Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Isotope selective activation: a new insight into the catalytic activity of urease
Sanchi Maithani†
a,
Mithun Pal†a,
Abhijit Maitya and
Manik Pradhan *ab
*ab
aDepartment of Chemical, Biological and Macromolecular Sciences, S. N. Bose National Centre for Basic Sciences, Salt Lake, JD Block, Sector III, Kolkata-700098, India. E-mail: manik.pradhan@bose.res.in; manik.pradhan@gmail.com; Fax: +91 33 2335 3477; Tel: +91 33 2335 5706 Tel: +91 33 2335 5708
bTechnical Research Centre (TRC), S. N. Bose National Centre for Basic Sciences, Salt Lake, JD Block, Sector III, Kolkata-700098, India
First published on 19th June 2017
Abstract
Urease, a metalloenzyme, requires carbon dioxide (CO2) for its activation. But, whether this activation is isotope-specific to 12CO2 or 13CO2, is not yet known and even the potential role of CO2 in the enzymatic activity of urease is poorly understood. Here, we provide direct experimental evidence that the catalytic activity of urease exhibits a unique isotope-specific response where the 12CO2 isotope is strongly preferred over the 13CO2 isotope during its catalytic activation. Moreover, this isotope-selective activation depends on different isotopic fractionations (12C:13C) of the reaction-environment as well as the substrate urea (13C-urea and 12C-urea), where the 12CO2 isotope in the reaction medium essentially facilitates the hydrolysis of 13C-enriched urea. This deepens our understanding of the isotope-specific urease activation and its potential role in hydrolytic reaction. Our findings thus may offer novel opportunities for a better fundamental understanding of isotope-specificity in chemical reactions involving metalloenzymes.
Introduction
Urease, the first enzyme to be isolated in crystalline form, was found to have a dinuclear nickel(II) active site.1,2 This Ni(II)-containing metalloenzyme is found in various soil microbes, bacteria, fungi and plants. It plays an important role in environmental nitrogen transformations.3 Urease is also a potent virulence factor in certain pathogens and is particularly known for its potential link to human diseases like peptic ulcers and stomach cancer.3,4 Moreover, this enzyme acts as a unique catalyst for its very specific action on urea [CO(NH2)2] and subsequently has found widespread applications in enzymatic assays and as urea sensors for routine clinical measurements of urea in blood, urine and different body fluids.5,6 Besides this, it is also widely used in waste water treatment for removal of urea5 and as a model enzyme for the study of enzymatic kinetics.5The urease-catalyzed hydrolysis of urea has extensively been studied in the past.5,7 Urease catalyzes the hydrolysis of urea to produce ammonium carbamate [H2NCO2NH4] which rapidly decomposes into bicarbonate [HCO3−] and ammonium ions [NH4+] in a non-enzymatic and buffer-mediated system. The bicarbonate finally gets converted into carbon dioxide (CO2). Early studies focused on several aspects of this reaction such as reaction kinetics,8 action of buffers9 and effect of temperature.10 But the mechanism underlying the activation of urease or its potential role in hydrolytic reaction still remains controversial. Moreover, it was demonstrated in the past that the metalloenzyme urease requires CO2 for its activation.11,12 Therefore, the product of the urease-catalyzed hydrolysis of urea and the activator of the catalyst (i.e. CO2) being the same entity makes the reaction mechanism more complex. In this context, the detailed study of isotopic signatures of CO2 (i.e. 13CO2 and 12CO2) would provide better insight into the reaction mechanisms. However, so far there have been no studies focused on the potential role of isotope-specific CO2 environment on the isotopic-fractionations of in situ CO2 production in the urease–urea reaction. Moreover, how the variation of isotopic compositions (12C:13C) of the substrate urea (13C-urea and 12C-urea) affects the catalytic activity of urease particularly in the environment of atmospheric CO2 concentration, has never been explored and therefore remains an open question. Consequently, unravelling the reaction mechanism involving the isotope-selective activation of urease would open new perspectives for a better understanding of the role of CO2 for urease activation kinetics.
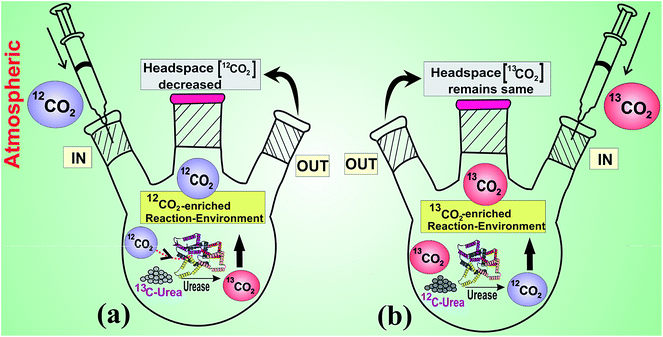
In this study, we report for the first time, that the enzymatic activity of urease exhibits a unique isotope-selective CO2 affinity. Furthermore, we have showed that the catalytic activity of urease depends on the isotopic compositions of both reaction-environment and substrate. Finally, we also demonstrate that the isotope-specific activation of urease is buffer-independent. This new knowledge is of great significance for fundamental understanding of isotope-specific chemical reactions involving metalloenzymes. Fig. 1 illustrates a scheme showing the isotope-specificity of urease enzyme and specific requirement of 12CO2 isotope for its activation.
Result and discussion
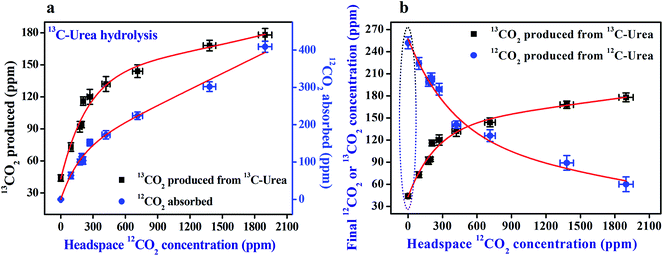
To investigate the urease (1 μM)-catalyzed hydrolysis of isotope enriched 12C and 13C-urea (4 mM) at different reaction environments, we first altered the headspace of the reaction with increasing concentration of 12CO2 (with ∼1.1% natural abundance of 13CO2) starting from CO2-free pure N2 environment. It is noteworthy to mention here that only the concentration of 12CO2 or 13CO2 isotopes is regulated keeping the total pressure constant at around atmospheric pressure above the reaction medium. We observed (Fig. 2a) that the headspace 12CO2 concentration was absorbed with simultaneous in situ generation of 13CO2 in the urease-catalyzed hydrolysis of 13C-urea. We also interestingly (Fig. 2a) observed that while 13C-urea derived in situ 13CO2 production is increased gradually with increasing initial headspace concentration of 12CO2, at the same time the final headspace 12CO2 concentration is decreased more in the reaction. Taken together, these observations indicate that there is a potential link between the enzymatic activity of urease and the requirement of specific isotope of CO2 (i.e. 12CO2). These observations are indistinguishable for urease-catalyzed hydrolysis of 12C-urea because the product and the headspace component are the same isotopic species (i.e. 12CO2).Another striking finding revealed in our observations (Fig. 2b) is that in CO2-free pure N2 environment, the in situ generation of 13CO2 concentration from 13C-urea was observed to be the lowest value, whereas the 12C-urea derived in situ production of 12CO2 concentration was found to be markedly enhanced. However, in absence of any 12CO2, the catalytic activity of urease diminishes and subsequently the hydrolysis of 13C-urea becomes very small, resulting in lower amount of 13CO2 production. In contrast, 12C-urea derived in situ 12CO2 itself facilitates the enzymatic activity of urease and thus urease-catalyzed hydrolysis of 12C-urea is not hindered in CO2-free pure N2 environment. However, 12C-urea derived in situ production of 12CO2 decreases with increase of initial headspace concentration of 12CO2 and this is likely to be the combined effect of partial pressure of the same species 12CO2 above the reaction medium as well as the over-saturation of urease enzyme in presence of excess headspace 12CO2.
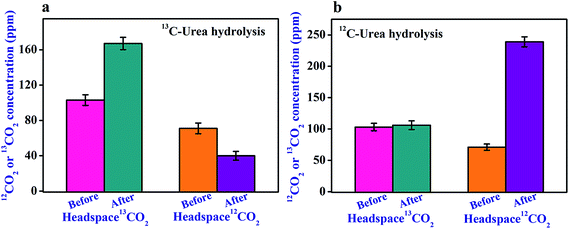
We next altered the headspace environment with ∼60% (103 ppm) isotope enriched 13CO2 (of 174 ppm total CO2), (instead of ∼1.1% natural abundance as in the previous case), to ensure the specific role of 12CO2 in the catalytic activity of urease. We found (Fig. 3a) that initial headspace 13CO2 concentration was not absorbed at all, while the headspace 12CO2 was markedly decreased in the usual way for urease (1 μM)-catalyzed hydrolysis of (4 mM) 13C-urea. Moreover, under this altered environmental conditions in headspace, no noticeable effect of enriched headspace 13CO2 concentration was observed (Fig. 3b) even for urease (1 μM)-catalyzed hydrolysis of (4 mM) 12C-urea. Our observation clearly manifests that urease preferentially links to 12CO2 for its activation, thus unveiling a missing link between the enzymatic activity of urease and the necessity for 12CO2 isotope.
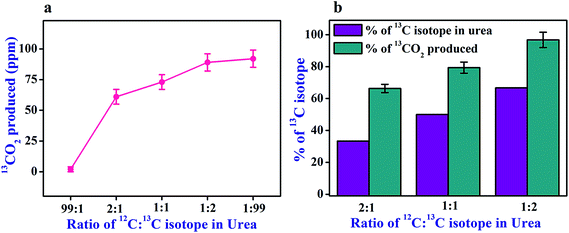
Next, we examined the catalytic activity of urease in response to the different isotopic-compositions (12C:13C) of the substrate urea to gain a better insight into the fundamental processes of isotope – selective nature of urease with a fixed headspace atmospheric CO2 concentration (∼360 ppm). The different isotopic-compositions (12C:13C) were achieved by suitably mixing 12C-urea and 13C-urea, while retaining the same concentration of urea (4 mM). We observed (Fig. 4a) that the in situ generation of the product 13CO2 from hydrolysis of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 (12C
2 (12C![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 13C) isotopic-composition of urea was almost identical to that of 1
13C) isotopic-composition of urea was almost identical to that of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 99 (12C
99 (12C![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 13C) isotopic-composition. However, 13C-isotopic enrichment of the substrate urea for 1
13C) isotopic-composition. However, 13C-isotopic enrichment of the substrate urea for 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 (12C
2 (12C![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 13C) isotopic-composition was significantly lower than that of 1
13C) isotopic-composition was significantly lower than that of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 99 (12C
99 (12C![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 13C) isotopic-compositions. This observation demonstrates that the in situ availability of 12CO2 derived from the 12C-urea, itself activates urease for higher hydrolysis of 13C-fraction of the 1
13C) isotopic-compositions. This observation demonstrates that the in situ availability of 12CO2 derived from the 12C-urea, itself activates urease for higher hydrolysis of 13C-fraction of the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 (12C
2 (12C![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 13C) isotopic-composition of urea, whereas the 13C-urea (12C
13C) isotopic-composition of urea, whereas the 13C-urea (12C![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 13C: 1
13C: 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 99) was not effectively hydrolysed in absence of in situ generation of 12CO2 in the reaction medium.
99) was not effectively hydrolysed in absence of in situ generation of 12CO2 in the reaction medium.
In order to gain a detailed and in-depth understanding of the role of 12CO2 derived from different isotopic fractionations of 12C-urea, we then normalized the in situ generation of 13CO2 from the different isotopic-compositions of urea (i.e. 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) to the percentage (%) of 13CO2 produced from 99% 13C-urea (i.e. 1
2) to the percentage (%) of 13CO2 produced from 99% 13C-urea (i.e. 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 99) and this is illustrated in Fig. 4b. It was found (Fig. 4b) that the percentage of in situ 13CO2 production was much higher than the 13C percentage actually present in all the different isotopic-compositions of urea, suggesting the inadequate hydrolysis of 13C-urea (1
99) and this is illustrated in Fig. 4b. It was found (Fig. 4b) that the percentage of in situ 13CO2 production was much higher than the 13C percentage actually present in all the different isotopic-compositions of urea, suggesting the inadequate hydrolysis of 13C-urea (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 99) in absence of sufficient in situ 12CO2 concentration in the reaction medium. Therefore, urease-catalyzed hydrolysis of urea was also found to be strongly affected due to the different isotopic fractionations of substrate urea in presence of CO2 environment. Taken together, our data clearly suggest that 12CO2 isotope plays a vital role that facilitates the enzymatic activity of urease enzyme.
99) in absence of sufficient in situ 12CO2 concentration in the reaction medium. Therefore, urease-catalyzed hydrolysis of urea was also found to be strongly affected due to the different isotopic fractionations of substrate urea in presence of CO2 environment. Taken together, our data clearly suggest that 12CO2 isotope plays a vital role that facilitates the enzymatic activity of urease enzyme.
The potential effect of 12C-urea and 13C-urea on the urease activation was further elucidated in response to different substrate (urea) and enzyme (urease) concentrations while maintaining the headspace at a typical atmospheric CO2 concentration (∼360 ppm). The product (12CO2 or 13CO2) of (1 μM) urease-catalyzed hydrolysis of 12C-urea and 13C-urea was found (Fig. 5a) to be gradually increased with increasing concentration of urea (12C-urea or 13C-urea) from 100 μM to 5 mM. The observation was followed by a sharp decline after 5 mM urea concentration, thus exhibiting the effect of substrate-inhibition on the urease-catalyzed hydrolysis. In contrast, keeping the urea concentration fixed (4 mM), there was no significant change of the concentration of the product (12CO2 or 13CO2) with increasing concentration of urease enzyme (Fig. 5b), thus indicating that urease still hydrolyzes urea in an efficient way and thereby indicates the steady catalytic activity of urease. However, it is noteworthy that the isotope-specific activation of urease was observed throughout the study. Our findings also indicate that the lower in situ generation of 13CO2 eventually signifies the hydrolysis of lesser amount of 13C-urea in absence of adequate activation of urease resulting from the headspace 12CO2 concentration.
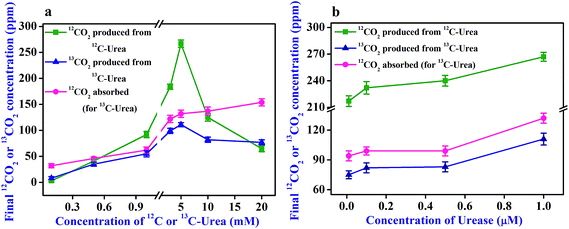 | ||
| Fig. 5 Concentration dependent study of urease-catalysed hydrolysis of 12C-urea and 13C-urea. (a and b) Elucidate the effect of different concentrations of urea and urease, respectively. | ||
Finally, we investigated the isotope-specific enzymatic activity of urease in two buffer mediums (Tris-buffer and phosphate buffer) to eliminate the potential effect of CO2 on the pH of the reaction medium. It is worth noting that the previous experiments in the present study were carried out in a buffer-free aqueous medium to avoid the effect of urease inhibition in presence of buffer ions.5 Fig. 6 depicts that this activation in the buffer mediums is not significantly changed compared to the buffer-free system. However, the diminished magnitude of the concentration of the products, 12CO2 and 13CO2 observed in the buffer mediums is exhibited to be the well-known inhibitory effect of buffer ions in the reaction.
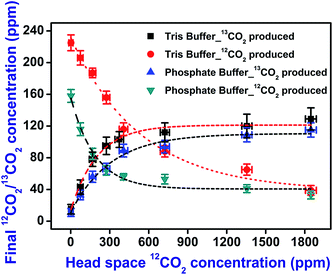 | ||
| Fig. 6 The effect of buffer medium. The hydrolysis of 12C-urea and 13C-urea in 12CO2 environment is performed in Tris buffer (pH 7.4) and phosphate buffer (pH 7). | ||
Conclusion
In conclusion, our findings provide new evidences that the catalytic activity of urease exhibits a unique isotope specific response. We have shown that the 12CO2 isotope is strongly preferred over the 13CO2 isotope by the urease enzyme during its catalytic activation. It was earlier reported11 that CO2 acts as a ligand in urease–urea reaction. Hence, the isotope preferential activation of urease enzyme may be attributed to the fact that 12CO2 isotope possibly offers lower interaction energy than 13CO2 isotope in this ligand–enzyme interaction. However, quantum-mechanical simulations of interaction energy for both the isotopes would provide better insight and further exploration of the isotope specific activation of urease enzyme. We also provide direct experimental evidences that deepens our understanding of how the activation of urease depends on the isotopic compositions (12C:13C) of reaction-environment as well as the substrate urea. Despite these new evidences, however, there still remains a great deal to be explored about the underlying mechanisms linking the specific requirement of 12CO2 isotope for the activation of urease. Moreover, new insights into the isotope-specific responses in urease-activation are fostering exploration of a new arena to study the chemical reactions involving metalloenzyme.Materials
Jack-bean urease enzyme (E.C. 3.5.1.5) was purchased from Sigma Aldrich and 13C-enriched urea (13C-urea, 99%) (CCLM-311-GMP) was obtained from Cambridge Isotopic Laboratories, Inc., USA. All other chemicals, including ACS grade urea with 99% 12C-enrichment (12C-urea), were acquired from Sigma Aldrich and were used without further purification. Milli-Q water was used to prepare the aqueous solutions.Method
The aqueous solutions of 12C-urea, 13C-urea and Jack-bean urease were utilized, unless stated otherwise, in the entire study. The reactions were carried out in sealed round-bottomed flasks wherein different concentrations of CO2 were maintained. To eliminate the interference due to air, the flasks were purged with CO2-free nitrogen before the experiments. The products (12CO2 or 13CO2) of urease-catalyzed hydrolysis of urea (12C or 13C-urea) were considered at the equilibrium of the reaction and the termination timing of the reaction was set to be at 60 minutes which was confirmed by Berthelot's experiment. After 60 minutes of the progress of the reaction, acidification was done using H3PO4 to extract the dissolved CO2 into headspace. The headspace CO2 was then collected from each flask and measured by a high-precision laser-based Integrated Cavity Output Spectroscopy (ICOS) technique. The net isotopic concentrations of CO2 produced from the hydrolysis reactions were calculated after subtracting the same from a blank flask containing only urease at equal initial CO2 concentration. All reactions were conducted at room temperature.Measurement and analysis
The measurements were made using a CO2 isotope analyser (CCIA 36-EP, LGR, USA) exploiting the ICOS technique as described in ref. 13–15. Briefly, a high-finesse optical cavity consisting of two very high-reflectivity mirrors (R ∼ 99.98%) provides an optical path length of ∼3 km thereby increasing the sensitivity considerably. The high-resolution absorption spectra of the stable isotopes 12C16O16O and 13C16O16O were acquired by probing the ro-vibrational lines at the wavenumbers 4874.448 cm−1 and 4874.086 cm−1, respectively in the vibrational combination band of CO2 molecule and subsequently the concentrations of the isotopic species were reported in parts per million (ppm).Author contributions
M. P. (Manik Pradhan) arranged the funding and supervised the whole study; M. P. (Manik Pradhan) and A. M. provided the conception of the study; S. M., M. P. (Mithun Pal) and A. M. designed the study; S. M., M. P. (Mithun Pal) and A. M. performed the experiments and analysed the data; all authors drafted the manuscript and critically reviewed.Conflict of interests
The authors declare no conflict of interests.Acknowledgements
The authors acknowledge the funding from the Technical Research Centre (TRC) (No. All1/64/SNB/2014(C)) of the S. N. Bose Centre, Kolkata for this work. S. Maithani, M. Pal and A. Maity acknowledge the Department of Science & Technology (DST, India) for Inspire Fellowships.References
- N. E. Dixon, T. C. Gazzola, R. L. Blakeley and B. Zermer, J. Am. Chem. Soc., 1975, 97(14), 4131 CrossRef CAS PubMed.
- J. B. Sumner, J. Biol. Chem., 1926, 69, 435 CAS.
- H. L. Mobley and R. P. Hausinger, Microbiol. Rev., 1989, 53(1), 85 CAS.
- A. Maity, M. Pal, S. Maithani, B. Ghosh, S. Chaudhuri and M. Pradhan, J. Breath Res., 2016, 10(3), 036007 CrossRef PubMed.
- Y. Qin and J. M. S. Cabral, Appl. Biochem. Biotechnol., 1994, 49(3), 217 CrossRef CAS PubMed.
- A. Maity, M. Pal, S. Som, S. Maithani, S. Chaudhuri and M. Pradhan, Anal. Bioanal. Chem., 2017, 409, 193–200 CrossRef CAS PubMed.
- B. Krajewska, J. Mol. Catal. B: Enzym., 2009, 59(1), 9–21 CrossRef CAS.
- T. C. Huang and D. H. Chen, J. Chem. Technol. Biotechnol., 1991, 52(4), 433 CrossRef CAS.
- N. D. Jespersen, J. Am. Chem. Soc., 1975, 97(7), 1662 CrossRef CAS PubMed.
- B. R. van Eldik and M. Brindell, J. Biol. Inorg Chem., 2012, 17(7), 1123 CrossRef PubMed.
- D. Walther, M. Ruben and S. Rau, Coord. Chem. Rev., 1999, 182, 67–100 CrossRef.
- I.-S. Park and R. P. Hausinger, Science, 1995, 267(5201), 1156 CrossRef CAS PubMed.
- E. R. Crosson, K. N. Ricci, B. A. Richman, F. C. Chilese, T. G. Owano, R. A. Provencal, M. W. Todd, J. Glasser, A. A. Kachanov, B. A. Paldus, T. G. Spence and R. N. Zare, Anal. Chem., 2002, 74, 2003 CrossRef CAS PubMed.
- A. Maity, S. Som, C. Ghosh, G. Dutta Banik, S. B. Daschakraborty, S. Ghosh, S. Chaudhuri and M. Pradhan, J. Anal. At. Spectrom., 2014, 29, 2251 RSC.
- A. Maity, G. D. Banik, C. Ghosh, S. Som, S. Chaudhuri, S. B. Daschakraborty, S. Ghosh, B. Ghosh, A. K. Raychaudhuri and M. Pradhan, J. Breath Res., 2014, 8(1), 016005 CrossRef PubMed.
Footnote |
| † Both authors contributed equally to this work and should be considered as joint first authors. |
| This journal is © The Royal Society of Chemistry 2017 |