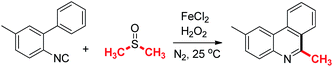Open Access Article
Open Access ArticleFree-radical initiated cascade methylation or trideuteromethylation of isocyanides with dimethyl sulfoxides†
Rui Zhang‡
a,
Xiaoqian Shi‡a,
Qinqin Yana,
Zejiang Li *ab,
Zheng Wanga,
Haifei Yua,
Xiaoke Wanga,
Jing Qi
*ab,
Zheng Wanga,
Haifei Yua,
Xiaoke Wanga,
Jing Qi *a and
Menglu Jianga
*a and
Menglu Jianga
aCollege of Chemistry & Environmental Science, Hebei University, Baoding, Hebei 071002, P. R. China. E-mail: lizejiang898@126.com; qijinghbu2013@126.com
bKey Laboratory of Medicinal Chemistry and Molecular Diagnosis of Ministry of Education, Baoding, Hebei 071002, P. R. China
First published on 8th August 2017
Abstract
The methylation or trideuteromethylation reaction of isocyanides with dimethyl sulfoxides in a radical way is developed, which offers a low-cost, easy-operation cascade methylation strategy for the synthesis of phenanthridines or isoquinolines.
The introduction of methyl or trideuteromethyl groups into organic compounds could affect their biological and chemical properties.1 Methylation reactions have been widely applied in synthetic organic chemistry.2 Among them methyl metal reagents,3 MeI,4 DMSO,5 MeCOOH,6 peroxides7 etc. are usually used as methylating reagents to complete the corresponding reactions. Meanwhile, deuterated iodomethane,8 deuterated reducing reagents9 or d6-DMSO10 are selected as deuterated methyl sources to develop trideuteromethylation reactions. Although considerable numbers of methods have been developed, more economic and practical methylation or trideuteromethylation strategies are highly desirable.
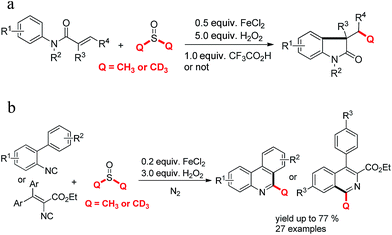
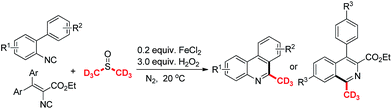
The preparation of phenanthridines or isoquinolines have been considered as a study topic recently.11,12 The methyl substituted phenanthridines which were produced by biphenyl isocyanides reacting with peroxides or methyl hydrazine have been developed by Liu,7 Mao13 and Cheng,14 et al. Antonchick15 and our group16 reported a cascade trideuteromethylation or methylation reaction of N-arylacrylamides with dimethyl sulfoxides respectively (Scheme 1a). However, a free radical cascade methylation or trideuteromethylation of isocyanides with dimethyl sulfoxides under analogous Fenton reaction condition was not finished now (Scheme 1b).17 Combined with our previous study,18 we wish to accomplish this reaction system.
Initially, 2-isocyano-5-methyl-1,1′-biphenyl and dimethyl sulfoxide were selected as model substrates to optimize the reaction systems (Table 1, also see ESI for detail†). It was shown that the yield of the final product in 6 hour is better than that in 3 and 10 hour (entries 1–3). Adding 1 mL, 2 mL and 4 mL of dimethyl sulfoxide gained the product in 30%, 40%, 50% yields, respectively (entries 4–6). No reaction was found without any iron salts (entry 7). Variation of the amount of iron(II) chloride and hydrogen peroxide greatly affected reaction efficiency (entries 8–14). Higher temperature slightly reduced reaction yield (entry 15). Finally, 2,6-dimethylphenanthridine was isolated in 70% yield under the optimum conditions: isonitriles (1 equiv., 0.25 mmol), FeCl2 (0.2 equiv., 0.05 mmol), H2O2 (30%, 3 equiv., 0.75 mmol) and DMSO (3 mL), 25 °C, N2, 6 h, sealed tube.
| Entry | Iron(II) chloride (equiv.) | Hydrogen peroxide (30%), (equiv.) | t/h | Yieldb (%) |
|---|---|---|---|---|
| a Reaction conditions: 2-isocyano-5-methyl-1,1′-biphenyl (1 equiv., 0.25 mmol), DMSO (3 mL), 25 °C, N2.b Isolated yields.c DMSO (1 mL).d DMSO (2 mL).e DMSO (4 mL).f 50 °C. | ||||
| 1 | 0.5 | 3 | 3 | 52 |
| 2 | 0.5 | 3 | 6 | 55 |
| 3 | 0.5 | 3 | 10 | 52 |
| 4c | 0.5 | 3 | 6 | 30 |
| 5d | 0.5 | 3 | 6 | 40 |
| 6e | 0.5 | 3 | 6 | 50 |
| 7 | — | 3 | 6 | n.r. |
| 8 | 0.1 | 3 | 6 | 63 |
| 9 | 0.2 | 3 | 6 | 70 |
| 10 | 0.3 | 3 | 6 | 55 |
| 11 | 0.4 | 3 | 6 | 55 |
| 12 | 0.2 | 1 | 6 | 20 |
| 13 | 0.2 | 2 | 6 | 36 |
| 14 | 0.2 | 4 | 6 | 56 |
| 15f | 0.2 | 3 | 6 | 60 |
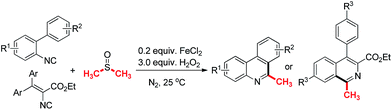
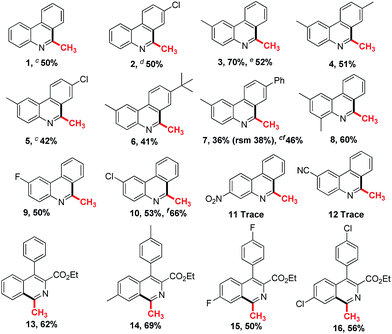
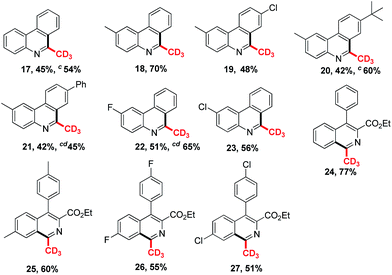
To investigate the substrate scope, a variety of isocyanides were prepared and tested in Table 2. As can be seen, many functional groups substituted substrates could react well in this reaction system (1–10). Halogen atoms such as F, Cl substituted isocyanides gave the corresponding products in moderate yields, respectively (2, 5, 9–10). The substrates with large groups such as t-butyl and phenyl on 4′-position of the aromatic ring obtained the product 6 and 7 in 41% and 36% yields, which indicate that the steric effect is slightly obvious. The products 7 and 10 were isolated in 46% and 66% yields respectively by adding potassium carbonate in the system. It indicates potassium carbonate could weaken the impact on the yields of final products from methylsulfinyl acid as the side product. The electronic effect also influences the reaction yields, and the raw material with electron-withdrawing group such as nitro, cyano could not result the desirable product (11–12). And vinyl isocyanides gained the desired isoquinolines in 50–69% yields (13–16). Notably, the product 3 was isolated in 52% yield, when the reaction system was scaled up to the gram level.
As can be seen in Table 3, diverse aryl isocyanides could react well with deuterated dimethyl sulfoxide in the typical condition (17–23). The halogen atoms such as F, Cl were tolerant in the system and gave the corresponding products in good yields (19, 22, 23). The t-butyl and phenyl substituted substrates obtained the product 20 and 21 both in 42% yields. Notably, the products 17, 20, 21 and 22 gave better yields by adding the base in the system under typical conditions. And vinyl isocyanides produced the trideuteromethyl substituted isoquinolines in 51–77% yields (24–27).
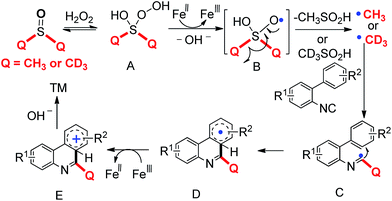
No reaction was deuterated when TEMPO was added to the reaction system, which suggests the reaction would be in process through a free radical way. According to the precedent literature15,16,19 and experimental study, we design a possible mechanism in Scheme 2. First, dimethyl sulfoxide or deuterated dimethyl sulfoxide reacts with hydrogen peroxide and generates the intermediate A. With the induction of iron(II) chloride, free radical B and hydroxyl ion are produced by intermediate A occurring heterolysis reaction. Radical B gives methyl radical or trideuteromethyl radical along with methylsulfinyl acid or detected methylsulfinyl acid produced in the process of β-cleavage reaction. Next, radical C is formed from adding methyl radical or trideuteromethyl radical to the isocyanides. Then, radical C adds to the aromatic ring and generates radical D. Radical D can be oxidized by iron and yields the carbocation E, which is pulled the proton by hydroxyl ion and gains the terminal product.
In summary, we have accomplished a free radical triggered cascade methylation or trideuteromethylation/cyclization reaction of isocyanides with dimethyl sulfoxides. It offers an economical and convenient protocol for preparing phenanthridines or isoquinolines. Further studies on cyclization reactions in a novel free radical way are ongoing in our laboratory.
Acknowledgements
This project is supported by the Natural Science Foundation of Hebei Province (B2017201043), the Fundamental Research Funds for The Midwest Universities Comprehensive Strength Promotion Project, Key Laboratory of Medicinal Chemistry and Molecular Diagnosis of Ministry of Education, Key Laboratory of Chemical Biology of Hebei Province and College of Chemistry & Environmental Science, Hebei University.Notes and references
- (a) E. J. Barreiro, A. E. Kümmerle and C. A. M. Fraga, Chem. Rev., 2011, 111, 5215 CrossRef CAS PubMed; (b) T. G. Gant, J. Med. Chem., 2014, 57, 3595 CrossRef CAS PubMed; (c) I. Kheterpal and R. Wetzel, Acc. Chem. Res., 2006, 39, 584 CrossRef CAS PubMed; (d) I. Lee, Chem. Soc. Rev., 1995, 24, 223 RSC.
- H. Schönherr and T. Cernak, Angew. Chem., Int. Ed., 2013, 52, 12256 CrossRef PubMed.
- (a) R. Giri, N. Maugel, J. Li, D. Wang, S. P. Breazzano, L. B. Saunders and J.-Q. Yu, J. Am. Chem. Soc., 2007, 129, 3510 CrossRef CAS PubMed; (b) J. A. Romero-Revilla, A. Garcia-Rubia, R. G. Arrayas, M. A. Fernandez-Ibanez and J. C. Carretero, J. Org. Chem., 2011, 76, 9525 CrossRef CAS PubMed; (c) H.-X. Dai, A. F. Stepan, M. S. Plummer, Y.-H. Zhang and J.-Q. Yu, J. Am. Chem. Soc., 2011, 133, 7222 CrossRef CAS PubMed; (d) X. Chen, J. Li, X. Hao, C. E. Goodhue and J.-Q. Yu, J. Am. Chem. Soc., 2006, 128, 78 CrossRef CAS PubMed; (e) S. R. Neufeldt, C. K. Seigerman and M. S. Sanford, Org. Lett., 2013, 15, 2302 CrossRef CAS PubMed; (f) Q. Chen, L. Ilies, N. Yoshikai and E. Nakamura, Org. Lett., 2011, 13, 3232 CrossRef CAS PubMed.
- (a) L. Barsky, H. W. Gschwend, J. McKenna and H. R. Rodriguez, J. Org. Chem., 1976, 41, 3651 CrossRef CAS; (b) S. J. Tremont and H. U. Rahman, J. Am. Chem. Soc., 1984, 106, 5759 CrossRef CAS; (c) Z. Zhao and G. Chen, Org. Lett., 2011, 13, 4850 CrossRef PubMed; (d) K. M. Engle, T.-S. Mei, M. Wasa and J.-Q. Yu, Acc. Chem. Res., 2012, 45, 788 CrossRef CAS PubMed.
- B. Yao, R.-J. Song, Y. Liu, Y.-X. Xie, J.-H. Li, M.-K. Wang, R.-Y. Tang, X.-G. Zhang and C.-L. Deng, Adv. Synth. Catal., 2012, 354, 1890 CrossRef CAS.
- F. Pan, Z.-Q. Lei, H. Wang, H. Li, J. Sun and Z.-J. Shi, Angew. Chem., Int. Ed., 2013, 52, 2063 CrossRef CAS PubMed.
- Z. Xu, C. Yan and Z.-Q. Liu, Org. Lett., 2014, 16, 5670 CrossRef CAS PubMed.
- (a) S. Komarapuri, K. Krishnan and D. F. Covey, J. Labelled Compd. Radiopharm., 2008, 51, 430 CrossRef CAS PubMed; (b) R. G. Gillis, Tetrahedron Lett., 1968, 9, 1413 CrossRef.
- For selected reviews, see: (a) J. H. Gui, Q. H. Zhou, C. M. Pan, Y. Yabe, A. C. Burns, M. R. Collins, M. A. Ornelas, Y. Ishihara and P. S. Baran, J. Am. Chem. Soc., 2014, 136, 4853 CrossRef CAS PubMed; (b) V. A. Khripach, V. N. Zhabinskii, A. P. Antonchick, O. V. Konstantinova and B. Schneider, Steroids, 2002, 67, 1101 CrossRef CAS PubMed; (c) M. Adamczyk, J. R. Fishpaugh and D. Johnson, J. Labelled Compd. Radiopharm., 1993, 33, 153 CrossRef CAS.
- (a) E. Jones-Mensah, M. Karki and J. Magolan, Synthesis, 2016, 48, 1421 CrossRef CAS; (b) X. F. Wu and K. Natte, Adv. Synth. Catal., 2016, 358, 336 CrossRef CAS; (c) X. Jiang, C. Wang, Y. W. Wei, D. Xue, Z. T. Liu and J. L. Xiao, Chem.–Eur. J., 2014, 20, 58 CrossRef CAS PubMed; (d) K. Kawai, Y. S. Li, M. F. Song and H. Kasai, Bioorg. Med. Chem. Lett., 2010, 20, 260 CrossRef CAS PubMed; (e) S. W. Peabody, B. Breiner, S. V. Kovalenko, S. Patil and I. V. Alabugin, Org. Biomol. Chem., 2005, 3, 218 RSC.
- For selected reviews on phenanthridine, see: (a) R. S. Theobald and K. Schofield, Chem. Rev., 1950, 46, 170 CrossRef; (b) S. Simeon, J. L. Rios and A. Villar, Pharmazie, 1989, 44, 593 CAS; (c) W. K. Brewster, D. E. Nichols, R. M. Riggs, D. M. Mottola, T. W. Lovenberg, M. H. Lewis and R. B. Mailman, J. Med. Chem., 1990, 33, 1756 CrossRef CAS PubMed; (d) T. Nakanishi, M. Suzuki, A. Saimoto and T. Kabasawa, J. Nat. Prod., 1999, 62, 864 CrossRef CAS PubMed; (e) L. Sripada, J. A. Teske and A. Deiters, Org. Biomol. Chem., 2008, 6, 263 RSC; (f) G. Qiu, Q. Ding and J. Wu, Chem. Soc. Rev., 2013, 42, 5257 RSC; (g) B. Zhang, C. Mück-Lichtenfeld, C. G. Daniliuc and A. Studer, Angew. Chem., Int. Ed., 2013, 52, 10792 CrossRef CAS PubMed; (h) D. Leifert, C. G. Daniliuc and A. Studer, Org. Lett., 2013, 15, 6286 CrossRef CAS PubMed; (i) Q. Wang, X. Dong, T. Xiao and L. Zhou, Org. Lett., 2013, 15, 4846 CrossRef CAS PubMed; (j) Y. Cheng, H. Jiang, Y. Zhang and S. Yu, Org. Lett., 2013, 15, 5520 CrossRef CAS PubMed; (k) B. Zhang, C. G. Daniliuc and A. Studer, Org. Lett., 2014, 16, 250 CrossRef CAS PubMed; (l) L. Gu, C. Jin, J. Liu, H. Ding and B. Fan, Chem. Commun., 2014, 50, 4643 RSC; (m) T.-H. Zhu, S.-Y. Wang, Y.-Q. Tao, T.-Q. Wei and S.-J. Ji, Org. Lett., 2014, 16, 1260 CrossRef CAS PubMed; (n) L. Wang, W. Sha, Q. Dai, X. Feng, W. Wu, H. Peng, B. Chen and J. Cheng, Org. Lett., 2014, 16, 2088 CrossRef CAS PubMed; (o) J.-J. Cao, T.-H. Zhu, S.-Y. Wang, Z.-Y. Gu, X. Wang and S.-J. Ji, Chem. Commun., 2014, 50, 6439 RSC; (p) T. Xiao, L. Li, G. Lin, Q. Wang, P. Zhang, Z.-W. Mao and L. Zhou, Green Chem., 2014, 16, 2418 RSC; (q) J. Liu, C. Fan, H. Yin, C. Qin, G. Zhang, X. Zhang, H. Yi and A. Lei, Chem. Commun., 2014, 50, 2145 RSC; (r) Z. Xia, J. Huang, Y. He, J. Zhao, J. Lei and Q. Zhu, Org. Lett., 2014, 16, 2546 CrossRef CAS PubMed; (s) J. Lei, J. Huang and Q. Zhu, Org. Biomol. Chem., 2016, 14, 2593 RSC; (t) B. Zhang and A. Studer, Chem. Soc. Rev., 2015, 44, 3505 RSC; (u) B.-R. Song and B. Xu, Chem. Soc. Rev., 2017, 46, 1103 RSC; (v) Z.-G. Xie, P.-H. Li, Y. Hu, N. Xu and L. Wang, Org. Biomol. Chem., 2017, 15, 4205 RSC; (w) H. Cao, S. Lei, N.-Y. Li, L.-B. Chen, J.-Y. Liu, H.-Y. Cai, S.-X. Qiu and J.-W. Tan, Chem. Commun., 2015, 51, 1823 RSC; (x) H. Jiang, Y. Cheng, R. Wang, M. Zheng, Y. Zhang and S. Yu, Angew. Chem., Int. Ed., 2013, 52, 13289 CrossRef CAS PubMed; (y) W. Sha, J.-T. Yu, Y. Jiang, H. Yang and J. Cheng, Chem. Commun., 2014, 50, 9179 RSC; (z) H.-Y. Tu, Y.-R. Liu, J.-J. Chu, B.-L. Hu and X.-G. Zhang, J. Org. Chem., 2014, 79, 9907 CrossRef CAS PubMed.
- For selected examples of preparation of isoquinolines and its derivatives, see: (a) P. Villuendas and E. P. Urriolabeitia, J. Org. Chem., 2013, 78, 5254 CrossRef CAS PubMed; (b) J. Jayakumar, K. Parthasarathy and C.-H. Cheng, Angew. Chem., Int. Ed., 2012, 51, 197 CrossRef CAS PubMed; (c) Y.-F. Wang, K. K. Toh, J.-Y. Lee and S. Chiba, Angew. Chem., Int. Ed., 2011, 50, 5927 CrossRef CAS PubMed; (d) P. C. Too, S. H. Chua, S. H. Wong and S. Chiba, J. Org. Chem., 2011, 76, 6159 CrossRef CAS PubMed; (e) N. Guimond and K. Fagnou, J. Am. Chem. Soc., 2009, 131, 12050 CrossRef CAS PubMed; (f) P. C. Too, Y.-F. Wang and S. Chiba, Org. Lett., 2010, 12, 5688 CrossRef CAS PubMed; (g) R. K. Chinnagolla, S. Pimparkar and M. Jeganmohan, Org. Lett., 2012, 14, 3032 CrossRef CAS PubMed; (h) H. Jiang, Y. Cheng, R. Wang, Y. Zhang and S.-Y. Yu, Chem. Commun., 2014, 50, 6164 RSC.
- T. Xiao, L. Li, G. Lin, Q. Wang, P. Zhang, Z.-W. Mao and L. Zhou, Green Chem., 2014, 16, 2418 RSC.
- Q. Dai, J. Yu, X. Feng, Y. Jiang, H. Yang and J. Cheng, Adv. Synth. Catal., 2014, 356, 3341 CrossRef CAS.
- R. Caporaso, S. Manna, S. Zinken, A. R. Kochnev, E. R. Lukyanenko, A. V. Kurkin and A. P. Antonchick, Chem. Commun., 2016, 52, 12486 RSC.
- Z.-J. Li, X. Cui, L. Niu, Y. Ren, M. Bian, X. Yang, B. Yang, Q.-Q. Yan and J. Zhao, Adv. Synth. Catal., 2017, 359, 246 CrossRef CAS.
- H. Fenton, J. Chem. Soc., Trans., 1984, 65, 899 RSC.
- (a) Z. J. Li, Y. Zhang, L. Z. Zhang and Z.-Q. Liu, Org. Lett., 2014, 16, 382 CrossRef CAS PubMed; (b) Z. J. Li, F. H. Fan, J. Yang and Z.-Q. Liu, Org. Lett., 2014, 16, 3396 CrossRef CAS PubMed; (c) L. Z. Zhang, Z. J. Li and Z.-Q. Liu, Org. Lett., 2014, 16, 3688 CrossRef CAS PubMed.
- K.-U. Schoening, W. Fischer, S. Hauck, A. Dichtl and M. Kuepfert, J. Org. Chem., 2009, 74, 1567 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7ra08484f |
| ‡ These authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2017 |