J., Strozak V., Varma-Nelson P. and Weiner M., (1996), Workshop chemistry: overcoming the barriers to student success, Chem. Educ., 1(1), 1–17.
- Gosser D. K., Kampmeier J. A. and Varma-Nelson P., (2010), Peer-led team learning: 2008 James Flack Norris award address, J. Chem. Educ., 87(4), 374–380.
- Holbrook S. Y. L. and Garneau-Tsodikova S., (2017), What is medicinal chemistry? – Demystifying a rapidly evolving discipline! Med. Chem. Commun., 8, 1739–1741.
- Kishbaugh T. L. S., Cessna S., Jeanne Horst S., Leaman L., Flanagan T., Graber Neufeld D. and Siderhurst M, (2012), Measuring beyond content: a rubric bank for assessing skills in authentic research assignments in the sciences, Chem. Educ. Res. Pract., 13, 268–276.
- Krathwohl D. R., (2002), A revision of Bloom's Taxonomy: an overview. Theory Pract., 41(4), 212–218.
- Li Q.-Y., Zu Y.-G., Shi R.-Z. and Yao L.-P., (2006), Review camptothecin: current perspectives, Curr. Med. Chem., 13(17), 2021–2039.
- Maton K., (2011), Theories and things: the semantics of discipinarity, in Christie F. and Maton K. (ed.), Disciplinarity: functional linguistic and sociological perspectives, pp. 62–84, London: Continuum.
- Maton K., (2013), Making semantic waves: a key to cumulative knowledge-building, Linguist. Educ., 24(1), 8–22.
- Mc Donnell C., O'Connor C. and Seery M. K., (2007), Developing practical chemistry skills by means of student-driven problem based learning mini-projects, Chem. Educ. Res. Pract., 8(2), 130–139.
- McInally T. and Macdonald S. J. F., (2017), Unusual undergraduate training in medicinal chemistry in collaboration between academia and industry, J. Med. Chem., 60(19), 7958–7964.
- Meisenheimer J. L., Bendall V. I. and Rood G. T., (1982), Design-a-drug: a medicinal chemistry computer game, J. Chem. Educ., 59(7), 600–601.
- Page E., (2013), Thinking out of the box – skills for work, Educ. Chem., (July), 22–25.
- Rafferty M. F., (2016), No denying it: medicinal chemistry training is in big trouble, J. Med. Chem., 59(24), 10859–10864.
- Ram P., (1999), Problem-based learning in undergraduate instruction. A sophomore chemistry laboratory, J. Chem. Educ., 76(8), 1122–1126.
- Roth B. D., (2002), The discovery and development of atorvastatin, a potent novel hypolipidemic agent, Prog. Med. Chem., 40(C), 1–22.
- Sevian H. and Talanquer V., (2014), Rethinking chemistry: a learning progression on chemical thinking, Chem. Educ. Res. Pract., 15, 10–23.
- Svogie A. L., Isaacs M., Hoppe H. C., Khanye S. D. and Veale C. G. L., (2016), Indolyl-3-ethanone-α-thioethers: a promising new class of non-toxic antimalarial agents, Eur. J. Med. Chem., 114(23), 79–88.
- Taber K. S., (2015), Exploring the language(s) of chemistry education, Chem. Educ. Res. Pract., 16, 193–197.
- Van Berkel B., De Vos W., Verdonk A. H. and Pilot A., (2000), Normal science education and its dangers: the case of school chemistry, Sci. Educ., 9, 123–159.
- Watson J., (2001), Social constructivism in the classroom, Support Learn., 16(3), 140–147.
- Watson-Verran H. and Turnbull D., (1995), Science and other indigenous knowledge systems, in Jasanoff T. J., Peterson S. and Pinch J. (ed.), Handbook of Science and Technology Studies, Thousand Oaks, CA: Sage Publications, pp. 115–139.
- Wilson S. B. and Varma-Nelson P., (2016), Small groups, significant impact: a review of peer-led team learning research with implications for STEM education researchers and faculty, J. Chem. Educ., 93(10), 1686–1702.
- Woodward A. E., Weiner M. and Gosser D., (1993), Problem solving workshops in general chemistry, J. Chem. Educ., 70(8), 651.
| This journal is © The Royal Society of Chemistry 2018 |