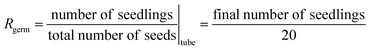Open Access Article
Open Access ArticlePromoting lentil germination and stem growth by plasma activated tap water, demineralized water and liquid fertilizer
S. Zhang,
A. Rousseau and
T. Dufour *
*
LPP, CNRS, UPMC Univ. Paris 06, Ecole Polytech., Univ. Paris-Sud, Observatoire de Paris, Université Paris-Saclay, Sorbonne Universités, PSL Research University, 4 Place Jussieu, 75252 Paris, France. E-mail: thierry.dufour@upmc.fr
First published on 16th June 2017
Abstract
Tap water, demineralized water and liquid fertilizer have been activated using an atmospheric pressure plasma jet (APPJ) to investigate their benefits for the germination rate and stem elongation rate of lentils from Puy-en-Velay (France). By plasma-activating tap water, we have obtained germination rates as high as 80% (instead of 30% with tap water). Also, higher stem elongation rates and final stem lengths were obtained using activated tap water compared with commercial fertilizer. We show that these rates of germination and stem growth strongly depend on the combination of two radicals generated in the liquids by the plasma: hydrogen peroxide and nitrate. This synergy appears to be a condition for releasing seed dormancy through the endogenous production of NO radicals.
Introduction
Cold atmospheric plasmas (CAP) have benefits for a wide variety of applications such as material processes,1,2 biomedical applications3–6 and micro-fabrication.7 They can be considered as cocktails containing chemical species and ions, broadband radiation, and even electric fields, making them effective and successful in such kinds of applications.CAP applications in agriculture have recently emerged due to the challenges in modern agriculture. Intensive agriculture offers the advantage of large yields at the expense of large inputs such as more employment of workers and higher levels of nonorganic fertilizer and pesticides that lead to worse environmental pollution and more pesticide residues. Moreover, organic agriculture is still contentious from the perspective of yields.8 Plasma agriculture, on the other hand, could be a solution to the current problems. As shown below, cold plasma could promote seed germination and stimulate plant growth in some crops without the use of nonorganic fertilizer. Additionally, it is known that the atmospheric pressure plasma jet (APPJ) can cure fungus in plants and improve resistance to some plant diseases, which means that lower amounts of pesticide are used.9 In this respect plasma agriculture offers a great potential to solve the problems of modern agriculture.
As for plasma treatment of seeds, nearly all the work to date has been performed by directly treating seeds or even plants by CAP.10 One advantage of direct treatment is that the short-lived species such as O, OH, NO, and even the electric field or UV produced by the plasma11,12 may possibly be effective; some reports have indicated that this type of plasma treatment could enhance or modify the germination and/or growth of oilseed rape seeds,13 bean and wheat seeds.14–16 Direct plasma treatments of tomato, radish and mulungu also gave positive effects.17–20 On the other hand, plasma activated liquids (PALs) (mainly different types of water) provide a much greater degree of mobility for CAP used in agriculture and could be much more convenient in the industry. So far, few studies have reported plasma processes dedicated to the activation of liquids for agricultural applications; however, researchers have recently developed a process to improve germination rates of radishes, tomatoes and sweet peppers.21–23 Hence, they obtained an increase as high as 80% in the germination rate in the case of radishes while stem elongation of 60% was obtained for tomatoes.
In this work, tap water, demineralized water and liquid fertilizer are used to irrigate seeds and seedlings from germination to early stage growth. These liquids are also exposed to an APPJ to obtain PALs. Since tap/demineralized waters have already shown differences for plant growth,24 we investigate here how these PALs can have important impacts on germination rates as well as on stem elongation. Two main radicals in the liquid phase are characterized: nitrates and hydrogen peroxide. Their respective effects on the agronomical parameters are discussed.
Experimental setup
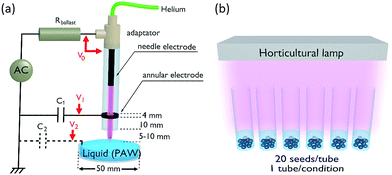
The plasma source utilized for liquid activation is an APPJ as shown in Fig. 1. The APPJ is composed of a quartz dielectric tube (inner diameter = 4.5 mm, outer diameter = 8 mm) with an inner rod electrode (AC high voltage) and an annular grounded electrode. The AC high voltage is delivered by a function generator (ELC Annecy France, GF467AF) and is amplified by a power amplifier (Crest Audio, 5500 W, CC5500). A ballast resistor (250 kΩ, 600 W) protects the power supply from excessive currents that may result from plasma glow-to-arc transitions. A capacitor (10 nF) is connected to the grounded electrode to monitor the plasma current. The APPJ is supplied with 500 sccm helium controlled by a flow controller (MKS, model 1259C-00500SV). A glass vessel is placed 5–10 mm down from the APPJ to collect liquids with typical volumes of 10–20 mL. After activation, the liquids are immediately poured onto the seeds or seedlings.As reported in Table 1, six experimental conditions are considered (three PALs and their respective controls), each one corresponding to a test tube containing 20 seeds (lentils from Puy-en-Velay, France). The chemical composition of tap water is reported in Table 2. It was evaluated from 63 samples by the Agence Régionale de Santé (Ile-de-France region) on public networks and interior building networks from the 5th district of Paris city, in February 2017.25 It is important to notice firstly the presence of nitrates (30.7 mg L−1) and secondly that the level of micro-organisms such as Enterococcus and Escherichia coli can be considered as negligible. The liquid fertilizer is a universal product commercialized by Truffaut©, dedicated to indoor plants and utilized following the supplier recommendations (dilution to 0.5%). The fertilizer brings the essential elements (nitrogen, phosphorus, potash) to the soil to feed seedling roots. The micro-organisms in the soil transform these elements into mineral salts, which can then be assimilated by the seedlings to constitute their nutritional intakes.
| Tube no. | Acronym | Description |
|---|---|---|
| 1 | TAP Ctrl | Tap water control |
| 2 | TAP PAL | Tap water plasma activated liquid |
| 3 | DEM Ctrl | Demineralized water control |
| 4 | DEM PAL | Demineralized water plasma activated liquid |
| 5 | FTZ Ctrl | Liquid fertilizer control |
| 6 | FTZ PAL | Liquid fertilizer plasma activated liquid |
| Parameter | Unit | Min | Mean | Max | Ref. limit of quality |
|---|---|---|---|---|---|
| pH | pH | 7.3 | 7.7 | 8.1 | 6.5–9 |
| Turbidity | NFU | 0.0 | 0.1 | 0.5 | 2 |
| Free chlorine | mg/LCI2 | 0.1 | 0.2 | 0.3 | — |
| Nitrates | mg L−1 | 23.6 | 30.7 | 37.4 | 50 |
| Conductivity | μS cm−1 | 520.0 | 571.9 | 637.0 | 200–1100 |
| Ammonia | mg L−1 | 0.0 | 0.0 | 0.0 | 0.1 |
| Escherichia coli | n/100 mL | 0.0 | 0.0 | 0.0 | 0.0 |
| Enterococcus | n/100 mL | 0.0 | 0.0 | 0.0 | 0.0 |
| Sulfato-reducing bacteria | n/100 mL | 0.0 | 0.0 | 0.0 | 0.0 |
| Coliforma bacteria | n/100 mL | 0.0 | 0.0 | 0.0 | 0.0 |
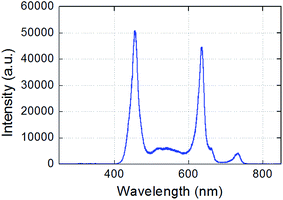
The seeds are irrigated daily with controlled liquid volumes as follows: (i) on days 1 and 2, 10 mL per day is poured into each test tube (and removed on the following day) so that all the seeds are immersed; and (ii) on day 3 and after, 1 mL per day of liquid is poured into each test tube without being removed on the following day. The experiments are performed at room temperature (22.5 °C ± 0.5 °C) with relative humidity ranging between 35 and 45%. Lighting is provided by the ambient light and reinforced 6 hours a day using a horticultural dimmable LED panel. The emission spectrum of this lamp is indicated in Fig. 2; it presents two main peaks at 460 and 636 nm, a continuum in between, and two minor peaks at 732 and 912 nm. Finally, it is important to underline that no soil or any organic substrate is utilized to clearly evidence the direct impact of the PALs on the seeds and seedlings, without any potential interference resulting from soils or solid carbon sources.
Two long-life radicals are quantified in the liquid phase: hydrogen peroxide species and nitrate ions. For the detection of H2O2, a solution of titanium(IV) oxysulfate (159.93 M, ∼15 wt% in dilute sulfuric acid, 99.99%, Sigma-Aldrich) is used. This acidic aqueous solution can then react in the presence of H2O2, according to the following reaction:
| [Ti(OH)3(H2O)3]+(aq) + H2O2(aq) ↔ [Ti(O2)(OH) × (H2O)3]+(aq) + 2H2O |
Hence a yellow peroxotitanium complex [Ti(O2)OH(H2O)3]+aq is formed, with an absorbance peak measured at 409 nm.26 Then the hydrogen peroxide concentration can be calculated using Beer's law. For the detection of NO3−, a PASCO scientific nitrate ion selective electrode is utilized. It allows the measurement of nitrate concentrations in the range 7 × 10−6 M to 1 M for pH = 2.5–11 and for temperatures between 273 K and 313 K. These concentration measurements are not performed in situ but post mortem, i.e. immediately after the plasma activation of the liquids. Some anions (e.g. Cl−, Br−, ClO3−) can interfere and cause electrode malfunction or drift, hence inducing an estimation error capped at 10%.
The impact of PAL on seeds and seedlings can be evaluated using two distinct methodological approaches (see Table 3): a single step method in which the growth rate (Rgrowth) is calculated according to eqn (1); and a two step method in which the germination rate (Rgerm) and average stem elongation rate (Rstem) are estimated according to eqn (2) and (3), respectively. In agriculture, Rgerm describes how many seeds of a plant species, variety or seed lot are likely to germinate over a given period. This parameter does not depend on the size of the seedling but only on its appearance.
These two methods are commonly utilized in agronomy. The single step approach synthesizes the information through a single parameter while the two step approach clearly deciphers the germination phenomenon from “real” seedling stem increases.
Results & discussion
The results dealing with agronomy are presented, followed by measurements of reactive species in the liquid phases. Finally, we discuss how the measured agronomical parameters can be related to plasma chemistry.Measurements of agronomical parameters
Photographs of lentil seeds and seedlings are taken every day, during the entire experiment. As an illustration, Fig. 3 shows seedlings on days 6, 12 and 18 for the six aforementioned conditions.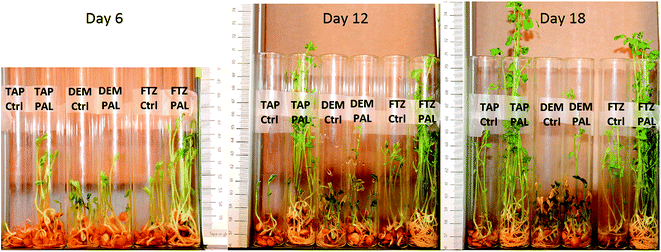 | ||
| Fig. 3 Pictures showing lentil seeds and seedlings on days 6, 12 and 18. TAP: tap water, DEM: demineralized water, FTZ: liquid fertilizer, Ctrl: control, PAL: plasma activated liquid. | ||
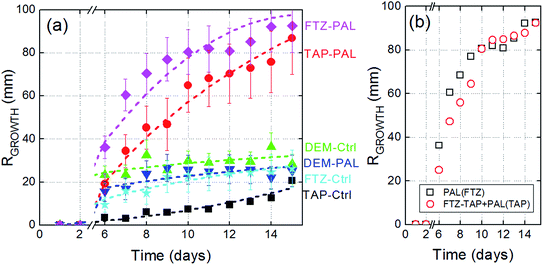
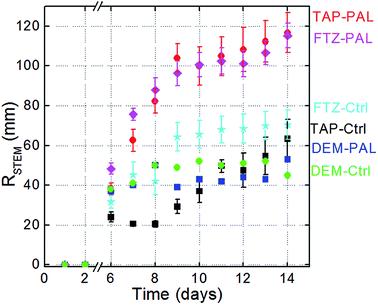
(i) When the seeds are irrigated with tap water (black curve, square symbols), seedlings grow quasi-linearly versus time to reach a mean length of 20 mm on day 15.
(ii) If seeds are irrigated using the commercial fertilizer (cyan curve, star symbols), one can clearly observe a growth rate higher than that obtained with tap water. This higher Rgrowth is particularly visible during days 6–10. Beyond this, Rgrowth tends to be limited and reaches the same value as in the tap water case, i.e. 20 mm. The fertilizer therefore has a boosting effect at the early stages of the seedlings' growth but not later.
(iii) The plasma activated tap water treatment (red circles) is of major interest. Rgrowth drastically increases during the early seedling stages (days 6–8) and reaches a value as high as 90 mm on day 15, i.e. a value at least four times higher than achieved when using tap water or even liquid fertilizer.
(iv) The plasma activation of the liquid fertilizer (purple diamonds) induces a drastic increase in Rgrowth in the early stages while this rise tends to slow down as time progresses and growth is limited to 90 mm, i.e. a value close to the one obtained using the plasma activated tap water.
(v) With demineralized water – as control or plasma activated – a growth rate close to 25 mm is reached on day 15. The absence of dissolved minerals seems not to be a hindrance for Rgrowth. Minerals could play an intermediate but crucial role in the interaction between seedling roots and the plasma-induced radical species in the liquid phase.
(vi) In Fig. 4a, the difference between the fertilizer curve (cyan stars) and the tap water curve (black squares) corresponds to the FTZ–TAP subtraction and stands for the gross contribution of the active elements contained in the liquid fertilizer, i.e. the nitrogen species, phosphorus and potash. The benefit of mixing these active elements with plasma activated tap water can be roughly estimated by adding the red circles curve to this difference; accordingly the FTZ–TAP + PAL(TAP) curve is plotted in Fig. 4b (open red circles). For the sake of comparison, we also report in the same graph, the curve of the plasma activated liquid fertilizer. The two curves are similar, at least after 10 days, hence allowing us to claim: PAL(FTZ) ≈ FTZ–PAL + PAL(TAP). Therefore, if tap water initially enriched in nitrogen species, phosphorus and potash is then activated by plasma, it turns out that similar Rgrowth values are obtained as for the fertilizer plasma activation. In Fig. 4b, the overlap of the two curves is less convincing between days 6 and 10. The resulting offset may be due to measurement uncertainties but could also indicate an unexpected effect in the liquid phase: namely an interaction between the plasma-induced radicals with the aforementioned active elements.
To summarize, if the benefits of plasma activated tap water and liquid fertilizer are compared, it turns out that the liquid fertilizer boosts Rgrowth only during the early stages without increasing final stem lengths, while the plasma activation process boosts Rgrowth during early and late stages as well as increasing final stem length. Finally, the plasma activation of the liquid fertilizer unifies two effects: an early stage boost (probably due to the fertilizer) and an enhancement of Rgrowth (probably due to the PAL).
The stem elongation rate parameter is represented versus time in Fig. 6. The observations reported for Fig. 5 still apply here although the trends are less pronounced. Overall, the values of Rstem are slightly higher than those of Rgrowth since the number of seeds considered can be lower than 20.
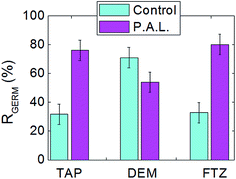 | ||
| Fig. 5 Germination rates of lentil seeds in control and PAL samples of tap water, demineralized water and liquid fertilizer (day 14). | ||
Chemistry of plasma activated liquids
Since the seeds and seedlings are not directly exposed to the plasma, only long-lived chemical species contained in a PAL can be involved in the enhanced rates of growth, germination and stem elongation. Since an exhaustive study of all the long-life species contained in the PAL is beyond the scope of the present article, we have focused our study on two species often considered as important and distinct markers: hydrogen peroxide (H2O2) and nitrates (NO3−).As shown in Fig. 7a, no hydrogen peroxide is detected in the controls while an increase of up to 160 μM is reached when tap and demineralized waters are plasma activated. The absence of a significant difference in H2O2 concentrations between these two media suggests that the presence of minerals (and eventually micro-organisms) in the tap water does not influence the production mechanisms for H2O2. In the case of the activated liquid fertilizer, the H2O2 concentration rises to 350 μM. In contrast to the tap and demineralized waters which are transparent, this liquid fertilizer presents a translucent appearance. Therefore, a dedicated calibration curve was generated so as to allow the utilization of spectrophotometry. As shown in Fig. 7b, the nitrates can be detected in the six liquids. Two observations are relevant:
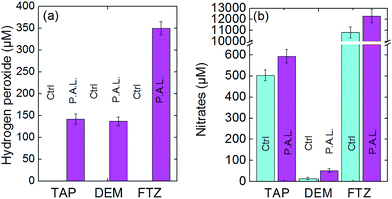 | ||
| Fig. 7 (a) H2O2 and (b) NO3− concentrations in control and PAL samples of tap water, demineralized water and liquid fertilizer. | ||
(i) The concentration of nitrates in the liquid fertilizer is greatly elevated at more than 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 μM versus only 470 μM for the tap water and 50 μM for the demineralized water. In the case of the tap water control, [NO3−] = 470 μM which is a value close to the one officially communicated by the Paris Town Hall, namely 29 mg L−1 = 467 μM.25
000 μM versus only 470 μM for the tap water and 50 μM for the demineralized water. In the case of the tap water control, [NO3−] = 470 μM which is a value close to the one officially communicated by the Paris Town Hall, namely 29 mg L−1 = 467 μM.25
(ii) The plasma exposure always increases the concentrations of nitrates in the liquid phases but to different extents. Indeed, in tap water and liquid fertilizer, the relative enrichments of nitrate are 18% and 14% respectively, while the enrichment is 286% in the demineralized water. This latter liquid is initially characterized by a very low electrical conductivity, and behaves as a floating counter-electrode unlike the two other liquids which behave as grounded electrodes owing to their much higher electrical conductivities (see Fig. 1). For this reason, overall radical production rates decrease in the plasma phase for the demineralized water, inducing a lower absolute rise in nitrates when treating demineralized water.
Relating agronomical rates to plasma activated liquid chemistry
Dormancy and germination are jointly controlled by two main hormones: abscisic acid (ABA) and gibberellic acid (GA). ABA is a positive regulator of dormancy induction and a negative regulator of germination. GA releases dormancy, promotes germination and counteracts the effects of ABA.27 The activity of these hormones can depend on the exogenous environment, in particular the concentration of reactive species such as hydrogen peroxide, nitrates and nitric oxide.Hydrogen peroxide is known to play a dual role in the physiological processes of seeds as well as in (a)biotic stress. As a signal molecule, it can promote the regulation of ABA and GA metabolism as well as hormonal balance.28 According to the model of Liu reported in Fig. 8,29 H2O2 can interrupt a seed's dormancy through two pathways. The first pathway corresponds to the enhancement of ABA catabolism and GA biosynthesis. The signaling molecule (NO) does not regulate GA biosynthesis directly but instead acts as a temporary signaling molecule involved in the H2O2 regulation of ABA catabolism. Therefore, H2O2 mediates the up-regulation of ABA catabolism through NO signaling and GA biosynthesis. The second pathway indicates that a high production of ABA can inhibit GA biosynthesis. H2O2 can therefore interrupt dormancy through GA signaling activation rather than by influencing ABA metabolism.30
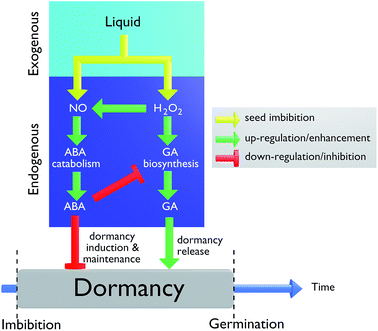 | ||
| Fig. 8 Model by Liu showing how H2O2, NO, ABA and GA can regulate seed dormancy and germination.29 | ||
Nitrate plays two main roles: (i) as a nutrient, it is assimilated by plant enzymes (e.g. nitrate reductase) leading to the production of amino acids and nitrogen compounds; and (ii) as a signal molecule, it can control numerous aspects of plant development and metabolism.31 The dormancy of viable seeds, i.e. germination failure despite optimal environmental conditions, can sometimes be broken by imbibing seeds with nitrate solutions such as NaNO3 or KNO3.32,33 These solutions promote the dormancy release of seeds only if NO3− can mediate nitric oxide radicals. Sodium nitro-prusside (SNP) as well as cyanide (CN) and nitrite can also be considered as NO donors. In contrast, the admixture of a NO scavenger such as 2-(4-carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide (c-PTIO) extends seed dormancy.34 In that respect, NO has to be considered as the key signaling molecule involved in seed dormancy release, even if others (such as hydroxylamine and nitrite) may also participate. It is worth mentioning that these radicals cannot be produced by cold plasma in the liquid phase, owing to their low solubility.35 They are biologically generated (e.g. with nitrate reductase) in the imbibed seed at the interface between the seed and the (plasma activated) water.
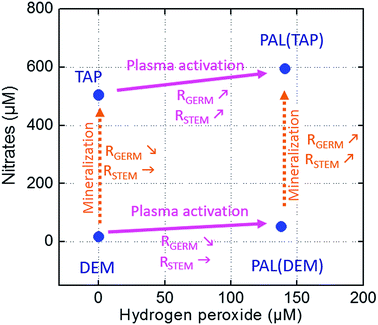
To relate our agronomy results (germination and stem elongation rates) to PAL chemistry (measurements of H2O2 and NO3−), our discussion is focused on the comparisons between tap and demineralized waters in the control samples and after plasma activation. Fig. 9 shows four data points corresponding to the control and activated tap and demineralized waters placed on the X and Y axes to reflect their respective concentrations of hydrogen peroxide and nitrate. The horizontal purple arrows represent the plasma activation process while the vertical dashed orange arrows represent virtual pollution (via mineralization and micro-organism charging). Rgerm and Rstem global trends are reported for each of these four arrows.
The plasma activation of demineralized water (bottom horizontal arrow) leads to a strong increase in its H2O2 concentration (from 0 to 140 μM) while the increase in nitrate remains comparatively negligible. H2O2 seems inconsequential for the promotion of stem elongation since Rstem remains unchanged. However, the increase in H2O2 significantly lowers Rgerm. Considering the model by Liu (Fig. 8 (ref. 29)), the increased production of H2O2 in the PAL sample could induce a disequilibrium between ABA and GA production. Here, the seed imbibition only produces exogenous H2O2 radicals and no nitric oxide. Therefore, even if high concentrations of exogenous H2O2 radicals are produced, they seem not to be involved into the production of endogenous radicals. As a result, the ABA catabolism remains low and ABA hormones remain at elevated levels so that dormancy is maintained. This first pathway is in competition with the second pathway in which exogenous H2O2 radicals participate in the biosynthesis of GA to release dormancy. It turns out that ABA is stronger than GA, meaning that the first pathway prevails over the second, hence explaining why elevated H2O2 levels cause the overall dormancy to be maintained and a lower germination rate to be observed.29
The mineralization of deionized water (left vertical arrow) corresponds to an increase in nitrates (from 30 to 470 μM) while the H2O2 concentration remains equal to zero. This operation is accompanied by an unchanged Rstem parameter while Rgerm is reduced, meaning that the germination process is promoted by a lower concentration of nitrates. At first sight, this inference seems in contradiction with conventional approaches in which nitrate solutions are known to promote germination. However, one must bear in mind that these solutions do not result from water plasma activation but from conventional biochemistry methods, i.e. using ionic salts such as KNO3.33 Moreover, and as previously stated, nitrate solutions shorten seed dormancy only if NO3− can produce nitric oxide radicals. The increasing uptake of nitrates could enhance the production of endogenous NO radicals, inducing a higher activity of ABA catabolism and therefore a lower level of ABA hormones. This pathway could hence cause dormancy release. However, since the demineralized sample (like the tap water) does not contain any H2O2 radicals, the simultaneous biosynthesis of GA is no longer stimulated. According to our results, the second pathway prevails over the first pathway since dormancy is longer and the germination rate is lower. In contrast, the obtained Rgerm decrease may result from the chemical composition of the tap water which has to be considered as a complex “cocktail” in which radicals and minerals (e.g. ammonium, free chlorine, calcium, bicarbonates) induce a non-optimal ABA–GA balance, giving rise to the longest dormancy and therefore delayed germination. The Rstem parameter remains constant despite the mineralization process (left vertical arrow), indicating that seedling elongation remains insensitive to radicals/minerals usually contained in tap water. The stem elongation mostly relies on the nutrients contained in the seeds.
The plasma activation of tap water (upper horizontal arrow) can be considered as an efficient process to produce H2O2 species but not nitrates since the nitrate concentration increases only from 550 μM to 600 μM, as shown in Fig. 9. However, both Rgerm and Rstem increase. If one refers to the Liu model in Fig. 8, this dormancy release could result from low ABA and high GA concentrations. This stable disequilibrium could result from a strong up-regulation of the ABA catabolism mediated by H2O2 through a strong NO signalization, i.e. through an elevated production of endogenous NO radicals. This point is underlined by Rajjou et al., who clearly explained that nitrates do not affect seed dormancy on their own but rather act through NO biosynthesis.36 These elevated levels of biosynthesized nitric oxide would hence require a PAL enriched both in NO3− and H2O2 radicals. This ‘two radicals’ synergy appears as a mandatory condition for germination and stem growth even if it must be considered as part of a more complex chemistry in which other long-life nitrogen radicals such as nitrites or peroxynitrites could also be involved at the interface of the seeds and PAL.
Practical aspects
In this research work, an APPJ device has been utilized to promote the germination and growth of lentil seeds by plasma-activating their irrigation liquids. A plasma jet requires a gas supply of argon or helium and an electrical source of energy (here AC high voltage) and it can only activate small volumes of liquids. These factors raise the question of whether cold plasma technology is an approach that can be transferred to farms. Here, it is important to bear in mind that the aim of this work was to perform a proof of concept, demonstrating that plasma activation of water (and even of liquid fertilizer) can drastically boost germination and growth of an agronomic model, namely lentils. However, there are many technological blocks that might be lifted to meet economic requirements: first, cold plasmas could be generated in dielectric barrier discharges (DBD) rather than in plasma jets. Indeed, no plasmagen gas is necessary in a DBD: the air could be used to directly generate almost the same reactive species in the liquid phase. Optimization work would nevertheless be necessary to process a larger surface of liquid, defining the optimal electrode-liquid interface gap and using thinner dielectric barriers with lower dielectric permittivities. The resulting lower electrical energy consumption combined with green electricity suggest a bright future for plasma agriculture.Moreover, the plasma activation of the seeds and seedlings has been performed here following an indirect approach: water is first plasma activated and second utilized to irrigate the seeds. Hence, only the long lifetime radicals such as nitrates and hydrogen peroxide can interplay with the physiological mechanisms of the seeds. A direct approach could also be explored, consisting of immersing the seeds in water while performing their in situ activation. Thereby short lifetime radicals such as NO and OH could also participate in seed germination.
Conclusion
In this article, a proof of concept has been demonstrated in which three PALs have shown distinct effects on the same lentil seed model.Concerning the germination process, higher Rgerm values were obtained solely in the case of plasma activated tap water in which both nitrates and hydrogen peroxide are present at concentrations as high as 500 μM and 150 μM, respectively. This synergetic combination seems a prerequisite to induce the endogenous production of nitric oxide at the PAL-seed interface. According to the Liu mechanism (Fig. 8), the resulting higher activity of the ABA catabolism would therefore reduce the down regulation of GA biosynthesis, hence releasing dormancy. The case of demineralized water activation is less clear owing to its lower electrical conductivity which may change the plasma properties depending upon the activation time. Overall it appears that the production of either nitrates or hydrogen peroxide alone using a plasma activation process does not permit to enhance the germination of lentil seeds.
Concerning the stem elongation process, plasma activated tap water presents at least two main benefits compared to the utilization of a commercial liquid fertilizer: Rstem and final stem length (day 15) are both higher. We have shown that H2O2 alone cannot promote stem elongation. The best conditions to increase Rstem involve the plasma generation of nitrate and hydrogen peroxide in the presence of minerals. This synergy appears as a mandatory condition even if not necessarily sufficient: other long-life nitrogen radicals such as nitrites or peroxynitrites might be involved. Furthermore, minerals could play a crucial role in this synergy, more specifically for nitrate production (see Fig. 7) even if not for H2O2. The PAL-induced higher Rstem and final stem length are of interest and highlight the strong agronomical and economic potential of PAL. Moreover, the plasma-generated species in the liquid phase could auto-degrade after a few hours while the liquid fertilizer can cumulate in the soil and induce long-term harmful pollution.
Acknowledgements
This work has been achieved within the LABEX Plas@Par project, and received financial state aid managed by the Agence Nationale de la Recherche, as part of the Programme Investissements d'Avenir (PIA) under the reference ANR-11-IDEX-0004-02. This work was also supported by grants from Région Ile-de-France (Sesame, Ref. 16016309).References
- M. A. Lieberman and A. J. Lichtenberg, Principles of plasma discharges and materials processing, John Wiley & Sons, 2005 Search PubMed.
- R. d’Agostino, P. Favia, C. Oehr and M. R. Wertheimer, Plasma Processes Polym., 2005, 2, 7–15 CrossRef.
- D. B. Graves, Plasma Processes Polym., 2014, 11(12), 1120–1127 CrossRef CAS.
- O. Lunov, V. Zablotskii, O. Churpita, E. Chánová, E. Syková, A. Dejneka and S. Kubinová, Sci. Rep., 2014, 4, 7129 CrossRef CAS PubMed.
- C. H. Park, J. S. Lee, J. H. Kim, D. K. Kim, O. J. Lee, H. W. Ju, B. M. Moon, J. H. Cho, M. H. Kim and P. P. Sun, et al., J. Phys. D: Appl. Phys., 2014, 47, 435402 CrossRef.
- M. Keidar, Plasma Sources Sci. Technol., 2015, 24, 033001 CrossRef.
- S. Samukawa, M. Hori, S. Rauf, K. Tachibana, P. Bruggeman, G. Kroesen, J. C. Whitehead, A. B. Murphy, A. F. Gutsol and S. Starikovskaia, et al., J. Phys. D: Appl. Phys., 2012, 45, 253001 CrossRef.
- J. Reganold and J. Wachter, Nat. Plants, 2016, 2, 15221 CrossRef PubMed.
- X. Zhang, D. Liu, R. Zhou, Y. Song, Y. Sun, Q. Zhang, J. Niu, H. Fan and S. Yang, Appl. Phys. Lett., 2014, 104, 043702 CrossRef.
- X. Zhang, D. Liu, R. Zhou, Y. Song, Y. Sun, Q. Zhang, J. Niu, H. Fan and S. z. Yang, Appl. Phys. Lett., 2014, 104, 043702 CrossRef.
- P. Bruggeman, M. J. Kushner, B. R. Locke, J. Gardeniers, W. Graham, D. B. Graves, R. Hofman-Caris, D. Maric, J. P. Reid and E. Ceriani, et al., Plasma Sources Sci. Technol., 2016, 25, 053002 CrossRef.
- D. Liu, Z. Liu, C. Chen, A. Yang, D. Li, M. Rong, H. Chen and M. Kong, Sci. Rep., 2016, 6, 23737 CrossRef CAS PubMed.
- L. Ling, L. Jiangang, S. Minchong, Z. Chunlei and D. Yuanhua, Sci. Rep., 2015, 5, 13033 CrossRef PubMed.
- E. Bormashenko, R. Grynyov, Y. Bormashenko and E. Drori, Sci. Rep., 2012, 2, 741 Search PubMed.
- A. Zahoranova, M. Henselova, D. Hudecová, B. Kalinakova, D. Kovacik, V. Medvecka and M. Cernak, Plasma Chem. Plasma Process., 2016, 36, 397–414 CrossRef CAS.
- R. Zhou, X. Zhang, J. Zhuang, S. Yang, K. Bazaka and K. K. Ostrikov, Sci. Rep., 2016, 6, 32603 CrossRef CAS PubMed.
- J. Jiang, Y. Lu, J. Li, L. Li, X. He, H. Shao and Y. Dong, PLoS One, 2014, 9, e97753 Search PubMed.
- Q. Lu, D. Liu, Y. Song, R. Zhou and J. Niu, Plasma Processes Polym., 2014, 11, 1028–1036 CrossRef CAS.
- S. Kitazaki, T. Sarinont, K. Koga, N. Hayashi and M. Shiratani, Curr. Appl. Phys., 2014, 14, S149–S153 CrossRef.
- C. A. Junior, J. de Oliveira Vitoriano, D. L. S. da Silva, M. de Lima Farias and N. B. de Lima Dantas, Sci. Rep., 2016, 6, 33722 CrossRef PubMed.
- D. P. Park, K. Davis, S. Gilani, C. A. Alonzo, D. Dobrynin, G. Friedman, A. Fridman, A. Rabinovich and G. Fridman, Curr. Appl. Phys., 2013, 13, S19–S29 CrossRef.
- A. Lindsay, B. Byrns, W. King, A. Andhvarapou, J. Fields, D. Knappe, W. Fonteno and S. Shannon, Plasma Chem. Plasma Process., 2014, 34, 1271–1290 CrossRef CAS.
- L. Sivachandiran and A. Khacef, RSC Adv., 2017, 7, 1822 RSC.
- S. Gvozdenac, D. Indic, S. Vukovic, M. Grahovac, M. Vrhovac, Z. Boskovic and N. Marinkovic, Acta agriculturae Serbica, 2011, XVI(31), 33–41 Search PubMed.
- Agence Régionale de Santé de la region Île-de-France, http://www.eaudeparis.fr/la-qualite-de-leau-a-paris/, http://www.eaudeparis.fr/fileadmin/contribution/UDI/udi_CENTRE.pdf.
- K. P. Reis, V. K. Joshi and M. E. Thompson, J. Catal., 1996, 161, 62–67 CrossRef CAS.
- B. Kucera, M. Alan Cohn and G. Leubner-Metzger, Seed Sci. Res., 2005, 15, 281–307 CAS.
- L. Wojtyla, K. Lechowska, S. Kubala and M. Garnczarska, Front. Plant Sci., 2016, 7, 66 Search PubMed.
- Y. Liu, N. Ye, R. Liu, M. Chen and J. Zhang, J. Exp. Bot., 2010, 61(11), 2979–2990 CrossRef CAS PubMed.
- E. Bahin, C. Bailly, B. Sotta, I. Kranner, F. Corbineau and J. Leymarie, Plant, Cell Environ., 2011, 34(6), 980–993 CrossRef CAS PubMed.
- R. Wang, M. Okamoto, X. Xing and N. M. Crawford, Plant Physiol., 2003, 132(2), 556–567 CrossRef CAS PubMed.
- S. B. Hendricks and R. B. Taylorson, Proc. Natl. Acad. Sci. U. S. A., 1975, 72(1), 306–309 CrossRef CAS.
- A. Atia, A. Debez, Z. Barhoumi, A. Smaoui and C. Abdelly, C. R. Biol., 2009, 332(8), 704–710 CrossRef CAS PubMed.
- P. C. Bethke, I. Libourel, V. Reinöhl and R. L. Jones, Planta, 2006, 223, 805–812 CrossRef CAS PubMed.
- R. Sander, Compilation of Henry's law constants (version 4.0) for water as solvent, Atmos. Chem. Phys., 2015, 15, 4399–4981, DOI:10.5194/acp-15-4399-2015.
- E. Arc, M. Galland, B. Godin, G. Cueff and L. Rajjou, Frontiers in Plant Science, 2013, 4, 346 Search PubMed.
| This journal is © The Royal Society of Chemistry 2017 |