Open Access Article
Open Access ArticleComprehensive investigation of the enzymatic oligomerization of esculin by laccase in ethanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/h2_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/h2_char_2009.gif) water mixtures†
water mixtures†
Abel Muñiz-Mouro ,
Isabel M. Oliveira,
Beatriz Gullón
,
Isabel M. Oliveira,
Beatriz Gullón ,
Thelmo A. Lú-Chau
,
Thelmo A. Lú-Chau ,
María Teresa Moreira
,
María Teresa Moreira ,
Juan M. Lema
,
Juan M. Lema and
Gemma Eibes
and
Gemma Eibes *
*
Dept. of Chemical Engineering, Institute of Technology, Universidade de Santiago de Compostela, Rua Constantino Candeira, Campus Vida, 15782 Santiago de Compostela, Spain. E-mail: gemma.eibes@usc.es; Fax: +34881816702; Tel: +34881816020
First published on 4th August 2017
Abstract
The enzymatic polymerization of phenolic compounds arouses increasing interest due to the production of derivatives with improved biological activity. The reaction yield, the molecular mass, the structure and the properties of synthesized polymers can be controlled by the reaction conditions such as solvent and type of enzyme and substrate. In this study, the oxidative oligomerization of esculin by laccase from Trametes versicolor was performed in the presence of ethanol, a biocompatible co-solvent for food and nutraceutical applications. The formation of a precipitate was associated with the oligomerization reaction except for the medium with 50% (v/v) ethanol, due to the low reaction yield. The evaluation of antioxidant activity of the monomer and products showed that the pellet fraction from the reaction with esculin at 2 g L−1 in acetate buffer led to the highest activities. The presence of esculin oligomers was confirmed by MALDI-TOF analysis, which identified a repetition unit of 338 Da with a degree of polymerization up to 9 as well as other oligomers, mainly in the pellet fraction, with a repetition unit of 176 Da which are attributed to be esculetin oligomers. Additionally, size exclusion chromatography (SEC) and Fourier transform infrared spectroscopy (FT-IR) were used to characterize the products.
1. Introduction
Coumarins are naturally occurring compounds belonging to a large class of phenolic substances which present an aromatic ring fused to a condensed 6-member lactone ring.1 They have a remarkable range of bioactivities, including the ability to inhibit xanthine oxidase (XO), act as radical scavengers and absorb harmful reactive oxygen species (ROS).2 Esculin is a glycosidic coumarin that can be extracted from the bark and leaves of Aesculus hippocastanum or horse-chestnut tree3 and it has been traditionally used as a natural UV-B protective agent and also for the treatment of various peripheral vascular disorders.4 Beyond these applications, esculin exhibits potential antiflogistic, cytostatic and antimutagenic properties.5 However, both its low solubility in organic and aqueous solvents and reduced thermal stability, also evidenced in other biologically active phenolic species such as rutin, catechin and epicatechin, limit its application.6 To overcome these major drawbacks, enzymatic polymerization can render it into high molecular weight polyphenols that present increased solubility, thermostability or antioxidant properties.7–10Oxidoreductases such as laccases (EC 1.10.3.2) or peroxidases (EC 1.11.1) have been reported to catalyze the oligomerization or polymerization of many phenolic compounds.7,8,11 The catalytic cycle of laccase requires oxygen as a substrate, which implies a clear advantage over peroxidases that use hydrogen peroxide.12,13 The advantages associated to laccase: the use of oxygen as the final electron acceptor and its commercial availability, have favored its applications in the production of polymers as well as in the food industry and/or pharmaceutical and cosmetics sectors, among others.13,14 Moreover, the use of laccase for the production of oligomers from flavonoids with improved properties has been demonstrated. Anthoni et al.15 reported the oligomerization of esculin by laccase with the production of derivatives with superior antioxidant activity than esculin. Moreover, the enhancement of the esculin antimutagenicity5 and the increase of its antibacterial potential after polymerization16 were proven. Despite the interesting properties of esculin oligomers, the enzymatic reaction conditions are far from being optimal for a practical use. As for instance, the reaction medium contained the toxic solvent methanol. The utilization of such organic solvent is incompatible with biotechnological applications, and its use is banned in cosmetics and food applications. So, the use of alternative solvents such as ethanol, which is considered as safe in accordance with the European Food Safety Authority (EFSA), could favor the application of oligomers in these sectors.
The aim of this work is to evaluate the presence of ethanol as co-solvent in order to increase the initial concentration of esculin in the enzymatic oligomerization mixture. As far as we know, there are no studies that rigorously evaluate the influence of the solvent in the production of oligomers from esculin. The products obtained were characterized in terms of chemical structure (MALDI-TOF, GPC-SEC, FT-IR, UV analysis), antioxidant activities by different assays (FRAP, CUPRAC, xanthine oxidase) and antimicrobial activity.
2. Experimental
2.1. Materials
Laccase from Trametes versicolor (E.C. 1.10.3.2., grade IV, ≥0.5 U mg−1), esculin hydrate (≥98%), esculetin (>99%), 2,2′-azino-bis(3methylbenzenothiazoline-6-sulphonic acid) diammonium salt (ABTS, ≥98%), iron(III) chloride hexahydrate (≥99%), 2,4,6-tri(2-pyridyl)-1,3,5-triazine (TPTZ, ≥99%), neocuproine (≥98%), 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (trolox, ≥97%), Folin Ciocalteu's phenol reagent, xanthine (≥99%), xanthine oxidase (E.C. 1.17.3.2., grade IV from bovine milk, 0.2 U mg−1 protein), gallic acid, dimethylsulfoxide (DMSO) absolute ethanol, were purchased from Sigma. Copper(II) chloride dihydrate was purchased from Merck.2.2. Laccase activity
Laccase activity was determined by monitoring the oxidation rate of 0.267 mM ABTS to its cation radical (ABTS˙+) at 420 nm (ε420 = 36![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 M−1 cm−1) in McIlvaine buffer (pH 3) at room temperature. One unit (U) of activity was defined as the amount of enzyme forming 1 μmol of ABTS˙+ per min. All spectrophotometric measurements were carried out on a Shimadzu UV-1800.
000 M−1 cm−1) in McIlvaine buffer (pH 3) at room temperature. One unit (U) of activity was defined as the amount of enzyme forming 1 μmol of ABTS˙+ per min. All spectrophotometric measurements were carried out on a Shimadzu UV-1800.
2.3. Effect of ethanol on laccase stability
In order to determine laccase stability in the presence of ethanol, the enzyme solution was incubated in acetate buffer (0.1 M) at pH 5 with ethanol concentrations ranging from 0% to 60% (v/v). In both cases, the solutions were stirred for 24 h at room temperature. The residual activity was measured according to the standard protocol and expressed as percentage of the initial activity.2.4. Solubility study of esculin in ethanol
The solubility of esculin in ethanol was determined by adding a sufficient amount of esculin in acetate buffer (pH 5, 0.1 M) with ethanol concentrations ranging from 0% to 60% (v/v). The solutions were stirred at 500 rpm for 24 h at 20 °C. After this time, the solutions were centrifuged and the esculin concentration in the supernatant was determined by high performance liquid chromatography (HPLC). The analysis was performed on a Jasco XLC HPLC (Jasco Analitica) equipped with a 3110 MD diode array detector (detection at 280 nm) and a Gemini reversed-phase column (150 × 4.6 mm, particle size: 3 μm) (Phenomenex, supplied by Jasco Analitica) maintained at 30 °C. Gradient elution (flow rate of 0.7 mL min−1) started with 5% acetonitrile in water (2% acetic acid), increased to 90% acetonitrile within 15 min, and then decreased back to the initial concentration after 17 min.2.5. Polymerization reaction
Polymerization of esculin was performed in 50 mL amber glass vials with Teflon-lined screw caps at different concentrations of esculin, buffer and solvent: (A) esculin at 2 g L−1 in 0.1 M acetate buffer (pH 5), (B) esculin at 5 g L−1 in 30/70 (v/v) ethanol/acetate buffer, and (C) esculin at 5 g L−1 in 50% (v/v) ethanol/acetate buffer. Laccase solution was added for a final activity of 1000 U L−1, except for the control experiments. The reaction medium (20 mL final volume) was stirred for 24 h at room temperature. Reactions were stopped by adding HCl (6 M) to acidify the reaction medium to a pH of 2–3.2.6. Separation and lyophilization of the polymers
The final reaction medium enriched with esculin polymers was separated by centrifugation (4500 rpm, 30 min) except for those reactions where no precipitates were obtained. The different fractions (pellet and supernatant) were lyophilized (Labconco FreeZone Benchtop Freeze Dry System, 0.098 Torr, 50 °C). Ethanol from the reaction media was removed by evaporation (Büchi Rotavapor R-205, 50–70 mbar, 40 °C, 30 rpm) before the lyophilization stage.2.7. UV, FT-IR and MALDI-TOF analysis
The UV spectra of reaction solutions in methanol were determined on a Shimadzu-UV-1800 spectrometer (Shimadzu). IR absorption spectra in attenuated total reflectance mode (FTIR-ATR) of freeze-dried control, pellet and supernatant fractions from reaction A were collected with a Varian 670IR spectrophotometer (Varian) fitted to a universal ATR sampling accessory GladiATR (PIKE Technologies). FTIR-ATR spectra were recorded in the 4000–400 cm−1 spectral range at 4 cm−1 resolution for 64 scans. Spectroscopic data was acquired using the software Agilent Resolution Pro (Agilent Technologies Inc). Spectral data was stored as csv files and analyzed using the software Spekwin32 (v.1.71.6.1).Absolute masses were determined by MALDI-TOF based on the protocol described by Anthoni et al.17 Lyophilized samples were dissolved at 1 g L−1 in acetonitrile/water (30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 70 v/v) with 0.1% TFA, except for the pellet fraction which was dissolved at 0.21 g L−1. The use of 2,5-dihydroxybenzoic acid (DHB) was considered at a concentration of 20 mg mL−1 in acetonitrile/water (30
70 v/v) with 0.1% TFA, except for the pellet fraction which was dissolved at 0.21 g L−1. The use of 2,5-dihydroxybenzoic acid (DHB) was considered at a concentration of 20 mg mL−1 in acetonitrile/water (30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 70 v/v) with 0.1% TFA. Sample solutions were mixed with DHB at a 1
70 v/v) with 0.1% TFA. Sample solutions were mixed with DHB at a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio. Then, 1 μL was spotted on the MALDI plate and crystallized at room temperature. MALDI-TOF analysis was performed on an Ultraflex III TOF/TOF mass spectrometer equipped with a Smartbeam® laser (Bruker Daltonics) in reflectron operation mode and positive polarity. The acceleration voltage was set to 25 kV and a total of 1200 laser shots per spot were automatically acquired. Data acquisition and data processing were performed by the Flex Analysis software (Bruker Daltonics).
1 ratio. Then, 1 μL was spotted on the MALDI plate and crystallized at room temperature. MALDI-TOF analysis was performed on an Ultraflex III TOF/TOF mass spectrometer equipped with a Smartbeam® laser (Bruker Daltonics) in reflectron operation mode and positive polarity. The acceleration voltage was set to 25 kV and a total of 1200 laser shots per spot were automatically acquired. Data acquisition and data processing were performed by the Flex Analysis software (Bruker Daltonics).
2.8. Total phenolic compounds assay
The total phenolic compounds (TPC) were determined by Folin–Ciocalteu assay.18 Aliquots of the diluted samples (75 μL) or gallic acid standards (from 15 to 100 mg L−1) were added to 625 μL of Folin–Ciocalteu chemical (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10, v/v) and 500 μL of Na2CO3 (7.5%). The mixture was kept for 1 h at room temperature and absorbance was measured at 760 nm. A blank was prepared in parallel with 75 μL of methanol instead of the sample. The results were expressed in mg of gallic acid equivalent (GAE) per g of lyophilized product as average of two replicates.
10, v/v) and 500 μL of Na2CO3 (7.5%). The mixture was kept for 1 h at room temperature and absorbance was measured at 760 nm. A blank was prepared in parallel with 75 μL of methanol instead of the sample. The results were expressed in mg of gallic acid equivalent (GAE) per g of lyophilized product as average of two replicates.
2.9. Ferric reducing antioxidant power (FRAP), cupric reducing antioxidant capacity (CUPRAC) and xanthine oxidase inhibition assay
FRAP was conducted according to the protocol described by Vázquez et al.19 Briefly, 0.1 mL of each sample was added to 3 mL of FRAP reagent (acetate buffer, 300 mM, pH 3.6; 10 mM of TPTZ in 40 mM HCl; 20 mM of FeCl3·6H2O, chemicals added with a ratio of 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). The absorbance was recorded after 6 min at 593 nm.
1). The absorbance was recorded after 6 min at 593 nm.
The cupric reducing antioxidant capacity was determined according to the method of Apak et al.20 Test solutions were comprised of 250 μL of 10−2 M copper(II) chloride solution, 250 μL of 7.5 × 10−3 M neocuproine (Nc) solution in 96% ethanol, 250 μL of ammonium acetate buffer (pH 7), 75 μL of sample and 200 μL of water. After 30 min, the absorbance was measured at 450 nm against a blank.
The relative activities of the samples were calculated from the calibration curve of Trolox (0 to 150 mg L−1 in FRAP assay and 0 to 255 mg L−1 in CUPRAC assay) and the results were expressed as mg of Trolox equivalent (TE) per g of lyophilized product. The assays were carried out in duplicate.
The xanthine oxidase activity was measured spectrophotometrically using a procedure based on the one reported by Chebil et al.6 Briefly, the samples were solubilized in phosphate buffer (pH 7.5, 0.1 M) with 10% DMSO. To measure the xanthine oxidase inhibition, 50 μL of a solution of xanthine oxidase (0.4 U mL−1) was added to 925 μL of the sample diluted in the minimum of DMSO (10%) and phosphate buffer (0.1 M, pH 7.5) and 25 μL of a solution of xanthine (1 mM) in a total reaction volume of 1 mL. The production of uric acid was monitored for 120 s at 295 nm on Shimadzu-UV-1800 spectrometer (molar absorption coefficient 1.22 × 104 M−1 cm−1). Results were given as the concentration leading to half-maximal enzyme activity (IC50) and were calculated by standard curve regression analysis. The assays were carried out in duplicate. The presence of inactivated enzyme did not affect the antioxidant activity measurements and the xanthine oxidase inhibition, as observed in control experiments with and without inactivated laccase.
2.10. Evaluation of antimicrobial activity
The microorganisms used in this study were food-related bacteria: Escherichia coli (ATCC 25922) and Salmonella enteritidis (ATCC 3076). Stock cultures were stored in cryovials with glycerol at 15% (v/v) and maintained at −80 °C before use. The bacterial strains were grown in Mueller–Hinton broth (MHB) at 37 °C for 10–12 h. The antimicrobial activities of the oligomers obtained: pellet and supernatant, were evaluated. In order to remove the salts, the supernatant solution was filtered on 45 μm membrane (3 times), passed through an ultrafiltration membrane of 10 kDa (Amicon, Millipore) and later lyophilized. The antimicrobial activity of esculin oligomers was determined according to Gullón et al.21 In brief, 980 μL of test compounds (concentrations obtained by serial dilution; 7.5, 5 and 2.5 g L−1 in Müller–Hinton growth medium with 9% DMSO) and 20 μL of bacterial cultures (E. coli and S. enteritidis) were added to sterilized vials. The cell mixtures were incubated overnight at 37 °C. Positive (bacterial cultures in Müller–Hinton medium with 9% DMSO) and negative (abiotic incubation of test compounds in Müller–Hinton growth medium with 9% DMSO) were run in parallel. Twenty-four hours after incubation, serial dilutions of the bacterial cultures were prepared and 20 μL of each bacterial solution was overlaid on the Müller–Hinton agar plate. Plates were incubated at 37 °C and the bacterial colonies on each plate (from 30 to 300 CFU) were counted after 24 h of incubation. The results were expressed as colony forming units (CFU) per mL. All the assays were carried out in duplicate.3. Results and discussion
Most studies of laccase polymerization reactions have focused on the influence of the biocatalyst activity, the presence of oxygen and/or mediator, the concentration of the monomer and the cosolvent.12 The present study focuses on the effect of the concentration of esculin and the cosolvent, while initial laccase activity was not varied.3.1. Ethanol concentration and enzymatic stability
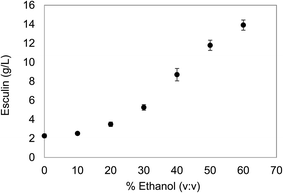
The initial concentration of the substrate is a critical issue to be considered before planning the polymerization reaction. On the one hand, high concentrations of the substrate are desirable in order to obtain high yields of the target product. However, esculin has a moderate aqueous solubility (≈3 g L−1) and, hence, the use of an organic solvent to increase the solubility of the substrate was considered. In the present work, ethanol was selected as a co-solvent, since it is compatible with cosmetic and pharmaceutical formulation guidelines and easy to scale up for industrial production.22With the purpose of selecting the oligomerization conditions, different ethanol concentrations were assessed to increase the solubility of esculin in aqueous matrix as well as to maintain satisfactory levels of laccase stability. The solubility of esculin in acetate buffer (0.1 M, pH 5) was 2.27 ± 0.20 g L−1 and the addition of ethanol increased exponentially the solubility of esculin, and rocketed to 5.2 times (11.8 ± 0.54 g L−1) when using 50% ethanol/acetate (Fig. 1).
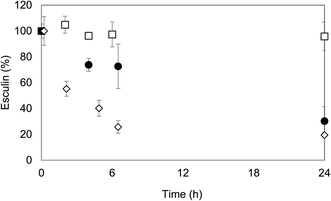
When it comes to evaluate the effect of ethanol on laccase stability, deactivation of laccase at the higher ethanol concentrations was evident (Fig. 2). Laccase was almost completely inactivated after 6 h of incubation at 60% ethanol, in agreement with other works.23 For lower ethanol concentrations of 40% and 50%, the effect was less dramatic, although laccase lost more than 90% of its activity after 24 h of incubation. Only at concentrations lower than 30% ethanol, laccase showed relatively satisfactory stability. After 6 h of incubation, the activity of laccase in 30% ethanol (v/v) was comparable to the one of the control (60% of the initial activity) and after 24 h, the enzyme lost approximately 70% of its activity, whereas for the control it was maintained slightly higher: 50% of the initial activity.
 | ||
Fig. 2 Influence of ethanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) acetate 100 mM (pH 5) mixtures on laccase stability at 0 h ( acetate 100 mM (pH 5) mixtures on laccase stability at 0 h ( ), 6 h ( ), 6 h ( ) and 24 h ( ) and 24 h ( ). ). | ||
From these results, the enzymatic oligomerization of esculin was subsequently performed at three initial concentrations of the substrate in ethanol/acetate buffer (pH 5) mixtures: 2, 5 and 10 g L−1. As a reference, the polymerization of 2 g L−1 of esculin in acetate buffer was performed (reaction A). Ethanol percentage of 30% allowed to increase 2.5 times the initial concentration of esculin (5 g L−1, reaction B), without a significantly detrimental effect on laccase activity. In order to increase esculin concentration up to 10 g L−1, 50% of ethanol was required (reaction C), and, in this case, a rapid laccase inactivation was expected. Laccase was added in one single initial pulse and the reactions were carried out under stirring conditions for 24 h at room temperature.
3.2. Polymerization reaction
The oxidation of substrates by laccase leads to the formation of free radicals that can react via non-enzymatic reactions i.e. oxidative condensation, and oxidative phenolic coupling or oxidative coupling, leading to the formation of soluble or insoluble reaction products, several of them of polymeric nature.24 Products from the coupling reactions can remain soluble and be substrates for further oxidative coupling, eventually forming larger polymers that precipitate.25 In this study, this phenomenon was observed in reactions A and B, where insoluble oligomers were produced and precipitated out of the solution. In the case of reaction C, no pellet was formed. Previous reports on esculin polymerization did not render into the formation of insoluble products, probably due to the presence of methanol as cosolvent.6,15The disappearance of esculin in the three experiments was monitored by following its residual concentration during the time-course of the oligomerization reaction (Fig. 3). Controls in absence of laccase showed that the initial concentration of esculin was maintained throughout the duration of the experiment (data not shown). It can be observed that higher concentration of esculin in reaction C led to lower conversion percentages (lower than 5%), probably due to the rapid laccase inactivation caused by the high concentration of ethanol. In this sense, laccase activity was below 15% after only 4 h and was negligible after 24 h (data not shown). The low conversion of esculin may explain the absence of precipitate in the reaction medium.
Reaction A took place mainly within the first hours and reached a final conversion of 80%. The pellet fraction accounted for 13.4% (w/w) of the total final products, as determined after lyophilization. In reaction B, the substrate concentration decreased more gradually and 70% of esculin was transformed after 24 h. In this case, although the formation of a small amount of precipitate was observed, its recovery was not possible, probably due to the presence of ethanol which permitted that most of the products remained soluble. A similar conversion of esculin (∼65% of a 3 g L−1 solution after 24 h) was obtained by Anthoni et al.8 using a mixture of methanol and water (30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 70, v/v).
70, v/v).
Polymerization reactions were monitored by UV-Vis spectrophotometry. During the development of the polymerization, the reaction media of A and B experiments changed their color to dark green. This change was associated with the enzymatic oxidation of esculin. This effect was also observed by Sun et al.26 for the catalytic oxidation by laccase of various phenolic compounds such as catechol, resorcinol and hydroquinone. The UV-Vis spectra of A and B reaction media showed a similar pattern (see Fig. S1 of the ESI†). A maximum absorption at 335 nm was recorded, which is attributed to esculin, and it decreased with incubation time. Furthermore, as the reaction proceeded, a bathochromic shift was observed, indicating a greater degree of π-conjugation and thus, an increase of the molar mass of the oligomer.27 Anthoni et al.15 and Chebil et al.6 also reported a similar profile in enzymatic synthesis of oligoesculin.
3.3. Evaluation of the total phenolic compounds of esculin transformation products
The number of hydroxyl groups on the ring structure of coumarins is correlated with ROS suppression effects. Laccase polymerization of esculin could occur through the formation of C–O bridges and might involve the phenolic part of the molecule.15 In order to study the evolution of hydroxyl phenolic moieties during the enzymatic reaction, Folin–Ciocalteau method was employed (Fig. S2†). The analysis revealed that the total phenolic compounds present in the different reaction media were closer to the control (medium without laccase). Only in the reaction B it was observed a progressive decay, and after 24 h of reaction the concentration of TPC was 25% lower than the control. Therefore, it was concluded that the action of laccase on esculin did not decrease significantly the phenolic moieties present in the media.3.4. Antioxidant activities of esculin transformation products determined as ferric reducing antioxidant power (FRAP), cupric reducing antioxidant capacity (CUPRAC) and xanthine oxidase inhibition assay
Coumarins have been associated with beneficial effects on human health and such effects are thought to be related to the radical scavenging effect, due to their antioxidant activities, along with other possible mechanisms, such as the interaction with several enzymes.1 CUPRAC and FRAP methods were used to evaluate the antioxidant activity of the lyophilized controls and products (Table 1). Reaction controls, lacking enzyme, contained acetate buffer as in the enzymatic reaction. Differences observed in the three controls derive from their salt content. The buffer concentration was the same in the three experiments (10 mM), but A consisted in 100% buffer, B contained 70% and C contained 50% buffer. Hence, after lyophilization, the salt content in medium A was double than in medium C. This resulted in significantly lower antioxidant activities for control A. Regarding the products from the enzymatic reactions, the pellet fraction from reaction A presented the strongest antioxidant activity, as represented by their higher values of trolox equivalents compared to each of the reaction controls: CUPRAC and FRAP values of TE were 7.4 and 3.2 folds higher than the controls and even higher than pure esculin (125.7 and 39.7 mg TE per g product for CUPRAC and FRAP assays, respectively).| Experiment | CUPRAC, mg TE per g product | FRAP, mg TE per g product | ||||
|---|---|---|---|---|---|---|
| A | B | C | A | B | C | |
| a Recovered pellet fraction was not sufficient to perform the antioxidant assays.b Not observed a pellet fraction. | ||||||
| Control | 43 ± 2 | 83 ± 3 | 110 ± 5 | 19 ± 2 | 30 ± 1 | 35 ± 2 |
| Pellet | 318 ± 15 | a | b | 60 ± 3 | a | b |
| Supernatant | 50 ± 2 | 169 ± 5 | 123 ± 16 | 15 ± 6 | 45 ± 1 | 35 ± 2 |
Therefore, comparing the results of antioxidant activity obtained for the different reactions, reaction A, with esculin at 2 g L−1 in acetate buffer (0.1 M, pH 5), led to products with enhanced antioxidant activity. These results were also consistent with the study of Chebil et al.6 where polyesculin presented better antioxidant properties than esculin.
Different authors reported that the position and the type of the substituent attached to the aromatic ring of coumarin molecules have a great influence on their antioxidant activity. The number of hydroxyl groups on the ring structure1 and the presence of an ortho-catechol moiety28 played a major role on effective radical-scavenging. Although few data are available about the behavior of these activities with polymerization, the presence or absence of these essential groups can explain the synthesis of different products during the enzymatic oligomerization of esculin.
Xanthine oxidase is not only an important biological source of ROS29 but also the enzyme responsible for the formation of uric acid associated with gout leading to painful inflammation in the joints.7 Hyperuricemia and gout are treated by either increasing the excretion of uric acid or reducing the uric acid production. Xanthine oxidase inhibitors are very useful for this because they reduce both vascular oxidative stress and circulating levels of uric acid.30
Table 2 shows xanthine oxidase inhibitory activity of esculin and its oligomeric fractions produced in the different reactions, assessed by the measurement of uric acid formation from xanthine oxidase. The values were calculated by standard curve regression analysis with R2 between 0.9704 and 1.0000. As described above, the differences observed in the three controls derive from their buffer content. All products led to IC50 values lower than their respective controls, and this was more evident for products from reaction A. The pellet fraction showed the lowest IC50 value (187 mg L−1), resulting in xanthine oxidase inhibition activity 5.3-fold higher than its control. In a previous paper, Chebil et al.6 found that the higher the molecular weight of the oligomers, the lower the IC50 value (molar concentration). In this sense, they observed that the fraction with a weight-average molecular mass of 6973 (g mol−1) had an IC50 of 141 μM (5-fold lower than the monomer). However, in terms of mass concentration, the IC50 value would be 983 mg L−1, indicating significantly lower inhibition capacity than the control (264 mg L−1). This is a critical issue for its practical application, since the transformation process should ideally lead to products with improved characteristics per gram of starting material. Our results differ from those by Chebil et al.6 since all products from the enzymatic oligomerization of esculin showed enhanced XO inhibitory activity in terms of mass concentration.
Lin et al.31 studied the structure–activity relationship of coumarin derivatives on xanthine oxidase inhibitory activity and their results suggest that the number of hydroxyl groups on the ring structure of coumarins is correlated with the effects of reactive oxygen species supression. Hofmann et al.2 reported that the most effective XO inhibitors carried a hydroxyl group in the C7 position of the coumarin scaffold. Hence, the higher XO inhibition showed by the products of the enzymatic transformation, suggest the formation of C–C bridges between esculin units during oligomerization, maintaining the hydroxyl group in the C7 position. Anthoni et al.15 performed molecular modeling simulations and their results suggest the preferential formation of C8–C8 linkages during the oligomerization reaction.
3.5. Evaluation of antimicrobial activity
The antimicrobial activity of pellet and the retentate of a 10 kDa membrane obtained from reaction A was evaluated against E. coli and S. enteritidis. Three different concentrations of the lyophilized products were tested (2.5, 5 and 7.5 mg mL−1), and the results are summarized in Table S1.† The esculin oligomer fractions did not exhibit antibacterial effects against the tested bacterial strains as the values obtained for the test compounds were similar for those of the positive control. E. coli and Salmonella are Gram-negative bacteria, characterized by the presence of an outer membrane and unique periplasmic space. The hydrophilic surface of the outer membrane is rich in lipopolysaccharide molecules and it represents a barrier to the penetration of numerous antibiotic molecules. Also, in the periplasmic space there are enzymes capable of breaking down the molecules introduced from outside.32 These characteristics could explain the absence of antibacterial effect of products obtained in the present study. However, Mokdad-Bzeouich et al.16 observed that oligomerization of esculin increased its antibacterial potential according to the degree of polymerization. The antimicrobial activity of esculin expressed as MIC (the lowest concentration of the tested compound that inhibit the bacterial growth) for E. coli was 2.5 g L−1 and for S. enteritidis and S. typhimurium was 625 mg L−1 and 5 g L−1, respectively. Moreover, the MIC values of retentate obtained after diafiltration of oligoesculin on a 5 kDa membrane for E. coli and S. typhimurium was 312 mg L−1 and for S. enteritidis was 625 mg L−1. Differences in the antimicrobial activity in both studies could be partially explained by a different degree of oligomerization, the formation of different products, strains sensitivity and variations in the antimicrobial protocols used.213.6. Molecular weight characterization of the different reaction products
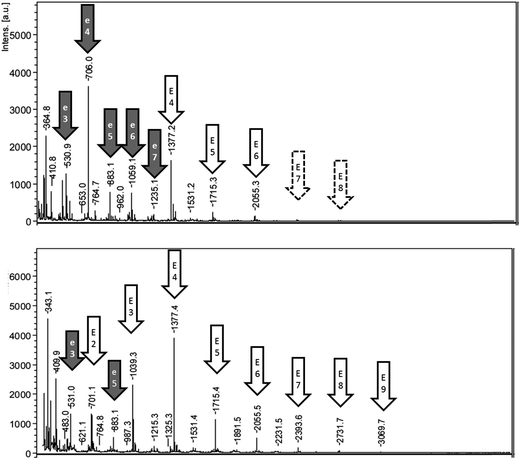
The MALDI-TOF analysis of fractions obtained from reaction A showed esculin oligomers with a common repeated unit of 338 Da (Fig. 4). Oligomers were mainly detected as sodium adducts, and also as protonated compounds (Tables S2 and S3†). The abstraction of two hydrogen atoms during oligomerization suggests that the connection mode between esculin units was a simple bridge. The longest esculin oligomer present in the pellet had a degree of polymerization (DP) of 8, and the highest intensity was obtained at DP 4 (Fig. 4). Similarly, the highest DP found in the supernatant fraction was 9, and the most abundant signal was at DP 4. The results are comparable with the values obtained by Anthoni et al.15,17 who determined that the longest oligomer was also a nonamer and the highest intensity was found at DP 5. Interestingly they observed that oligomerization stabilized at the weight-average mass corresponding to DP 5 and did not increase with time, although enzyme was still active and substrate was not limiting.15 This could be explained by a higher reactivity of low molecular weight oligomers in comparison to larger ones.Other significant signals were observed, especially in the pellet fraction, with a repetition unit of 176.1 m/z. This mass, after the abstraction of two hydrogen atoms, corresponds to the coumarin aglycon esculetin (178.1 Da), which is esculin lacking the sugar moiety. The presence of oligomers with this common repetition may suggest the hydrolysis of esculin oligomers to produce esculetin oligomers. In this sense, the sugar group of the oxidized species may have increased susceptibility toward hydrolysis. Esculetin is very poor soluble in water due to the absence of glycosylation as compared with the glycoside esculin.33 Hence, it should be expected that this compound and its oligomers would precipitate in aqueous medium. Accordingly, as can be observed in Fig. 4, the oligomers of esculetin were mainly distributed in the pellet fraction. The presence of esculetin oligomers has not been reported in previous research of esculin oligomerization by laccase where methanol was used in the reaction medium.15,17 In the present work, the absence of ethanol in reaction A favored the formation of a pellet, rich in these oligomers, which permitted a detailed analysis between both soluble and insoluble fractions.
The increased XO inhibitory potential and antioxidant activities of the pellet fraction could be related to the presence of esculetin oligomers. In a related study, Hofmann et al.2 evaluated the XO inhibition activity of different coumarins and concluded that esculetin was the most potent inhibitor by a large margin, with an IC50 of 1.33 mg L−1. Additionally, esculetin was reported as the most potent radical scavenger among eight tested coumarin derivatives, as well as the most potent agent at protecting cells against ROS-mediated Aβ-damage.31 In order to reinforce the hypothesis that the presence of oligoesculin oligomers could be related to the increased XO inhibitory potential, laccase catalyzed oligomerization of commercially available esculetin was performed. In this sense, the products obtained after 24 h of reaction showed an IC50 of 91 mg L−1, significantly lower than that of esculin and its oligomers. Other characteristics of these esculetin oligomers deserve careful future investigations.
Other signals with a repetition unit of 338 Da but with masses different from esculin oligomers were also detected, as presented in ESI Tables S2 and S3.† This could correspond to oligomers of esculin lacking one glucose molecule (162.1), as was observed in the supernatant for 2E-G (dimer of esculin lacking one sugar moiety) to 7E-G molecules or esculin oligomers with an additional molecule of glucose (Tables S2 and S3†). Fig. 5 shows the structure of the suggested metabolites from the enzymatic transformation of esculin, considering C8–C8 linkages between esculin units, as suggested by Anthoni et al.15
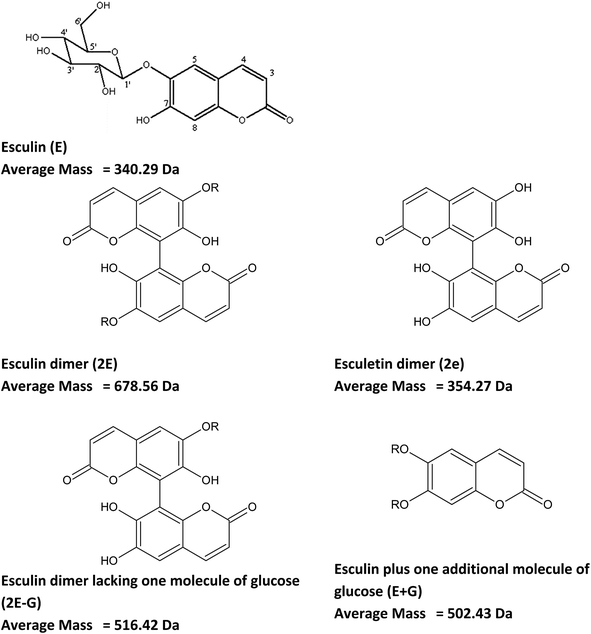 | ||
| Fig. 5 Chemical structure of esculin and possible metabolites from its laccase catalyzed transformation. C8–C8 linkages between esculin units are suggested accordingly to Anthoni et al.15 R: glucose. | ||
The MALDI-TOF spectrum of the supernatant from reaction B (Fig. S3 of ESI†) was very similar to that of reaction A, observing oligomers with DP 7 and the maximum intensity for DP 4. As observed with reaction A, oligomers with a repetition unit of 176.1 m/z, suggested as esculetin oligomers, were also detected. Furthermore, other signals with a repetition unit of 338 Da, which could correspond to oligomers of esculin lacking one sugar moiety, were observed (data not shown). Finally, the low extent of reaction C was evidenced by the limited presence of oligomers (detected only DP 2 and DP 3) (Fig. S3†).
Relative masses of polymers formed in reaction A were also evaluated by size exclusion chromatography as described in ESI (Fig. S4†). The pellet and supernatant fractions showed oligomers with molecular mass between 8100 and 1220 g mol−1, whereas the control only showed the peak of esculin. These results indicated that the polymerization of esculin occurred and led to polymers with molecular mass higher than those observed by MALDI analyses. This would suggest that MALDI analysis underestimated molecular mass distribution, and could be related to a weak ionization of the oligomers.17
3.7. FT-IR analysis
The FT-IR spectra of the supernatant and pellet fractions from the reaction A and its control are shown in Fig. 6. The FT-IR spectra of both products were considerably smoother, probably due to the limited mobility of the side substituents especially in case of polymers, leading to a more rigid final product.34 A new peak in 1475 cm−1 was observed for the pellet fraction. This could correspond with new C–C bonds formed during oligomerization reactions (typically around 1450 and 1512 cm−1), but only observed in the pellet fraction. This is in accordance with the hypothesis that high XO inhibition of the oligomers suggests the formation of C–C bridges between esculin units during oligomerization.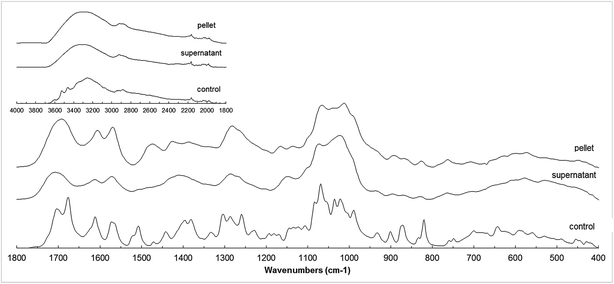 | ||
| Fig. 6 FT-IR spectra of control, pellet and supernatant fractions of reaction A (esculin at 2 g L−1 in acetate buffer). | ||
The peak in 1506 cm−1 observed in the control was absent in both products, and could be attributed to the disappearance of a C–H bond. The intensity of the ether band around 1015 cm−1 increased in both products (C–O–C stretching vibration typically located around 1010–1270 cm−1), and indicate that polymerization may occur through ether bond. Due to the high antioxidant activity of the products and considering that the phenolic content did not decrease throughout the reaction, a linkage between aromatic part and glycosidic part of esculin (C4–O2′) is considered more probable than the O7–C4 bridge.15
The comparison of pellet and supernatant spectra showed more intense bands in 1550–1650 cm−1 in the pellet fraction, corresponding to aromatic C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds, and also higher intensity for the peak in 1700 cm−1 corresponding to the C
C bonds, and also higher intensity for the peak in 1700 cm−1 corresponding to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond. This could suggest that aromatic compounds, although present in both fractions, were more abundant in the pellet fraction. This is in agreement with findings from MALDI analysis, which suggested the presence of esculetin (aglycon of esculin) in the pellet fraction.
O bond. This could suggest that aromatic compounds, although present in both fractions, were more abundant in the pellet fraction. This is in agreement with findings from MALDI analysis, which suggested the presence of esculetin (aglycon of esculin) in the pellet fraction.
Summarizing, the data obtained from FTIR analysis suggested that supernatant oligomers were mainly formed through C–O bonds, whereas the pellet fraction presented both C–O and C–C linkages. These results differ from those from Rhouma et al.5 who reported that oligoesculin was obtained through C–C linkages.
4. Conclusions
The oligoesculin molecules were enzymatically synthesized by an oxidative polymerization of esculin by laccase from T. versicolor. The presence of ethanol in the reaction medium was required to promote esculin solubilization above 2 g L−1. However, the highest concentration of ethanol (50%, v/v) led to a rapid inactivation of the enzyme and the transformation of esculin was minor. The evaluation of antioxidant activity and the xanthine oxidase inhibitory effect of the products obtained in the different reactions showed that the reaction with esculin in absence of ethanol produced the most interesting polymers. Particularly, the pellet fraction of this reaction showed the most potent activities, as demonstrated by the values of trolox equivalents: 7.4 and 3.2 folds higher than the controls, for CUPRAC and FRAP assays, respectively. Additionally, the pellet showed the lowest IC50 value for xanthine oxidase inhibition (178 mg L−1), being 5.4-fold higher than the control. Contrarily to previous published research, esculin oligomer fractions did not exhibit antibacterial effects against E. coli and Salmonella. The MALDI-TOF analysis indicated the presence of esculin oligomers with a similar degree of polymerization in the pellet fraction and the supernatant (DP 9). However, the pellet fraction was rich in polymers with a repetition unit of 176 Da, which may correspond with esculetin oligomer. The presence of these oligomers in the pellet could be related with its high antioxidant potential. SEC spectra of the products showed a greater degree of polymerization than that observed by MALDI analyses. FTIR analysis revealed that oligomerization in the supernatant occurred through C–O linkages, meanwhile oligomers present in the pellet were formed via C–C and C–O linkages, the latter probably via the glycosidic part of esculin.This is the first study that reports the formulation of an insoluble esculin derivative by its laccase-catalyzed oligomerization, which can be carried out using a simple process compatible with food, cosmetic and pharmaceutical guidelines. This product has shown excellent antioxidant properties and may have great potential use as nutraceutical or pharmaceutical agent. Further research should focus on how to shift the enzymatic reaction to obtain the oligomers with the most interesting properties.
Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
This work was financially supported by the Spanish Ministry of Economy and Competitiveness (CTQ2014-58879-JIN). Authors belong to the Galician Competitive Research Group GRC 2013-032 and to the strategic group CRETUS (AGRUP2015/02). All these programmes are co-funded by FEDER. B. G. thanks the Spanish Ministry of Economy and Competitiveness for her postdoctoral fellowship (Grant reference FPDI-2013-17341).References
- G. Bubols, D. Vianna, A. Medina-Remón, G. von Poser, R. Lamuela-Raventos, V. Eifler-Lima and S. Garcia, The antioxidant activity of coumarins and flavonoids, Mini-Rev. Med. Chem., 2013, 13(3), 318–334 CAS.
- E. Hofmann, J. Webster, T. Kidd, R. Kline, M. Jayasinghe and S. Paula, Coumarins with xanthine oxidase inhibiting and radical scavenging properties: tools to combat oxidative stress in cells, Int. J. Biosci., Biochem. Bioinf., 2014, 4(4), 234–239 CAS.
- G. Stanić, B. Jurišić and D. Brkić, HPLC Analysis of esculin and fraxin in horse-chestnut bark (Aesculus hippocastanum L), Croat. Chem. Acta, 1999, 72(4), 827–834 Search PubMed.
- E. Bellini and S. Nin, Horse Chestnut: Cultivation for ornamental purposes and non-food crop production, J. Herbs, Spices Med. Plants, 2005, 11(1–2), 93–120 CrossRef.
- G. B. Rhouma, L. Chebil, M. Krifa, M. Ghoul and L. Chekir-Ghedira, Evaluation of mutagenic and antimutagenic activities of oligorutin and oligoesculin, Food Chem., 2012, 135, 1700–1707 CrossRef PubMed.
- L. Chebil, G. B. Rhouma, L. Chekir-Ghedira and M. Ghoul, in Enzymatic polymerization of rutin and esculin and evaluation of the antioxidant capacity of polyrutin and polyesculin, Ed. D. Ekinci, INTECH Biotechnology, 2015 Search PubMed.
- M. Kurisawa, J. E. Chung, Y. J. Kim, H. Uyama and S. Kobayashi, Amplification of antioxidant activity and xanthine oxidase inhibition of catechin by enzymatic polymerization, Biomacromolecules, 2003, 4(3), 469–471 CrossRef CAS PubMed.
- M. Kurisawa, J. E. Chung, H. Uyama and S. Kobayashi, Enzymatic synthesis and antioxidant properties of poly(rutin), Biomacromolecules, 2003, 4, 1394–1399 CrossRef CAS PubMed.
- K. Racicot, N. Favreau, S. Fossey, A. R. Grella, T. Ndou and F. F. Bruno, Antioxidant potency of highly purified polyepicatechin fractions, Food Chem., 2012, 13, 902–907 CrossRef.
- M. Ghoul and L. Chebil, Enzymatic polymerization of phenolic compounds by oxidoreductases, Springer Briefs in Green Chemistry for Sustainability, Springer, Netherlands, 2012 Search PubMed.
- S. Shoda, H. Uyama, J. Kadokawa, S. Kimura and S. Kobayashi, Enzymes as green catalysts for precision macromolecular synthesis, Chem. Rev., 2016, 116, 2307–2413 CrossRef CAS PubMed.
- F. Hollmann and I. W. C. E. Arends, Enzyme initiated radical polymerizations, Polymers, 2012, 4(1), 759–793 CrossRef.
- S. B. Jadhav and R. S. Singhal, Laccase–gum Arabic conjugate for preparation of water-soluble oligomer of catechin with enhanced antioxidant activity, Food Chem., 2014, 150, 9–16 CrossRef CAS PubMed.
- A. Kunamneni, J. F. Plou, A. Ballesteros and M. Alcalde, Laccases and their applications: A patent review, Recent Pat. Biotechnol., 2008, 2(1), 10–24 CrossRef CAS PubMed.
- J. Anthoni, C. Humeau, E. R. Maia, L. Chebil, J. M. Engasser and M. Ghoul, Enzymatic synthesis of oligoesculin: structure and biological activities characterizations, Eur. Food Res. Technol., 2010, 231, 571–579 CrossRef CAS.
- I. Mokdad-Bzeouich, N. Mustapha, F. Chaabane, Z. Ghedira, K. Ghedira, M. Ghoul, L. Chebil and L. Chekir-Ghedira, Oligomerization of esculin improves its antibacterial activity and modulates antibiotic resistance, J. Antibiot., 2014, 68(3), 148–152 CrossRef PubMed.
- J. Anthoni, L. Chebil, F. Lionneton, J. Magdalou, C. Humeau and M. Ghoul, Automated analysis of synthesized oligorutin and oligoesculin by laccase, Can. J. Chem., 2011, 89, 964–970 CrossRef CAS.
- V. L. Singleton and J. A. Rossi, Colorimetric of total phenolics with phosphomolybdic–Phosphotungstic acid reagents, Am. J. Enol. Vitic., 1965, 16, 144–158 CAS.
- G. Vázquez, J. Santos, M. S. Freire, G. Antorrena and J. González-Álvarez, Extraction of antioxidants from eucaliptus (Eucalyptus globulus) bark, Wood Sci. Technol., 2012, 46, 443–457 CrossRef.
- R. Apak, K. Güçlü, B. Demirata, M. Özyürek, S. E. Çelik, B. Bektaşoğlu, K. I. Berker and D. Özyurt, Comparative Evaluation of various total antioxidant capacity assays applied to phenolic compounds with the CUPRAC assay, Molecules, 2007, 12, 1496–1547 CrossRef CAS PubMed.
- B. Gullón, M. E. Pintado, J. A. Pérez-Álvarez and M. Viuda-Martos, Assessment of polyphenolic profile and antibacterial activity of pomegranate peel (Punica granatum) flour obtained from co-product of juice extraction, Food Control, 2016, 59, 94–98 CrossRef.
- R. G. Strickley, Solubilizing excipients in oral and injectable formulations, Pharm. Res., 2004, 21(2), 201–230 CrossRef CAS.
- J. Rogalski, A. Dawidowicz, E. Jóźwik and A. Leonowicz, Immobilization of laccase from Cerrena unicolor on controlled porosity glass, J. Mol. Catal. B: Enzym., 1999, 6, 29–39 CrossRef CAS.
- C. Hautphenne, M. Penninckx and F. Debaste, Product formation from phenolic compounds removal by laccases: A review, Environmental Technology & Innovation, 2016, 5, 250–266 Search PubMed.
- Q. Huang, J. Tang and W. J. Weber, Precipitation of enzyme-catalyzed phenol oxidative coupling products: Background ion and pH effects, Water Res., 2005, 39, 3021–3027 CrossRef CAS PubMed.
- X. Sun, R. Bai, Y. Zhang, Q. Wang, X. Fan, J. Yuan, L. Cui and P. Wang, Laccase-catalyzed oxidative polymerization of phenolic compounds, Appl. Biochem. Biotechnol., 2013, 171, 1673–1680 CrossRef CAS PubMed.
- G. R. Strobl, The Physics of Polymers: Concepts for Understanding their Structures and Behavior, Springer, 2007 Search PubMed.
- T. Kaneko, N. Baba and M. Matsuo, Protection of coumarins against linoleic acid hydroperoxide-induced cytotoxicity, Chem.–Biol. Interact., 2003, 142, 239–254 CrossRef CAS PubMed.
- J. M. McCord and I. Fridovich, The Reduction of cytochrome c by milk xanthine oxidase, J. Biol. Chem., 1968, 243(21), 5753–5760 CAS.
- D. A. Kostić, D. S. Dimitrijević, G. S. Stojanović, I. R. Palić, A. S. Đorđević and J. D. Ickovski, Xanthine oxidase: isolation, assays of activity, and inhibition, J. Chem., 2015, 2015, 294858 Search PubMed.
- H. C. Lin, S. H. Tsai, C. S. Chen, Y. C. Chang, C. M. Lee, Z. Y. Lai and C. M. Lin, Structure–activity relationship of coumarin derivatives on xanthine oxidase-inhibiting and free radical-scavenging activities, Biochem. Pharmacol., 2008, 75, 1416–1425 CrossRef CAS PubMed.
- Y. Gao, M. J. van Belkum and M. E. Stiles, The outer membrane of gram-negative bacteria inhibits antibacterial activity of brochocin-C, Appl. Environ. Microbiol., 1999, 65(10), 4329–4333 CAS.
- M. Tattini, M. Di Ferdinando, C. Brunetti, A. Goti, S. Pollastri, C. Bellasio, C. Giordano, A. Fini and G. Agati, Esculetin and esculin (esculetin 6-O-glucoside) occur as inclusions and are differentially distributed in the vacuole of palisade cells in Fraxinus ornus leaves: A fluorescence microscopy analysis, J. Photochem. Photobiol., B, 2014, 140, 28–35 CrossRef CAS PubMed.
- A. Zerva, N. Manos, S. Vouyiouka, P. Christakopoulos and E. Topakas, Bioconversion of biomass-derived phenols catalyzed by Myceliophthora thermophila laccase, Molecules, 2016, 21, 550 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7ra06972c |
| This journal is © The Royal Society of Chemistry 2017 |