Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Zinc dopant inspired enhancement of electron injection for CuInS2 quantum dot-sensitized solar cells†
Qinqin Wua,
Chunqi Caia,
Lanlan Zhai a,
Jiantao Wanga,
Fantai Kongb,
Yun Yang
a,
Jiantao Wanga,
Fantai Kongb,
Yun Yang a,
Lijie Zhanga,
Chao Zou
a,
Lijie Zhanga,
Chao Zou *a and
Shaoming Huang*a
*a and
Shaoming Huang*a
aZhejiang Key Laboratory of Carbon Materials, College of Chemistry and Material Engineering, Wenzhou University, Wenzhou 325027, Zhejiang, People's Republic of China. E-mail: zouchao@wzu.edu.cn; smhuang@wzu.edu.cn
bKey Laboratory of Novel Thin Film Solar Cells, Hefei Institute of Physics Science, Chinese Academy of Sciences, Hefei 230088, Anhui, People's Republic of China
First published on 14th August 2017
Abstract
After being doped with zinc, CuInS2 quantum dots (QDs) exhibit desired tunable optical and electronic properties, more specifically, photoluminescence emission and band gap. The former is mainly due to the intrinsic donor–acceptor transition, which, together with the improved quantum yield and large longest decay time, accounts for 95% of the whole emission profiles. The latter results in an enhanced ket value of 2.99 × 1010 s−1, greater than that for pure CuInS2 QDs by an order of magnitude. Functioning as light harvesting materials in quantum dot sensitized solar cells, zinc doped CuInS2 QDs show broadened photoresponse up to ∼950 nm. Incident photon-to-current conversion efficiency of quantum dot sensitized solar cells achieves a maximum of 69% at 500 nm and can be maintained over 50% within the window below 750 nm. After the doping with zinc under optimized conditions, the average power conversion efficiency of solar cells under one full sun illumination demonstrates an increase of 13.2%, from 5.21% for pure CuInS2 QDs to 5.90% for doped CuInS2 QDs. It is proven that the improved performance can be attributed to a broadened optoelectronic response range and accelerated electron injection.
1. Introduction
Due to quantum confinement and enhanced surface to volume ratios, colloidal quantum dots (QDs) provide a unique platform for designing new applications in solar cells, LEDs, and photocatalysts.1 The flexibility of QDs may lead to controllable band edge energies and composition, tunable bandgap onset energies, strong optical transitions, facile doping and synthesis strategies, and enhanced multiple exciton generation (MEG).2–6 Recently, quantum dot-sensitized solar cells (QDSCs) have drawn great attention with the use of QDs for the development of a suitable sensitizer in the next generation of photovoltaic technologies.7,8 Power Conversion Efficiencies (PCE) up to 11.16% and 11.61% based on CdSeTe and Zn–Cu–In–Se QDSCs, respectively, were reported by Zhong and coworkers.9,10 Functioning as sensitizers, QDs play vital roles in terms of light harvesting and corresponding electron injection.11–13 The recent emergence of ternary I–III–VI2 QDs offers a promising alternative to the binary chalcogenide QDs for their enhanced tunable optical and electronic properties, in addition to ecofriendliness.14,15As a typical I–III–VI2 semiconductor,16 CuInS2 possessing direct band gap displays high absorbing coefficient and good stability under solar radiation, and is compatible with the AM0 solar spectrum (Eg = 1.5 eV).17–19 These properties make CuInS2 QDs a good candidate as the sensitizer in quantum dot-sensitized solar cells.8 In order to meet the increasingly demand of effective solar cells, considerable efforts have been made to improve the performance of CuInS2 QDs based QDSCs.8,18,20–23 Zhong and Hyeon groups have reported high performance QDSCs with PCE of 7.04% (ref. 23) for CuInS2/ZnS and 8.10% for CuInSe2,24 respectively. However, the potential of QDSCs has yet to be fully demonstrated. Beneficial from MEG, the theoretical PCE of QDSCs could reach up to 44%.2,25
To further boost the efficiency of QDSCs, it is desired to obtain suitable QDs, which are featured by wide absorption range, high conduction band edge, and limited trap state defects, using simple and reproducible processes.26,27 It has been demonstrated that type-I core/shell structured QDs developed by the overgrowth of wider band gap ZnS or CdS shell on QDs have enhanced luminescent emission efficiency and stability.28 For example, Zhong and coworkers showed type-I core/shell QDs based QDSCs with PCE over 9% for CdSeTe/CdS29 and 7.04% for CuInS2/ZnS,23 respectively. However, the overgrowth of shell on QDs core may reduce charge recombination and act as injection barrier, which have conflicting effects on effective electron transportation.23 It is difficult to make a compromise between these two effects. In addition, the conventional syntheses by two separated steps for core and shell growths using Schlenk technology are neither easy nor reproducible, even after decades from the appearance of colloidal method.28,30 Doped QDs provide a promising alternative.31 Dopant could alter electron wave in orbitals of host QDs, producing moderate modulation on band gaps and further on conduction band edges,30,32 hence, promoting electron injection from QDs to TiO2 photoanodes. Additionally, ternary CuInS2 QDs tolerate a large range of off-stoichiometric compositions and complex crystal structure relevant with donor and acceptor trap states.15,33–36 Donor and acceptor sub-bandgap states are originated from Cu and In vacancies (VCu and VIn) and replacing defects (CuIn and InCu).35–37 It has been shown that donor and acceptor trap states in QDs could be adapted by the incorporation of dopant into the host structure. The successes of Zn–Cu–In–Se,10 Cu–In–Ga–Se,38 CuInSexS2−x (ref. 18) and Zn–Ag–In–Se39 QDs have been validated. Noted is that both of the two reports on QDSCs with PCE above 11% were conducted under the assistant of highly catalytic Ti/C counter electrodes rather than on conventional Cu2S/brass.
Herein, a facile synthesis of zinc doped CuInS2 QDs with tunable photoluminescence is presented. A series of zinc doped CuInS2 QDs that is capped by oleylamine were synthesized by thermal decomposition of organometallic precursors. It was shown that tunable photoluminescence (PL), long lifetime and enhanced quantum yield (QY) of zinc doped CuInS2 QDs are mainly attributed to the donor–acceptor pair recombination, which accounts for over 95% of the whole emission profiles. Water-soluble zinc doped CuInS2 QDs were tethered on mesoporous TiO2 film electrode after ligand exchange. The fabricated zinc doped CuInS2 QDs-based QDSCs exhibit photoresponse extended to ∼950 nm and achieve average PCE of 5.90% under one full sun illumination.
2. Experimental
2.1 Materials
All chemicals were used as received without further purification. Copper diethyldithiocarbamate (Cu(dedc)2, 97.0%) and zinc diethyldithiocarbamate (Zn(dedc)2, 99%) were obtained from TCI; indium nitrate (In(NO3)3, 99.9%) from Alfa Aesar; sodium diethyldithiocarbamate trihydrate (Na(dedc), 99%), and n-hexane (95%) from J&K; oleylamine (>80%), dodecanethiol (98%), and mercaptopropionic acid (MPA, >99%) from Acros.2.2 Synthesis of In(dedc)3 precursors
The In(dedc)3 precursors were synthesized according to the previous reports.40 In a typical synthesis of In(dedc)3, Na(dedc) (6 mmol) and In(NO3)3 (2 mmol) were respectively dissolved in 100 mL and 50 mL de-ionized water, then the In(NO3)3 solution was added dropwise to the Na(dedc) solution with magnetic stirring. The white product was washed 3 times with de-ionized water and dried under a vacuum at 60 °C for 3 h. As-synthesized precursors were stored in desiccator at room temperature.2.3 Synthesis of zinc doped CuInS2 QDs with tunable composition
Zinc doped CuInS2 QDs with tunable compositions can be achieved by varying the relative molar ratio of precursors in the source materials. In a typical synthesis of zinc doped CuInS2 QDs (ZCIS-10), Cu(dedc)2 (0.05 mmol), In(dedc)3 (0.1 mmol) and Zn(dedc)2 (0.01 mmol) were loaded into a 50 mL rounded-bottom flask, which was then filled with 4 mL of oleylamine. After ultrasonic dispersing at room temperature, magnetic stirring at room temperature was followed. The flask was immersed into oil bath and maintained at 180 °C for 20 min and then allowed to cool to room temperature by water bath. The red dark product was collected by centrifugation, washed several times with absolute ethanol and n-hexane. Pure CuInS2 QDs were synthesized with the absence of zinc precursors. For simplicity, the sample for zinc doped CuInS2 QDs with the ratio of Zn/In precursors 0.025 was referred ZCIS-2.5 and so on.2.4 Ligand exchange of QD and fabrication of TiO2 photoanodes
Ligand exchange of oil-soluble ZCIS QD to water-soluble QDs was performed according to a literature method. Typically, 0.4 mmol MPA was first dissolved in 1.0 mL methanol, then the pH of the solution was adjusted to 11 with the use of 30% NaOH aqueous solution. The MPA solution was added into 15 mL ZCIS QDs dichloromethane solution and stirred for 30 min to get the precipitation of the ZCIS QDs. Then 10.0 mL de-ionized water was added into the mixture and kept the stirring for another 20 min. The solution was separated into two phases and the ZCIS QDs were transferred into the superincumbent water from the underlying chloroform, the underlying phase was discarded and the aqueous phase containing the MPA-capped ZCIS QDs was collected. The aqueous dispersion was further purified by centrifugation and decantation with the addition of acetone, and the precipitate was redissolved in 1.0 mL de-ionized water.The double layered mesoporous TiO2 photoanodes were fabricated on well-cleaned FTO glass. Before the 10.0 μm thick transparent 20 nm-TiO2 layer was coated on the FTO substrate by successive screen-printing of TiO2 paste, the cleaned FTO glass was treated with 40 mM TiCl4 aqueous solution for the formation of a compact TiO2 layer on substrate, followed by another screen-printing of 2.0 μm thick light scattering 200 nm-TiO2 layer. Finally, the film was heat treated in a hot plate at 500 °C for 30 min.
The doped CuInS2 QD sensitizers were immobilized on the TiO2 mesoporous films by pipetting 30 μL QD aqueous dispersion (absorbance of 3.0 and pH of 11.0) onto the film surface and maintaining 4 h before rinsed sequentially with de-ionized water and ethanol and then dried with nitrogen. After the immobilization, the sensitized TiO2 films were coated with ZnS, alternately dipping into 0.1 M Zn(NO3)2 and 0.1 M Na2S solution for 1 min, rinsing with ethanol between dips.
2.5 Assembling solar cells
The sandwich-type cells were constructed by assembling the photoanode and the Cu2S/brass counter electrode using a 50 μm thick Scotch spacer. To prepare Cu2S/brass counter electrode, brass foil was immersed in HCl solution (1.0 M) at 80 °C for 15 min and subsequently soaked into polysulfide electrode solution for 2 min. The polysulfide electrode solution was obtained by the dissolution of 2.0 M Na2S, 2.0 M S, and 0.2 M KCl in de-ionized water. A droplet (10 μL) of polysulfide electrolyte was injected into the cell device. The area of the cells was 0.25 cm2. For QDSCs fabricated under each condition, several cells were performed and tested in parallel.2.6 Characterization
The as-synthesized sample were drop cast on the Si low background sample holders and dried at room temperature in the air, then the powder X-ray diffraction (XRD) patterns of the prepared samples were recorded on Bruker D8 advance X-ray diffractometer with graphite monochromatized Cu Kα (λ = 1.5405 Å) radiation with a step of 0.02° at a scanning speed of 4° min−1 in 2θ ranging from 10° to 80°. Scanning electron microscopy (SEM) images were taken using a FEI Nova NanoSEM200 microscope. Composition analysis was performed by EDS (oxford INCA). The transmission electron microscopy (TEM) was carried out under JEOL JEM-2100F microscope operating at an accelerating voltage of 200 kV. UV-visible (UV-vis) absorption spectra of the samples were recorded on a SHIMADZU UV-1800 spectrophotometer. Steady-state photoluminescence (PL) spectra and absolute quantum yield (QY) were measured using a Fluoromax-4 spectrofluorometer (HORIBA Jobin Yvon Inc.) equipped with a 150 W xenon lamp as the excitation source. The absolute QYs of the samples were determined by measuring emission and scattered light from the sample and reference in an integrating sphere.41,42 All the samples were dispersed in dichloromethane and placed in a cuvette inside the integrating sphere. The emitted and scatted radiation was collected at 90° angle from the excitation, and a baffle was placed beside the sample on the emission monochromator side to avoid the collection of directly scatted light. The PL decay dynamics were measured using time-correlated single photon counting (TCSPC) set-up from Jobin Yvon equipped with a 454 nm LED excitation source. Photocurrent density–photovoltage curves (J–V) of QDSCs were derived with a Keithley 2400 digital source meter (Keithley, USA) under AM 1.5G illumination (100 mW cm−2) by Oriel Sol 3A Solar Simulator (94023A, Newport Stratford Inc., USA), calibrated with a standard crystalline silicon solar cell. The incident photon-to-current conversion efficiency (IPCE) was measured by using solar cell quantum efficiency measurement system (QEX10, PV Measurements, Inc.), and was calibrated with a NREL-certified Si diode before measurement.3. Results and discussion
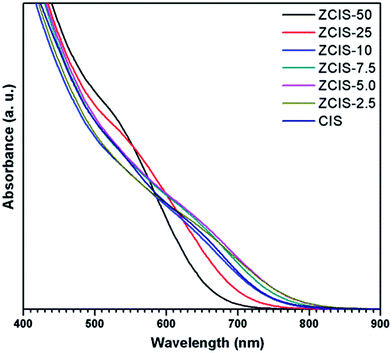
Ternary CuInS2 QDs tolerate a large range of off-stoichiometric compositions and complex crystal structures relevant with donor and acceptor trap states, which have significant effects on their optical and electronic properties.16 Doping zinc ionic into QDs could disturb electron waves in orbits and enhance electronic coupling, hence, also affecting the optoelectronic properties of host CuInS2 QDs. The absorbance spectra of zinc doped CuInS2 QDs with different zinc contents are shown in Fig. 1. While they don't exhibit obvious exciton absorption peaks, the doped QDs apparently have a series of absorbance edges ranging from ca. 800 nm to ca. 670 nm. A substantial blue shift of the optical absorbance band edges occurs with increased concentration of doped zinc in CuInS2 QDs, indicating an increase in the determined optical band gaps of the doped QDs. The optical band gaps of doped QDs can be derived in the range of 1.55–1.85 eV. High-resolution TEM images (Fig. 2a and b) show that the doped CuInS2 QDs have uniform diameters around 5 nm, independent of zinc concentration, suggesting minor quantum confinement effects. Therefore, the observed blue shift is mainly attributed to the widening of band gaps as a result of the doped zinc. Quantitative elemental analyses of the QDs by EDS (Table 1) suggested the compositions of the QDs were roughly but systemically consistent with the fed Cu/In molar ratios.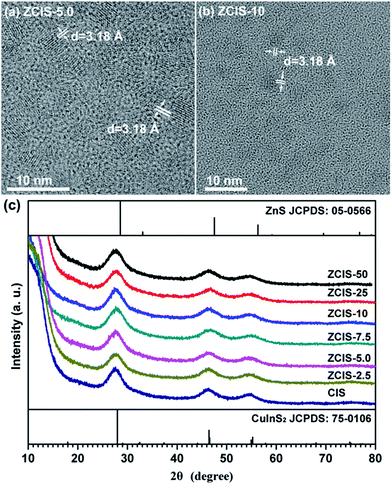 | ||
| Fig. 2 (a, b) Typical TEM image of doped CuInS2 QDs, and (c) XRD patterns of doped CuInS2 QDs with different zinc contents. | ||
| QDs | Cu (atom%) | In (atom%) | S (atom%) | Zn (atom%) | Zn/In ratios in QDs (%) |
|---|---|---|---|---|---|
| ZCIS-50 | 21.41 | 18.64 | 56.33 | 3.63 | 19.5 |
| ZCIS-25 | 23.67 | 20.65 | 53.46 | 2.22 | 10.8 |
| ZCIS-10 | 22.42 | 22.94 | 53.53 | 1.11 | 4.8 |
| ZCIS-7.5 | 24.05 | 22.08 | 52.72 | 1.15 | 5.2 |
| ZCIS-5.0 | 23.23 | 21.28 | 54.71 | 0.79 | 3.7 |
| ZCIS-2.5 | 24.38 | 22.50 | 52.53 | 0.60 | 2.7 |
| CIS | 22.53 | 22.02 | 55.46 | — | — |
TEM image also shows that an individual QD has a clear lattice fringe with interplanar spacing of 3.1 Å, which can be resolved as (112) lattice fringes and agrees well with those determined from diffraction peak at 27.88° in the XRD patterns, demonstrating crystalline nature of doped CuInS2 QDs. Furthermore, XRD patterns (Fig. 2c) exhibit three broad peaks at 2θ = 27.88°, 47.28°, and 55.06°, which are assigned to the diffractions of the (112), (204), and (312) planes of the chalcopyrite CuInS2 crystals, respectively. The three main peaks in XRD pattern shows wider full-width at half-maximum (FWHM) than bulk CuInS2, which can be explained by the broadening effect of nanometer size of QDs. XRD analysis demonstrates that zinc doped CuInS2 QDs with different contents have a chalcopyrite phase. With increased doped zinc concentration, the major diffraction peaks essentially keep stable and no splitting appears. This indicates the incorporation of zinc ions into CuInS2 crystals and rules out phase separation or individual nucleation of ZnS during the synthesis. It may therefore be concluded that the doped zinc is homogeneous distributed in CuInS2 QDs matrix.
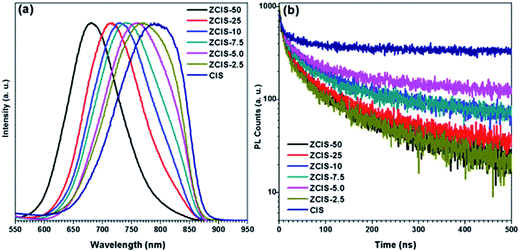
PL spectra of zinc doped CuInS2 QDs (Fig. 3a) display broad blue shift emission peaks with higher zinc contents, from 760 nm for ZCIS-2.5 to 680 nm for ZCIS-50. It should be noted that the blue shifted PL peaks for ZnS shell was within a smaller degree than that for zinc alloying QDs. By introducing zinc ions, ZnS–CuInS2 alloy compounds would be formed via alloying ZnS with a wider band gap and CuInS2 with a narrower band gap. The band gap of doped CuInS2 QDs becomes wider with the increased contents of doped zinc. The PL peaks of doped CuInS2 QDs exhibit large FWHM from 137 nm for ZCIS-2.5 to 104 nm for ZCIS-50. Usually, large FWHM is observed in the ternary I–III–VI semiconductor QDs due to the characteristic donor–acceptor pair transition or surface defect states.8,34
The sensitivity of QY with zinc concentration was investigated (Fig. S1†). The results showed that a minor content of zinc (ZCIS-2.5) may increase QY from 0.2% for pure CuInS2 QDs to 1.9%, approximately by a magnitude order. QY keeps increasing with increased zinc content to 3.1% for ZCIS-5.0, and 4.7% for ZCIS-7.5, 4.2% for ZCIS-10, 4.2% for ZCIS-25, and 4.9% for ZCIS-50. The low QY of pure CuInS2 QDs indicates the extensive existence of surface defect and/or internal trap state, the latter of which can be effectively suppressed by the doping of zinc. It has been reported that QY can also be increased by overcoating wider band gap ZnS or CdS layer on CuInS2 QDs. Klimov et al. demonstrated QY of CuInS2 nanocrystals could reach as high as 80% after CdS overcoating growth.28 Zhong and coworkers reported CuInS2/ZnS core/shell structured QDs with QY 10-fold higher than that of CuInS2 QDs.23 Many studies have reported QY of ternary nanocrystals with widely ranged values,17,43–45 which are affected by several factors such as synthesis strategy, types of ligands, cation ratios, and core/shell structure. Deng et al.44 reported Cu–Zn–In–S nanocrystals with QY as high as over 70–80%. According to those strategies on synthesis, dodecanethiol was used as capping agent. Most studies on semiconductor nanocrystals used dodecanethiol and oleylamine, which controls the reactivity of metal ions by tailoring the phase and shape of targets.15,17,28,30,40,44 However, dodecanethiol would hinder electron transport for tight adsorbance on QD surface in photovoltaic application,23,46 and thus was avoided in the present work on purpose.
The intrinsic defects, surface defects, and size-dependent band gap of QDs are involved in the PL emission.34 Different electron–hole recombination mechanisms may correspond to different PL decay lifetimes. To understand the mechanisms underlying the dependence of QY on zinc contents, PL decay dynamics of doped CuInS2 QDs were investigated by time correlated single photon counting (TCSPC) technique. As shown in Fig. 3b, their PL decay curves can be fitted by biexponential equation I(t) = α1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp(−t/τ1) + α2
exp(−t/τ1) + α2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp(−t/τ2).47 Also, the average lifetimes τave is used to estimate the PL decay time, which can be defined by τave = (α1τ12 + α2τ22)/(α1τ1 + α2τ2). The fractional intensities F1, defined as (α1τ1)/(α1τ1 + α2τ2), gives the weights of the two components and signifies the proportion of the excited state population that radiatively decays through each pathway. As shown in Table 2, the PL relaxations can be decomposed into fast and slow decay components. The fast decay components are within tens of nanoseconds with contributions of less than 5%, while the slow decay parts are hundreds of nanoseconds and account for more than 95% to the whole emission profiles. With increased zinc content, the lifetimes of fast components keep stable with enlarged shares, while the lifetimes of slow components decrease with reduced shares. The fast decay (19–25 ns, depending on doped zinc contents) can be attributed to the intrinsic recombination of core states and surface defect states. The slow decay (160–180 ns) is due to donor–acceptor pair transition, which can be verified by the aforementioned broad emission peaks of the doped CuInS2 QDs (FWHM of the peaks is distributed within range of 104–137 nm). It is well known that intrinsic defects in ternary CuInS2 semiconductors are deep trap states for their enhanced configurable degree of freedom in atomic packing models.34,48 Zhang et al.45 also reported similar results that, in ZnS–CuInS2 alloyed nanocrystals, donor–acceptor transition accounts for a large fraction of the total PL emission spectra. Castro et al.49 attributed some donor and acceptor states for the origin of fluorescence of CuInS2 QDs to sulfur vacancy and copper indium substitution as the donor, cupper vacancy as the acceptor. Sulfur vacancy and interstitial copper create donor levels, and copper vacancy and interstitial sulfur act as acceptor levels in CuInS2 QDs.50–52 Therefore, cation vacancy plays a fundamental role in donor–acceptor pair mechanism of PL.
exp(−t/τ2).47 Also, the average lifetimes τave is used to estimate the PL decay time, which can be defined by τave = (α1τ12 + α2τ22)/(α1τ1 + α2τ2). The fractional intensities F1, defined as (α1τ1)/(α1τ1 + α2τ2), gives the weights of the two components and signifies the proportion of the excited state population that radiatively decays through each pathway. As shown in Table 2, the PL relaxations can be decomposed into fast and slow decay components. The fast decay components are within tens of nanoseconds with contributions of less than 5%, while the slow decay parts are hundreds of nanoseconds and account for more than 95% to the whole emission profiles. With increased zinc content, the lifetimes of fast components keep stable with enlarged shares, while the lifetimes of slow components decrease with reduced shares. The fast decay (19–25 ns, depending on doped zinc contents) can be attributed to the intrinsic recombination of core states and surface defect states. The slow decay (160–180 ns) is due to donor–acceptor pair transition, which can be verified by the aforementioned broad emission peaks of the doped CuInS2 QDs (FWHM of the peaks is distributed within range of 104–137 nm). It is well known that intrinsic defects in ternary CuInS2 semiconductors are deep trap states for their enhanced configurable degree of freedom in atomic packing models.34,48 Zhang et al.45 also reported similar results that, in ZnS–CuInS2 alloyed nanocrystals, donor–acceptor transition accounts for a large fraction of the total PL emission spectra. Castro et al.49 attributed some donor and acceptor states for the origin of fluorescence of CuInS2 QDs to sulfur vacancy and copper indium substitution as the donor, cupper vacancy as the acceptor. Sulfur vacancy and interstitial copper create donor levels, and copper vacancy and interstitial sulfur act as acceptor levels in CuInS2 QDs.50–52 Therefore, cation vacancy plays a fundamental role in donor–acceptor pair mechanism of PL.
| QD | τ1/ns | τ2/ns | α1/% | α2/% | F1/% | F2/% | τave/ns |
|---|---|---|---|---|---|---|---|
| ZCIS-50 | 21.7 | 160.9 | 24.88 | 75.12 | 4.3 | 95.7 | 154.9 |
| ZCIS-25 | 20.2 | 161.0 | 23.53 | 76.47 | 3.7 | 96.3 | 155.8 |
| ZCIS-10 | 22.4 | 182.7 | 19.63 | 80.37 | 2.9 | 97.1 | 178.0 |
| ZCIS-7.5 | 21.0 | 172.7 | 20.52 | 79.48 | 3.0 | 97.0 | 168.1 |
| ZCIS-5.0 | 20.5 | 178.5 | 17.88 | 82.12 | 2.4 | 97.6 | 174.6 |
| ZCIS-2.5 | 24.4 | 185.9 | 23.37 | 77.89 | 3.8 | 96.2 | 179.8 |
| CIS | 19.4 | 222.7 | 16.81 | 83.19 | 1.7 | 98.3 | 219.2 |
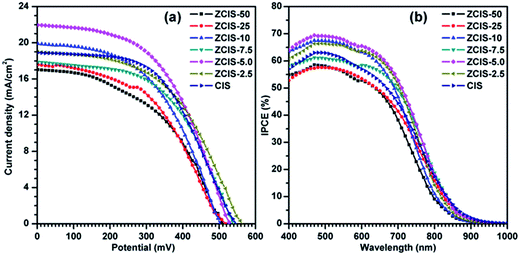
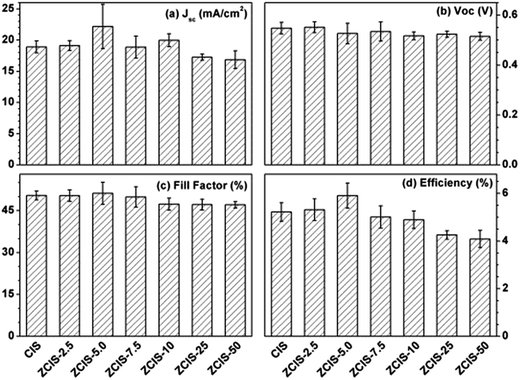
During water-soluble ligand exchange in base circumstance, the reactivity of the MPA was enhanced by removing the thiolic hydrogen with a strong base, which ensured a dense and compact coverage of the MPA ligand on the surface of QDs by the thiolic end.53 MPA-capped doped CuInS2 QDs were deposited and absorbed onto the surface of TiO2 mesoporous films, facilitated by their interaction with carboxyl groups.54 Then, three cycles of ZnS passivation coating using SILAR method were carried out. Sandwich-type cells were constructed by assembling QD-sensitized TiO2 film electrode and Cu2S/brass counter electrode, which were separated with hollowed Scotch spacer. Then polysulfide electrolyte was injected and inhaled to the layers by capillary effect. The J–V curves of the solar cells under the illumination of an AM 1.5G solar simulator with an intensity of 100 mW cm−2 (1 full sun) are shown in Fig. 4a, and the extracted photovoltaic parameters are collected in Table S1.† It is noted that for the photovoltaic performance measurement at least three cells were constructed. The trends of the photovoltaic parameters are shown in Fig. 5 as functions of zinc contents. FF about 51.1 (±3.9)% and Voc about 0.526 (±0.04) V are not sensitive to zinc contents where as Jsc has a maximum of 22.1 (±3.5) mA cm−2 at ZCIS-5.0 due to the compromising between charge recombination and injection barrier effects. Correspondingly, a maximum PCE of 5.9 (±0.5)% was achieved at the doped CuInS2 QDs of ZCIS-5.0. After the doping with zinc at optimized conditions, the average PCE of QDSCs under one full sun illumination is increased from 5.21% for pure QDs to 5.90% for doped QDs, a remarkable increase of 13.2%. As a comparison, Jsc and PCE of pure CuInS2 QDSCs were measured to be 18.9 (±0.9) mA cm−2 and 5.21 (±0.3)%, respectively. Although the obtained efficiencies are lower than the values of 7.06% for glaring CuInS2/ZnS QDSCs23 and 11.6% for Zn–Cu–In–Se QDSCs,10 the improvement of photovoltaic performance in doped CuInS2 QDSCs is still encouraging, especially, taking the facile synthesis into consideration. The remarkable enhanced Jsc for QDSCs of ZCIS-5.0 (from 18.9 to 22.1 mA cm−2) was thought introduced by increased efficient electron injection from both extended photoresponse range, which confirmed by red shifted absorption band edges in UV-vis absorption spectra (Fig. 1), and moderate lifetime PL with increased QY (Fig. 3a and S1†), while other QDSCs possessed either or neither of the two sources.
IPCE represents the percentage of incident photons that are converted to charge carriers and collected at the electrode surface. The IPCE spectra shown in Fig. 4b exhibit photoresponses roughly matching with the absorption spectra of QDs. The photoresponse ranges in the IPCE spectra are wider than the corresponding absorption ranges of doped CuInS2 QDs, which can be ascribed to the light scattering effect by 200 nm TiO2 particles in mesoporous TiO2 layer. The IPCE spectrum of ZCIS-5.0 based QDSCs exhibits a strong photoresponse over 50% within the window below 750 nm and reaches the maximum value of 69% at 500 nm, while the other QDSCs show the weak photoresponse either in shorter wavelength window or low convert efficiency, consistent with the aforementioned Jsc trend (Fig. 4a). The Jsc values obtained by integrating IPCE spectra are 12.15, 13.14, 14.68, 14.49, 15.81, 15.07 and 14.10 mA cm−2, respectively. They are generally smaller than the corresponding values by J–V characteristics. The systematic discrepancy can be may be explained by limited charge separation and collection efficiencies due to lower light intensities from monochromator in IPCE than that of AM 1.5G illumination in J–V characteristics.21 Previously reported photoresponse range of CdSe and CdS/CdSe QDs sensitizers in QDSCs are typically less than 700 nm.54–57 In the current work, the ranges are extended to near-infrared region (ca. 950 nm), which were realized by properly controlling band gap of doped CuInS2, resulting in effective light harvesting utilization and reinforced photocurrents.
Electron injection rate from absorber to the conduction band of TiO2 is an important parameter in QDSCs.58 It has been demonstrated that the excited-state features of QDs depend on the substrate to which they are attached.23,59 To evaluate the kinetics of charge transfer from QDs to TiO2, PL decay measurements were carried out for QDs deposited on both TiO2 and insulating SiO2 substrate.60 An additional deactivation route was formed after doped CuInS2 and pure CuInS2 QDs deposited on TiO2 mesoporous films, in which electrons could easily transfer to TiO2 for their lower conduction band edge. As compared in Fig. 6, the recoveries of bleaching of doped CuInS2 of ZCIS-5.0 and pure CuInS2 QDs on insulating TiO2 are relatively faster than those on SiO2, which. These results confirm promoted electron injections from QDs to TiO2.60
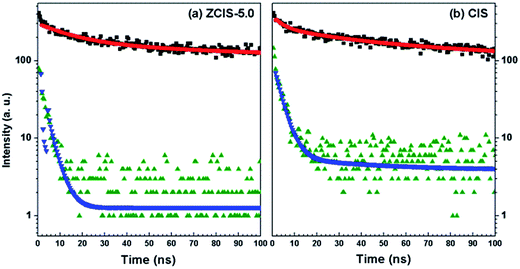 | ||
| Fig. 6 PL emission decay plots of doped (a) and pure (b) CuInS2 QDs on TiO2 (olive for decay and blue for fit) and SiO2 (black for decay and red for fit) films, respectively. | ||
Biexponential decay model was used to fit the lifetime traces. The fitting results and calculated average lifetime (τav) are listed in Table 3. The rate constants of electron transfer from QD to TiO2, ket, can be estimated by comparing the PL decay processes of the QDs on TiO2 to those on SiO2, following eqn (1).
| ket = 1/τav(TiO2) − 1/τav(SiO2) | (1) |
| Sample | τ1/ns | α1/% | τ2/ns | α2/% | τave/ns | ket (×1010/s−1) |
|---|---|---|---|---|---|---|
| ZCIS-5.0@TiO2 | 0.22 | 32.72 | 3.4 | 67.28 | 3.3 | 2.99 |
| ZCIS-5.0@SiO2 | 17.3 | 9.39 | 238.1 | 90.61 | 236.4 | |
| CIS@TiO2 | 3.55 | 80.68 | 41.4 | 19.32 | 31.4 | 0.276 |
| CIS@SiO2 | 23.05 | 9.92 | 240.12 | 90.08 | 237.8 |
4. Conclusion
In this paper we report a facile synthesis of doped CuInS2 QDs under air circumstance by the organometallic high temperature method. Owing to the modulation on doped zinc contents, features of tunable photoluminescence of doped CuInS2 QDs with improved lifetime and QY have been demonstrated. It was shown that donor–acceptor pair recombination contributes to above 95% of the whole emission profiles. The optical and electronic properties of doped CuInS2 QDs have significant effects on the performance of QDSCs. ZCIS-5.0 QDs based QDSCs exhibited PCE of 5.90% under one full sun illumination, higher than the value of 5.21% for pure CuInS2 QDs. The improvement is mainly originated from broadened optoelectronic response range up to ∼950 nm. IPCE values are over ∼50% within the window below 750 nm and a maximum of 69% is obtained at 500 nm. Furthermore, faster electron injection of doped CuInS2 from QDs to TiO2 films, with ket increased from 0.276 × 1010 s−1 to 2.99 × 1010 s−1, plays another important role in increasing PCE. The facile synthetic approach combined with composition modulation on dopant provides practical guidance to the future development of high performance QDSCs.Acknowledgements
This work was financially supported by the funds from the National Natural Science Foundation of China (51572199, 51420105002, 51302194, 61471270) and the Key Lab of Novel Thin Film Solar Cells, Chinese Academy of Sciences (KF201603).References
- A. D. Yoffe, Adv. Phys., 2001, 50, 1–208 CrossRef CAS.
- O. E. Semonin, J. M. Luther, S. Choi, H.-Y. Chen, J. Gao, A. J. Nozik and M. C. Beard, Science, 2011, 334, 1530–1533 CrossRef CAS PubMed.
- C.-H. M. Chuang, P. R. Brown, V. Bulović and M. G. Bawendi, Nat. Mater., 2014, 13, 796–801 CrossRef CAS PubMed.
- D. Bera, L. Qian, T.-K. Tseng and P. H. Holloway, Materials, 2010, 3, 2260 CrossRef CAS.
- K. Chen, J. Zhou, W. Chen, Q. Chen, P. Zhou and Y. Liu, Nanoscale, 2016, 8, 5146–5152 RSC.
- K. Chen, J. Zhou, W. Chen, P. Zhou, F. He and Y. Liu, Part. Part. Syst. Charact., 2015, 32, 999–1005 CrossRef CAS.
- I. Robel, M. Kuno and P. V. Kamat, J. Am. Chem. Soc., 2007, 129, 4136–4137 CrossRef CAS PubMed.
- D. H. Jara, S. J. Yoon, K. G. Stamplecoskie and P. V. Kamat, Chem. Mater., 2014, 26, 7221–7228 CrossRef CAS.
- Z. Du, Z. Pan, F. Fabregat-Santiago, K. Zhao, D. Long, H. Zhang, Y. Zhao, X. Zhong, J.-S. Yu and J. Bisquert, J. Phys. Chem. Lett., 2016, 7, 3103–3111 CrossRef CAS PubMed.
- J. Du, Z. L. Du, J. S. Hu, Z. X. Pan, Q. Shen, J. K. Sung, D. H. Long, H. Dong, L. T. Sun, X. H. Zhong and L. J. Wan, J. Am. Chem. Soc., 2016, 138, 4201–4209 CrossRef CAS PubMed.
- P. V. Kamat, J. Phys. Chem. Lett., 2013, 4, 908–918 CrossRef CAS PubMed.
- F. Liu, J. Zhu, J. F. Wei, Y. Li, L. H. Hu and S. Y. Dai, Prog. Chem., 2013, 25, 409–418 CAS.
- H. Y. Wei, G. S. Wang, H. J. Wu, Y. H. Luo, D. M. Li and Q. B. Meng, Acta Phys.-Chim. Sin., 2016, 32, 201–213 CAS.
- H. K. Jun, M. A. Careem and A. K. Arof, Renewable Sustainable Energy Rev., 2013, 22, 148–167 CrossRef CAS.
- H. Zhong, Z. Bai and B. Zou, J. Phys. Chem. Lett., 2012, 3, 3167–3175 CrossRef CAS PubMed.
- S. R. Kodigala, in Thin Films and Nanostructures, ed. K. Subba Ramaiah, Academic Press, 2010, vol. 35, pp. 319–391 Search PubMed.
- R. G. Xie, M. Rutherford and X. G. Peng, J. Am. Chem. Soc., 2009, 131, 5691–5697 CrossRef CAS PubMed.
- H. McDaniel, N. Fuke, J. M. Pietryga and V. I. Klimov, J. Phys. Chem. Lett., 2013, 4, 355–361 CrossRef CAS PubMed.
- Y. Liu, T. Chen, Z. Peng, L. Wu, K. Chen, P. Zhou, L. Wang and W. Chen, J. Alloys Compd., 2016, 658, 76–84 CrossRef CAS.
- P. K. Santra, P. V. Nair, K. George Thomas and P. V. Kamat, J. Phys. Chem. Lett., 2013, 4, 722–729 CrossRef CAS PubMed.
- T.-L. Li, Y.-L. Lee and H. Teng, Energy Environ. Sci., 2012, 5, 5315–5324 CAS.
- Z. Peng, Y. Liu, W. Chen, K. Chen, J. Chen and J. Chen, J. Alloys Compd., 2017, 701, 131–137 CrossRef CAS.
- Z. X. Pan, I. Mora-Sero, Q. Shen, H. Zhang, Y. Li, K. Zhao, J. Wang, X. H. Zhong and J. Bisquert, J. Am. Chem. Soc., 2014, 136, 9203–9210 CrossRef CAS PubMed.
- J.-Y. Kim, J. Yang, J. H. Yu, W. Baek, C.-H. Lee, H. J. Son, T. Hyeon and M. J. Ko, ACS Nano, 2015, 9, 11286–11295 CrossRef CAS PubMed.
- M. C. Hanna and A. J. Nozik, J. Appl. Phys., 2006, 100, 074510 CrossRef.
- Z. Yang, C.-Y. Chen, P. Roy and H.-T. Chang, Chem. Commun., 2011, 47, 9561–9571 RSC.
- S. Rühle, M. Shalom and A. Zaban, ChemPhysChem, 2010, 11, 2290–2304 CrossRef PubMed.
- L. Li, A. Pandey, D. J. Werder, B. P. Khanal, J. M. Pietryga and V. I. Klimov, J. Am. Chem. Soc., 2011, 133, 1176–1179 CrossRef CAS PubMed.
- J. W. Yang, J. Wang, K. Zhao, T. Izuishi, Y. Li, Q. Shen and X. H. Zhong, J. Phys. Chem. C, 2015, 119, 28800–28808 CAS.
- A. M. Smith and S. Nie, Acc. Chem. Res., 2010, 43, 190–200 CrossRef CAS PubMed.
- Y. C. Cao, Science, 2011, 332, 48–49 CrossRef PubMed.
- D. V. Talapin, J.-S. Lee, M. V. Kovalenko and E. V. Shevchenko, Chem. Rev., 2010, 110, 389–458 CrossRef CAS PubMed.
- T. Omata, K. Nose, K. Kurimoto and M. Kita, J. Mater. Chem. C, 2014, 2, 6867–6872 RSC.
- D. H. Jara, K. G. Stamplecoskie and P. V. Kamat, J. Phys. Chem. Lett., 2016, 7, 1452–1459 CrossRef CAS PubMed.
- B. K. Chen, H. Z. Zhong, W. Q. Zhang, Z. A. Tan, Y. F. Li, C. R. Yu, T. Y. Zhai, Y. S. Bando, S. Y. Yang and B. S. Zou, Adv. Funct. Mater., 2012, 22, 2081–2088 CrossRef CAS.
- Y. K. Kim, S. H. Ahn, K. Chung, Y. S. Cho and C. J. Choi, J. Mater. Chem., 2012, 22, 1516–1520 RSC.
- W. Y. Liu, Y. Zhang, J. Zhao, Y. Feng, D. Wang, T. Q. Zhang, W. Z. Gao, H. R. Chu, J. Z. Yin, Y. D. Wang, J. Zhao and W. W. Yu, J. Lumin., 2015, 162, 191–196 CrossRef CAS.
- W. Peng, J. Du, Z. Pan, N. Nakazawa, J. Sun, Z. Du, G. Shen, J. Yu, J.-S. Hu, Q. Shen and X. Zhong, ACS Appl. Mater. Interfaces, 2017, 9, 5328–5336 CAS.
- G. Halder and S. Bhattacharyya, J. Mater. Chem. A, 2017, 5, 11746–11755 CAS.
- D. C. Pan, L. J. An, Z. M. Sun, W. Hou, Y. Yang, Z. Z. Yang and Y. F. Lu, J. Am. Chem. Soc., 2008, 130, 5620–5621 CrossRef CAS PubMed.
- M. Grabolle, M. Spieles, V. Lesnyak, N. Gaponik, A. Eychmuller and U. Resch-Genger, Anal. Chem., 2009, 81, 6285–6294 CrossRef CAS.
- H. Zhong, Z. Wang, E. Bovero, Z. Lu, F. C. J. M. van Veggel and G. D. Scholes, J. Phys. Chem. C, 2011, 115, 12396–12402 CAS.
- J. Zhang, R. Xie and W. Yang, Chem. Mater., 2011, 23, 3357–3361 CrossRef CAS.
- D. Deng, Y. Chen, J. Cao, J. Tian, Z. Qian, S. Achilefu and Y. Gu, Chem. Mater., 2012, 24, 3029–3037 CrossRef CAS.
- W. Zhang and X. Zhong, Inorg. Chem., 2011, 50, 4065–4072 CrossRef CAS PubMed.
- C.-C. Chang, J.-K. Chen, C.-P. Chen, C.-H. Yang and J.-Y. Chang, ACS Appl. Mater. Interfaces, 2013, 5, 11296–11306 CAS.
- Principles of Fluorescence Spectroscopy, ed. J. R. Lakowicz, Springer, US, Boston, MA, 2006, pp. 97–155, DOI:10.1007/978-0-387-46312-4_4.
- V. K. Komarala, C. Xie, Y. Wang, J. Xu and M. Xiao, J. Appl. Phys., 2012, 111, 124314 CrossRef.
- S. L. Castro, S. G. Bailey, R. P. Raffaelle, K. K. Banger and A. F. Hepp, J. Phys. Chem. B, 2004, 108, 12429–12435 CrossRef CAS.
- S. B. Zhang, S.-H. Wei, A. Zunger and H. Katayama-Yoshida, Phys. Rev. B: Condens. Matter Mater. Phys., 1998, 57, 9642–9656 CrossRef CAS.
- C. Rincón and R. Márquez, J. Phys. Chem. Solids, 1999, 60, 1865–1873 CrossRef.
- B. J. Stanbery, Crit. Rev. Solid State Mater. Sci., 2002, 27, 73–117 CrossRef CAS.
- B.-K. Pong, B. L. Trout and J.-Y. Lee, Langmuir, 2008, 24, 5270–5276 CrossRef CAS PubMed.
- H. Zhang, K. Cheng, Y. M. Hou, Z. Fang, Z. X. Pan, W. J. Wu, J. L. Hua and X. H. Zhong, Chem. Commun., 2012, 48, 11235–11237 RSC.
- Z. Pan, H. Zhang, K. Cheng, Y. Hou, J. Hua and X. Zhong, ACS Nano, 2012, 6, 3982–3991 CrossRef CAS PubMed.
- F. Liu, J. Zhu, J. Wei, Y. Li, L. Hu, Y. Huang, O. Takuya, Q. Shen, T. Toyoda, B. Zhang, J. Yao and S. Dai, J. Phys. Chem. C, 2014, 118, 214–222 CAS.
- F. Huang, L. Zhang, Q. Zhang, J. Hou, H. Wang, H. Wang, S. Peng, J. Liu and G. Cao, ACS Appl. Mater. Interfaces, 2016, 8, 34482–34489 CAS.
- K. Tvrdy, P. A. Frantsuzov and P. V. Kamat, Proc. Natl. Acad. Sci. U. S. A., 2011, 108, 29–34 CrossRef CAS PubMed.
- G. Wang, H. Wei, Y. Luo, H. Wu, D. Li, X. Zhong and Q. Meng, J. Power Sources, 2016, 302, 266–273 CrossRef CAS.
- J. Wang, I. Mora-Sero, Z. X. Pan, K. Zhao, H. Zhang, Y. Y. Feng, G. Yang, X. H. Zhong and J. Bisquert, J. Am. Chem. Soc., 2013, 135, 15913–15922 CrossRef CAS PubMed.
- I. Robel, V. Subramanian, M. Kuno and P. V. Kamat, J. Am. Chem. Soc., 2006, 128, 2385–2393 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Quantum yields of ZCIS QDs, photovoltaic parameters of corresponding QDSCs. See DOI: 10.1039/c7ra06659g |
| This journal is © The Royal Society of Chemistry 2017 |