Open Access Article
Open Access ArticleSynthesis and characterisation of push–pull flavin dyes with efficient second harmonic generation (SHG) properties†
Nabeel Mohammed‡
a,
Alan A. Wiles‡ a,
Michael Belsley‡
a,
Michael Belsley‡ b,
Sara S. M. Fernandesc,
Michele Carielloa,
Vincent M. Rotello
b,
Sara S. M. Fernandesc,
Michele Carielloa,
Vincent M. Rotello d,
M. Manuela M. Raposo
d,
M. Manuela M. Raposo *c and
Graeme Cooke
*c and
Graeme Cooke *a
*a
aGlasgow Centre for Physical Organic Chemistry, School of Chemistry, University of Glasgow, WestCHEM, Glasgow, G12 8QQ, UK
bCenter of Physics, University of Minho, Campus de Gualtar, 4710-057 Braga, Portugal
cCenter of Chemistry, University of Minho, Campus de Gualtar, 4710-057 Braga, Portugal. E-mail: mfox@quimica.uminho.pt
dDepartment of Chemistry, University of Massachusetts, Amherst, MA 01003, USA
First published on 5th May 2017
Abstract
Organic compounds displaying second-order non-linear optical properties are key materials for optoelectronics. Here, three push–pull flavin derivates (FLA-A–C) have been synthesised and their optical, redox and second harmonic generation (SHG) properties have been investigated. In particular, we describe the role differing electron donor units (N,N-dimethylaniline-, N,N-diphenylaniline- and ferrocenyl-) attached through the C(8) position of the flavin unit have on the optical and redox properties of the latter. Hyper-Rayleigh scattering in dioxane solutions using a fundamental wavelength of 1064 nm was employed to evaluate their second-order nonlinear optical properties. Our experiments have indicated that their first hyperpolarisabilities (β) are influenced by the electronic nature and strength of the donor groups. N,N-dimethylaniline-functionalised derivative FLA-A, exhibited the largest first hyperpolarisability (β = 9550 × 10−30 esu, using the T convention) and high decomposition temperature (Td = 312 °C), thus indicating its potential application as a useful material for incorporation into non-linear optical devices.
Introduction
Nonlinear optical (NLO) materials represent one of the principal branches of optoelectronics.1 Applications of NLO materials encompass a range of scientific sub-disciplines including medicine (e.g. second harmonic generation (SHG)-imaging, high-resolution optical microscopy for biological imaging) and materials science (e.g. frequency conversion of lasers, waveguides and electro-optic modulators, optical transistors, fibres and lenses).2Organic NLO chromophores possess several advantages over their inorganic brethren including convenient synthesis, tunability, straightforward processability, ultrafast response times, lower dielectric constants and enhanced NLO responses.3 Accordingly, during the last four decades, extensive progress has been made in the improvement of the SHG properties of organic donor–acceptor π-conjugated systems.4,5
Organic push–pull NLO chromophores are promising materials, and typically comprise of a strong electron donor (D) (e.g. arylamino groups) and strong electron acceptor (A) group (e.g. cyano, nitro, di- or tricyanovinyl) linked through a π-bridge (aromatic or heterocyclic) (D–π–A). This D–π–A arrangement promotes efficient intramolecular charge-transfer (ICT) between the D and A groups and generates dipolar push–pull systems possessing an intense, low-energy CT absorption, and the propensity to display strong molecular SHG. The NLO response correlates strongly with the strength of the attached D and A groups as well as the electronic nature and length of the π-conjugated spacer.3
Amines (NR2), such as 4-N,N-dimethylaniline- or 4-N,N-diphenylaniline-, are commonly used donor groups in NLO chromophores. The latter moieties tend to have a lower electron donating ability due to the partial delocalisation of the nitrogen nonbonding lone pair to the conjugated phenyl units that are usually organised in a nonplanar fashion.5 On the other hand, N,N-diarylaniline moieties usually impart significantly higher thermo- and photostabilities compared to their N,N-dialkylaniline analogues. Consequently, chromophores featuring N,N-dialkylaniline donor groups tend to show much higher nonlinearities compared to their N,N-diarylaniline brethren, although they generally have much lower thermostabilities.6 Organometallic7 groups such as ferrocene have also been utilised as D moieties in NLO chromophores.8 More recently, electron-rich (e.g. thiophene and pyrrole) and electron-poor (e.g. azoles and diazines) five-membered heterocycles have also been used as efficient “non-conventional” electron donor or acceptor moieties, respectively, due to their auxiliary D–A heterocycle effect.9
Flavins are electron deficient heterocycles and are important cofactors in a range of flavoenzymes.10 Synthetic flavin derivatives due to their convenient synthesis, photostability and tunable optical and redox properties have found application in a diverse range of areas including: supramolecular chemistry11 and photonics such as solvatochromic probes12 and photon-induced energy and charge transfer systems.13 However, their application as A units in NLO materials has not been widely reported. For example, Stanley and co-workers have reported that a push–pull flavin derivative displayed a hyperpolarisability (β0) of 720 × 10−30 esu, estimated with the aid of Stark spectroscopy.14
Here we report the synthesis and characterisation of three flavin-based dyes FLA-A–C featuring N,N-dimethylaniline-, N,N-diphenylaniline- and ferrocenyl-units attached through the C(8) position of the flavin unit to ensure good electronic communication between the differing D units and the flavin A moiety.15 FLA-C features an acetylene linker unit as these moieties have been shown to increase planarity and charge-transfer in D–A push–pull systems5b which in turn increases SHG efficiency.16 We have investigated their optical and redox properties and have determined their molecular first hyperpolarisabilities (β) using the hyper-Rayleigh scattering (HRS) method which has revealed that these push–pull systems display efficient SHG properties.
Results and discussion
Synthesis
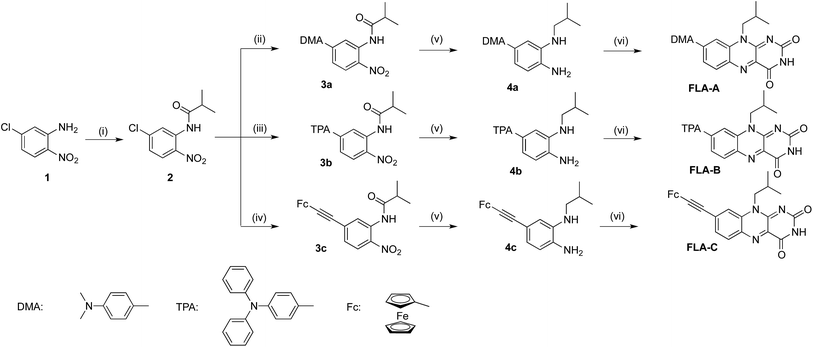
The three push–pull flavins (FLA-A–C) have been synthesised from 5-chloro-2-nitroaniline 1 through a simple 4-step route in moderate to high yields (Scheme 1). Firstly, compound 1 was acylated to give compound 2 in high yield. The differing donor moieties were then added to this building block using either Suzuki (compounds 3a and 3b) or Sonogashira (compound 3c) coupling methodologies. Simultaneous reduction of both the carbonyl- and nitro-moieties with LiAlH4 gave compounds 4a–c. Finally, formation of the flavin moiety was achieved through condensation of 4a–c with alloxan monohydrate to give the flavin-based dyes FLA-A–C.Optical properties
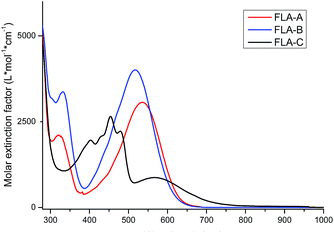
The UV-vis spectra of the three flavin dyes were recorded to determine their optical properties and solvatochromic behaviour, as the latter has been shown to have strong correlation to the ability of compounds to display SHG properties.17 Fig. 1 shows that the three dyes have a broad absorption in the visible region. FLA-A and FLA-B show the highest absorbance values at 536 nm (3066 L mol−1 cm−1) and 517 nm (4009 L mol−1 cm−1) nm, respectively. On the other hand, FLA-C shows a highest absorbance at 454 nm (2657 L mol−1 cm−1) nm and a weaker but longer wavelength absorption at 566 nm (873 L mol−1 cm−1), which we assign as π-LUMO and metal-LUMO bands, respectively.18 Investigation of the influence solvent polarity has on the absorbance of dyes FLA-A–C (Fig. 2) has revealed that significant solvatochromic behaviour exists for this series of dyes. In accordance with previously reported data for a related push–pull flavin derivative, the λmax values for FLA-A–B did not display a linear correlation with solvent polarity (dielectric constant or ET(30)), suggesting that solute–solvent interactions are occuring.12 Interestingly, the position of the metal-LUMO absorption bands of FLA-C are in broad correlation with the dielectric constants for the different solvents.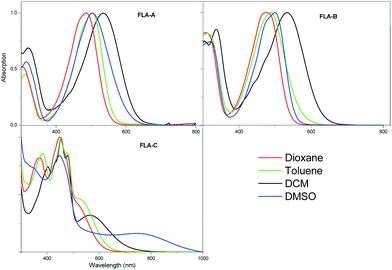 | ||
| Fig. 2 Normalised UV-vis absorption spectra of FLA-A–C recorded in four different solvents (1 × 10−4 M). | ||
Electrochemistry
The redox properties of the dyes have been investigated using cyclic voltammetry (see ESI, Fig. S4†). Each dye displays a pseudo-reversible oxidation and reduction wave. The differing donor abilities of the D units play a predictable effect on the electron affinities of the dyes (FLA-A −3.55 eV, FLA-B −3.88 eV, FLA-C −3.76 eV),15 whereas the ionisation potentials of these moieties show a more marked variation (FLA-A −5.32 eV, FLA-B −5.49 eV, FLA-C −4.99 eV) in line with their structurally and electronically differing D moieties.NLO properties
The molecular first hyperpolarisabilities β of flavin derivatives FLA-A–C were measured by the hyper-Rayleigh scattering (HRS) method19 at a fundamental wavelength of 1064 nm and the data are summarised in Table 1. It is clear that the electronic donor ability of the moieties attached at the position C(8) of the flavin derivative for compounds FLA-A–C modulates the red-shifted absorption maxima and nonlinearities as anticipated from the donor strengths of the substituents (ferrocenyl- < 4-N,N-diphenylaniline- < 4-N,N-dimethylaniline-). These results are not unexpected bearing in mind the Hammett σp constants of N,N-dimethylaniline (σp = −0.83), N,N-diphenylaniline (σp = −0.56) and ferrocenyl (σp = −0.18), donor groups20 as well as data provided in several earlier studies.6 Therefore, 4-N,N-dimethylaniline, being the strongest electron-donor group, gives rise to a higher β value for compound FLA-A.21 On the other hand, the ferrocenyl derivative FLA-C exhibits the lowest hyperpolarisability (β = 355 × 10−30 esu) compared to FLA-A and B (β = 9550 and 8660 × 10−30 esu, respectively).| Comp. | Donor | λmax (nm) | εmax (M−1 cm−1) | βb (10−30 esu) | β0c (10−30 esu) | Tdd (°C) |
|---|---|---|---|---|---|---|
| a Experimental first hyperpolarisabilities β and spectroscopic data measured in dioxane solutions are reported using the T convention.b All compounds are transparent at the 1064 nm fundamental wavelength.c Data corrected for resonance enhancement at 532 nm using the two-level model with β0 = β[1 − (λmax/1064)2][1 − (λmax/532)2]; damping factors not included 1064 nm.22d Decomposition temperature (Td) measured at a heating rate of 10 °C min−1 under a nitrogen atmosphere, obtained by thermogravimetric analysis (TGA). | ||||||
| FLA-A | DMA | 487 | 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 087 087 |
9550 | 1220 ± 120 | 309 |
| FLA-B | TPA | 473 | 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 520 520 |
8660 | 1460 ± 180 | 329 |
| FLA-C | Fc | 449 | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 720 720 |
355 | 85 ± 15 | 310 |
| pNA | — | 352 | — | 40.1 | 20.1 | — |
Thermal stabilities
In order to be readily incorporated into efficient optoelectronic devices, organic NLO-phores should exhibit large molecular quadratic hyperpolarisability, good optical transparency and excellent thermal and chemical stabilities. Derivatives that feature alkyl- and aryl-amine moieties as their donor units have been shown to possess these important properties.5a,6 The thermal stability of chromophores FLA-A–C was studied by thermogravimetric analysis (TGA) and by differential scanning calorimetry (DSC) (see Fig. S1–S3, ESI†). FLA-A–C exhibit high decomposition temperatures of 312, 329 and 319 °C respectively. Comparison of the thermal stability of N,N-dimethylaniline derivative FLA-A with flavin FLA-B shows that the N,N-diphenylaniline-substitution results in an enhanced thermal stability which is in agreement with previous findings.5a,6Theoretical calculations
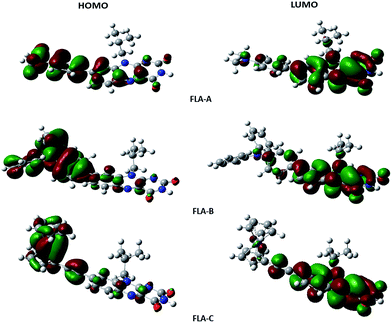
To further our understanding of the properties of these dyes and establish a comparative computational basis for this series, density functional theory (DFT) calculations were undertaken. The HOMO and LUMO maps of FLA-A–C were computed in a polarized solvent continuum of 1,4-dioxane (Fig. 3) and show diffuse and overlapping HOMO and LUMO densities. FLA-A shows the most extensive overlap of HOMO/LUMO densities which is consistent with good CT between the strongest N,N-dimethylaniline donor and flavin moieties. The estimated dipole moments for these molecules range between 13 and 17 Debye (in 1,4-dioxane), and follows the same trend as the experimentally determined hyperpolarisabilities (Tables 1 and 2). Interestingly, the magnitude of the calculated hyperpolarisabilities have little correlation with the experimentally determined values for FLA-A and B in this solvent.| Comp. | Donor | μ(D) | βtot (10−30 esu) | β∥ (10−30 esu) | HOMO (eV) | LUMO (eV) |
|---|---|---|---|---|---|---|
| FLA-A | DMA | 17.01 | 804 | 477 | −5.64 | −3.14 |
| FLA-B | TPA | 14.30 | 1448 | 870 | −5.57 | −3.24 |
| FLA-C | Fc | 13.66 | 615 | 370 | −5.89 | −3.36 |
The geometry of each of the dyes were also optimised under differing solvent continua and the dipole moments were calculated. For all dyes the largest calculated dipole moment was obtained in DMSO, with 1,4-dioxane providing the lowest value (Tables 3 and S2, ESI†). Interestingly, for each solvent studied the magnitude of the calculated dipole moment follows the same general trend as the experimentally determined hyperpolarizability values of the dyes. Furthermore, solvent polarity also seems to affect the magnitude of the HOMO more than the LUMO of these compounds (Table S2, ESI†). Bearing in mind the important relationship between solvatochromism, polarizability and the extrapolation to hyperpolarizability values in second order NLO materials,17 the data suggest that for these systems DFT predictions of dipole moment (and its solvent dependency) may offer a better yardstick for designing new analogues with large hyperpolarisability values than their calculated hyperpolarisabilities.
Experimental
General methods
All reactions were undertaken under inert atmosphere. Solvents were purified using a Pure Solv™ solvent purifier system. Reagents were purchased from Sigma Aldrich, and were used without further purification. NMR spectra were obtained with either a Bruker AVIII 400 MHz or a Bruker AVIII 500 MHz spectrometer (relative to TMS). UV-vis spectra were recorded on a Perkin Elmer Lamda 25 instrument. Optical band gaps (EG,OPT) were estimated using the onset of the longest wavelength absorption (λ) using EG,OPT (eV) = (1240/λ (nm)).Synthesis
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5) and then recrystallised from hot ethanol to yield title compound 2 as yellow needles (2.69 g, 95%). Mp 91–92 °C; δH (500 MHz, CDCl3, TMS) 10.56 (1H, s), 8.96 (1H, d, J 2.3), 8.18 (1H, d, J 9.0), 7.13 (1H, dd, J 9.0, 2.3), 2.66 (1H, sept, J 6.9), 1.31 (6H, d, J 6.9); δC (125 MHz, CDCl3, TMS) 176.3, 143.0, 136.3, 134.5, 127.1, 123.4, 121.9, 37.8, 19.4; HRMS (EI+, m/z): [M]+ found, 242.0455; calc. for (C10H11ClN2O3+), 242.0458.
5) and then recrystallised from hot ethanol to yield title compound 2 as yellow needles (2.69 g, 95%). Mp 91–92 °C; δH (500 MHz, CDCl3, TMS) 10.56 (1H, s), 8.96 (1H, d, J 2.3), 8.18 (1H, d, J 9.0), 7.13 (1H, dd, J 9.0, 2.3), 2.66 (1H, sept, J 6.9), 1.31 (6H, d, J 6.9); δC (125 MHz, CDCl3, TMS) 176.3, 143.0, 136.3, 134.5, 127.1, 123.4, 121.9, 37.8, 19.4; HRMS (EI+, m/z): [M]+ found, 242.0455; calc. for (C10H11ClN2O3+), 242.0458.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20) affording 3a as an orange solid (2.08 g, 65%). Mp 182–185 °C; δH (500 MHz, CDCl3, TMS) 10.75 (1H, s), 9.12 (1H, d, J 2.0), 8.24 (1H, d, J 8.9), 7.64–7.63 (2H, m), 7.35 (1H, dd, J 8.9, 2.0), 6.77–6.74 (2H, m), 3.02 (6H, s), 2.68 (1H, sept, J 6.9), 1.33 (6H, d, J 6.9); δC (125 MHz, CDCl3, TMS) 176.5, 151.4, 149.2, 136.1, 133.7, 128.5, 126.6, 125.4, 120.1, 118.0, 112.5, 40.4, 37.8, 19.6; HRMS (ESI+, m/z): [M + Na]+ found, 350.1475; calc. for (C18H21N3NaO3+), 350.1481.
20) affording 3a as an orange solid (2.08 g, 65%). Mp 182–185 °C; δH (500 MHz, CDCl3, TMS) 10.75 (1H, s), 9.12 (1H, d, J 2.0), 8.24 (1H, d, J 8.9), 7.64–7.63 (2H, m), 7.35 (1H, dd, J 8.9, 2.0), 6.77–6.74 (2H, m), 3.02 (6H, s), 2.68 (1H, sept, J 6.9), 1.33 (6H, d, J 6.9); δC (125 MHz, CDCl3, TMS) 176.5, 151.4, 149.2, 136.1, 133.7, 128.5, 126.6, 125.4, 120.1, 118.0, 112.5, 40.4, 37.8, 19.6; HRMS (ESI+, m/z): [M + Na]+ found, 350.1475; calc. for (C18H21N3NaO3+), 350.1481.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20) affording 3b as an orange solid (2.78 g, 67%). Mp 95–98 °C; δH (400 MHz, CDCl3, TMS) 10.67 (1H, s), 9.12 (1H, d, J 2.0), 8.27 (1H, d, J 8.9), 7.57–7.53 (2H, m), 7.35 (1H, dd, J 8.9, 2.0), 7.32–7.27 (4H, m), 7.15–7.06 (8H, m), 2.68 (1H, sept, J 6.9), 1.32 (6H, d, J 6.9); δC (100 MHz, CDCl3, TMS) 176.5, 149.3, 148.7, 147.3, 136.0, 134.4, 131.5, 129.6, 128.5, 126.6, 125.2, 123.9, 122.9, 120.8, 119.3, 37.8, 19.6; HRMS (ESI+, m/z): [M + Na]+ found, 474.1799; calc. for (C28H25N3NaO3+), 474.1794.
20) affording 3b as an orange solid (2.78 g, 67%). Mp 95–98 °C; δH (400 MHz, CDCl3, TMS) 10.67 (1H, s), 9.12 (1H, d, J 2.0), 8.27 (1H, d, J 8.9), 7.57–7.53 (2H, m), 7.35 (1H, dd, J 8.9, 2.0), 7.32–7.27 (4H, m), 7.15–7.06 (8H, m), 2.68 (1H, sept, J 6.9), 1.32 (6H, d, J 6.9); δC (100 MHz, CDCl3, TMS) 176.5, 149.3, 148.7, 147.3, 136.0, 134.4, 131.5, 129.6, 128.5, 126.6, 125.2, 123.9, 122.9, 120.8, 119.3, 37.8, 19.6; HRMS (ESI+, m/z): [M + Na]+ found, 474.1799; calc. for (C28H25N3NaO3+), 474.1794.FLA-A
Flash column chromatography (DCM/acetone; 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10) afforded FLA-A as a dark purple red solid (0.195 g, 38%), from 3a (0.408 g, 1.00 mmol). Mp > 300 °C; δH (400 MHz, CDCl3, TMS) 8.40 (1H, s), 8.26 (1H, d, J 8.7), 7.86 (1H, dd, J 8.7, 1.8), 7.68 (1H, d, J 1.8), 7.66–7.62 (2H, m), 6.86–6.82 (2H, m), 4.70 (2H, bs), 3.09 (6H, s) 2.51(1H, sept, J 6.7), 1.09 (6H, d, J 6.7); δC (100 MHz, CDCl3, TMS) 159.9, 155.3, 151.7, 151.5, 149.4, 135.2, 135.1, 134.1, 133.8, 128.7, 125.5, 125.5, 112.8, 111.1, 51.4, 40.3, 27.8, 20.3; HRMS (NSI+, m/z): [M + H]+ found, 390.1922; calc. for (C22H24N5O2+), 390.1925.
10) afforded FLA-A as a dark purple red solid (0.195 g, 38%), from 3a (0.408 g, 1.00 mmol). Mp > 300 °C; δH (400 MHz, CDCl3, TMS) 8.40 (1H, s), 8.26 (1H, d, J 8.7), 7.86 (1H, dd, J 8.7, 1.8), 7.68 (1H, d, J 1.8), 7.66–7.62 (2H, m), 6.86–6.82 (2H, m), 4.70 (2H, bs), 3.09 (6H, s) 2.51(1H, sept, J 6.7), 1.09 (6H, d, J 6.7); δC (100 MHz, CDCl3, TMS) 159.9, 155.3, 151.7, 151.5, 149.4, 135.2, 135.1, 134.1, 133.8, 128.7, 125.5, 125.5, 112.8, 111.1, 51.4, 40.3, 27.8, 20.3; HRMS (NSI+, m/z): [M + H]+ found, 390.1922; calc. for (C22H24N5O2+), 390.1925.
FLA-B
Flash column chromatography (DCM/acetone; 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10) afforded FLA-B as a dark purple solid (0.132 g, 42%), from 3b (0.275 g, 0.610 mmol). Mp > 300 °C; δH (400 MHz, CDCl3, TMS) 8.37 (1H, s), 8.31 (1H, d, J 8.6), 7.85 (1H, dd, J 8.6, 1.7), 7.71 (1H, s), 7.58–7.54 (2H, m), 7.36–7.31 (4H, m), 7.20–7.12 (8H, m) 4.70 (2H, bs), 2.51 (1H, sept, J 6.7), 1.09 (6H, d, J 6.7); δC (100 MHz, CDCl3, TMS) 159.6, 155.1, 151.5, 150.0, 148.7, 146.9, 136.0, 135.3, 133.9, 130.8, 129.8, 128.9, 128.5, 125.8, 125.7, 124.5, 122.2, 112.3, 51.5, 27.8, 20.3; HRMS (NSI+, m/z): [M + H]+ found, 514.2249; calc. for (C32H28N5O2+), 514.2238.
10) afforded FLA-B as a dark purple solid (0.132 g, 42%), from 3b (0.275 g, 0.610 mmol). Mp > 300 °C; δH (400 MHz, CDCl3, TMS) 8.37 (1H, s), 8.31 (1H, d, J 8.6), 7.85 (1H, dd, J 8.6, 1.7), 7.71 (1H, s), 7.58–7.54 (2H, m), 7.36–7.31 (4H, m), 7.20–7.12 (8H, m) 4.70 (2H, bs), 2.51 (1H, sept, J 6.7), 1.09 (6H, d, J 6.7); δC (100 MHz, CDCl3, TMS) 159.6, 155.1, 151.5, 150.0, 148.7, 146.9, 136.0, 135.3, 133.9, 130.8, 129.8, 128.9, 128.5, 125.8, 125.7, 124.5, 122.2, 112.3, 51.5, 27.8, 20.3; HRMS (NSI+, m/z): [M + H]+ found, 514.2249; calc. for (C32H28N5O2+), 514.2238.
FLA-C
Flash column chromatography (Et2O/EtOAc; 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) afforded FLA-C as a green solid (0.268 g, 34%), from 3c (0.683 g, 1.65 mmol). Mp > 300 °C; δH (500 MHz, CDCl3, TMS) 8.50 (1H, s), 8.22 (1H, d, J 8.5), 7.65 (1H, dd, J 8.5, 1.4), 7.62 (1H, d, J 1.4), 4.65 (2H, bs), 4.62 (2H, t, J 1.9), 4.40 (2H, t, J 1.9), 4.30 (5H, s), 2.49 (1H, sept, J 6.9), 1.09 (6H, d, J 6.9); δC (125 MHz, CDCl3, TMS) 170.2, 154.9; 151.5, 135.2, 133.6, 133.4, 132.6, 129.9, 117.4, 113.2, 98.8, 72.3, 70.4, 70.3, 63.1, 51.5, 31.1, 27.7, 20.2; HRMS (FAB+, m/z): [M + H]+ found, 479.1176; calc. for (C26H23N456FeO2+), 479.1171.
1) afforded FLA-C as a green solid (0.268 g, 34%), from 3c (0.683 g, 1.65 mmol). Mp > 300 °C; δH (500 MHz, CDCl3, TMS) 8.50 (1H, s), 8.22 (1H, d, J 8.5), 7.65 (1H, dd, J 8.5, 1.4), 7.62 (1H, d, J 1.4), 4.65 (2H, bs), 4.62 (2H, t, J 1.9), 4.40 (2H, t, J 1.9), 4.30 (5H, s), 2.49 (1H, sept, J 6.9), 1.09 (6H, d, J 6.9); δC (125 MHz, CDCl3, TMS) 170.2, 154.9; 151.5, 135.2, 133.6, 133.4, 132.6, 129.9, 117.4, 113.2, 98.8, 72.3, 70.4, 70.3, 63.1, 51.5, 31.1, 27.7, 20.2; HRMS (FAB+, m/z): [M + H]+ found, 479.1176; calc. for (C26H23N456FeO2+), 479.1171.
Nonlinear optical measurements using the hyper-Rayleigh scattering (HRS) method
Hyper-Rayleigh scattering (HRS) was used to measure the angle-averaged first hyperpolarisability β response of the molecules studied. The experimental set-up for hyper-Rayleigh measurements has previously been described in detail.19b A Q-switched Nd:YAG laser operating at a 10 Hz repetition rate with approximately 10 mJ of energy per pulse and a pulse duration (FWHM) close to 12 ns is used to excite hyper-Rayleigh scattering with an incident wavelength of 1064 nm. The hyper-Rayleigh signal was normalized at each pulse by using a small fraction of the laser pulse to generate a second harmonic signal from a KDP crystal to compensate for fluctuations in the temporal profile of the laser pulses due to longitudinal mode beating. Dioxane was used as a solvent, and the β values were calibrated using a reference solution of p-nitroaniline (pNA)23 also dissolved in dioxane at a concentration of 1 × 10−2 mol dm−3 (external reference method). The hyperpolarisability of pNA dissolved in dioxane is known from EFISH measurements carried out at the same fundamental wavelength. We have chosen to report our values using the so-called T (Taylor expansion) convention23b in which the β333 of pNA in dioxane at 1064 nm is 40 × 10−30 esu. This value includes a correction factor of 1.88 that accounts for the most recent measurement of the CCl4 hyper-Rayleigh scattering signal which was used as a reference.24All solutions were filtered (0.2 μm porosity) to avoid spurious signals from suspended impurities. The small hyper Rayleigh signal that arises from dioxane was taken into account. We took particular care to avoid reporting artificially high hyperpolarisabilities due to a possible contamination of the hyper-Rayleigh signal by molecular fluorescence near 532 nm. Measurements were carried out using two different interference filters with different transmission pass bands centred near the second harmonic at 532 nm allowing us to estimate and correct for any fluorescence emitted near 532 nm.
TGA/DSC
Thermogravimetric analysis (TGA) and differential scanning calorimetry (DSC) were performed using a TA Instruments SDT Q-600 series thermal analyser. Gas flow was 100 mL min−1. The DSC curves show exotherms as up and endotherms as down.Theoretical calculations
Gaussian 09 (ref. 25) was used to estimate solvent dependence and hyperpolarisability factors of the chromophores. The geometry of each dye was initially obtained semi-empirically (PM6). These geometries were then re-optimised at DFT level using the hybrid B3LYP level by employing the 6-31+G** basis set using polarizable continuum model (keyword: scrf = solvent). Frequency calculations were performed to ensure the absence of negative frequencies. Hyperpolarisability factors were estimated using an incident wavelength of 1064 nm (keywords: freq = raman, cphf = rdfreq, polar) and with a polarized solvent continuum using dioxane as the solvent (Table 2).26Conclusions
In conclusion, flavins FLA-A–C were readily synthesised from 5-chloro-2-nitroaniline using a simple 4-step synthetic route in moderate to high yields. Evaluation of the first hyperpolarisabilities of FLA-A–C using the hyper-Rayleigh scattering technique showed that derivatives FLA-A and FLA-B bearing stronger donor groups exhibit the higher hyperpolarisabilities (β = 8660–9550 × 10−30 esu) and higher thermal stabilities thereby indicating their potential future application in NLO devices. The convenient synthesis, good thermal stability and efficient SHG properties of this class of flavin derivatives suggest that related molecules may prove to be interesting candidates for further development. Moreover, the well-documented ability to accurately predict the physical properties of flavins using linear free energy relationships and, as we have shown here DFT calculations, will help ensure that push–pull flavin derivatives of this type, once embedded into acentric structures,27 will become an important class of NLO materials with predictable properties.Acknowledgements
GC thanks EPSRC (EP/E036244/1). VR thanks the NSF (CHE-1307021). NM thanks the Ministry of Higher Education and Scientific Research of Iraq and the University of Kirkuk. MMR thanks the Funds provided by FCT through the Chemistry Research Centre of the University of Minho (Ref. UID/QUI/00686/2013 and UID/QUI/0686/2016) and a PhD grant to S. S. M. Fernandes (SFRH/BD/87786/2012). The NMR spectrometer Bruker Avance III 400 is part of the National NMR Network and was purchased within the framework of the National Program for Scientific Re-equipment, with funds from FCT.References
- D. S. Chemla and J. Zyss, Nonlinear Optical Properties of Organic Molecules and Crystals, Academic Press, New York, 1987 Search PubMed.
- F. Meyers, S. R. Marder and J. W. Perry, in Chemistry of Advanced Materials: An Overview, ed. L. V. Interrante and M. J. Hampden-Smith, Wiley-VCH, New York, 1998, pp. 207–269 Search PubMed.
- (a) P. N. Prasad and D. J. Williams, in Introduction to Nonlinear Optical Effects in Molecules and Polymers, ed. J. Klafter and J. M. Drake, Wiley, New York, 1991, pp. 132–174 Search PubMed; (b) H. S. Nalwa and S. Miyata, Nonlinear Optics of Organic Molecules and Polymers, CRC Press, New York, 1997 Search PubMed; (c) L. R. Dalton, P. A. Sullivan, D. Bale, B. Olbricht, J. Davies, S. Benight, I. Kosilkin, B. H. Robinson, B. E. Eichinger and A. K. Y. Jen, in Organic Thin Films for Photonic Applications, ed. W. N. Herman, S. R. Flom and S. H. Foulger, American Chemical Society, Washington DC, 2010, vol. 1039, ch. 2, pp. 13–33 Search PubMed; (d) T. Verbiest, S. Houbrechts, M. Kauranen, K. Clays and A. Persoons, J. Mater. Chem., 1997, 7, 2175–2189 RSC; (e) H. S. Nalwa and S. Miyata, Nonlinear Optics of Organic Molecules and Polymers, CRC Press, New York, 1997 Search PubMed.
- (a) J. Zyss, Molecular Nonlinear Optics: Materials, Physics, Devices, Academic Press, Boston, 1994 Search PubMed; (b) G. S. He, L.-S. Tan, Q. Zheng and P. N. Prasad, Chem. Rev., 2008, 108, 1245–1330 CrossRef CAS PubMed; (c) M. M. M. Raposo, in The Encyclopedia of colour science and technology, ed. R. Luo, Springer, New York, 2014, pp. 1–14 Search PubMed.
- (a) L. R. Dalton, P. A. Sullivan and D. H. Bale, Chem. Rev., 2010, 110, 25–55 CrossRef CAS PubMed; (b) F. Bureš, RSC Adv., 2014, 4, 58826–58851 RSC.
- (a) F. Liu, H. Xiao, Y. Yang, H. Wang, H. Zhang, J. Liu, S. Bo, Z. Zhen, X. Liu and L. Qiu, Dyes Pigm., 2016, 130, 138–147 CrossRef CAS; (b) J. Wu, B. A. Wilson, D. W. J. Smith and S. O. Nielsen, J. Mater. Chem. C, 2014, 2, 2591–2599 RSC; (c) Y.-J. Cheng, J. Luo, S. Hau, D. H. Bale, T.-D. Kim, Z. Shi, D. B. Lao, N. M. Tucker, Y. Tian, L. R. Dalton, P. J. Reid and A. K.-Y. Jen, Chem. Mater., 2007, 19, 1154–1163 CrossRef CAS; (d) S. Suresh, H. Zengin, B. K. Spraul, T. Sassa, T. Wada and D. W. Smith, Tetrahedron Lett., 2005, 46, 3913–3916 CrossRef CAS; (e) C. Cai, I. Liakatas, M.-S. Wong, M. Bosch, C. Bosshard, P. Günter, S. Concilio, N. Tirelli and U. W. Suter, Org. Lett., 1999, 1, 1847–1849 CrossRef CAS.
- N. J. Long, Angew. Chem., Int. Ed., 1995, 34, 21–38 CrossRef CAS.
- For representative examples see: (a) M. L. H. Green, S. R. Marder, M. E. Thompson, J. A. Bandy, D. Bloor, P. V. Kolinsky and R. J. Jones, Nature, 1987, 330, 360–362 CrossRef CAS; (b) I. Asselberghs, K. Clays, A. Persoons, A. M. McDonagh, M. D. Ward and J. A. McCleverty, Chem. Phys. Lett., 2003, 368, 408–411 CrossRef CAS; (c) J. Chiffre, F. Averseng, G. G. A. Balavoine, J.-C. Daran, G. Iftime, P. G. Lacroix, E. Manoury and K. Nakatani, Eur. J. Inorg. Chem., 2001, 9, 2221–2226 CrossRef; (d) N. Tsuboya, R. Hamasaki, M. Ito, M. Mitsuishi, T. Miyashita and Y. Yumansoto, J. Mater. Chem., 2003, 13, 511–513 RSC; (e) Y. Liao, B. E. Eichinger, K. A. Firestone, M. Haller, J.-D. Luo, W. Kaminsky, J. B. Benedict, P. J. Reid, A. K.-Y. Jen, L. R. Dalton and B. H. Robinson, J. Am. Chem. Soc., 2005, 127, 2758–2766 CrossRef CAS PubMed; (f) C. Arbez-Gindre, B. R. Steele, G. A. Heropoulos, C. G. Screttas, J.-E. Communal, W. J. Blau and I. Ledoux-Rak, J. Organomet. Chem., 2005, 690, 1620–1626 CrossRef CAS; (g) I. Janowska, J. Zakrzewski, K. Nakatani, M. Palusiak, M. Walak and H. Scholl, J. Organomet. Chem., 2006, 691, 323–330 CrossRef CAS; (h) T. L. Kinnibrugh, S. Salman, Y. A. Getmanenko, V. Coropceanu, W. W. Porter III, T. V. Timofeeva, A. J. Matzger, J.-L. Brédas, S. R. Marder and S. Barlow, Organometallics, 2009, 28, 1350–1357 CrossRef CAS PubMed; (i) B. J. Coe, S. P. Foxon, M. Helliwell, D. Rusanova, B. S. Brunschwig, K. Clays, G. Depotter, M. Nyk, M. Samoc, D. Wawrzynczyk, J. Garín and J. Orduna, Chem.–Eur. J., 2013, 19, 6613–6629 CrossRef CAS PubMed; (j) M. Zaarour, A. Singh, C. Latouche, J. A. G. Williams, I. Ledoux-Rak, J. Zyss, A. Boucekkine, H. Le Bozec, V. Guerchais, C. Dragonetti, A. Colombo, D. Roberto and A. Valore, Inorg. Chem., 2013, 52, 7987–7994 CrossRef CAS PubMed; (k) S. Kaur, S. Dhoun, G. Depotter, P. Kaur, K. Clays and K. Singh, RSC Adv., 2015, 5, 84643–84656 RSC; (l) L. E. R. Buckley, B. J. Coe, D. Rusanova, S. Sánchez, M. Jirásek, V. D. Joshi, J. Vávra, D. Khobragade, L. Pospíšil, Š. Ramešová, I. Císařová, D. Šaman, R. Pohl, K. Clays, N. V. Steerteghem, B. S. Brunschwig and F. Teplý, Dalton Trans., 2017, 46, 1052–1064 RSC.
- (a) I. D. L. Albert, T. J. Marks and M. A. Ratner, J. Am. Chem. Soc., 1997, 119, 6575–6582 CrossRef CAS; (b) S. Bradamante, A. Facchetti and G. A. Pagani, J. Phys. Org. Chem., 1997, 10, 514–524 CrossRef CAS; (c) M. M. M. Raposo, A. M. R. C. Sousa, G. Kirsch, F. Ferreira, M. Belsley, E. Matos Gomes and A. M. C. Fonseca, Org. Lett., 2006, 8, 3681–3684 CrossRef CAS PubMed; (d) R. M. F. Batista, S. P. G. Costa, E. L. Malheiro, M. Belsley and M. M. M. Raposo, Tetrahedron, 2007, 63, 4258–4265 CrossRef CAS; (e) J. Pina, S. Seixas de Melo, R. M. F. Batista, S. P. G. Costa and M. M. M. Raposo, Phys. Chem. Chem. Phys., 2010, 12, 9719–9725 RSC; (f) M. C. R. Castro, M. Belsley, A. M. C. Fonseca and M. M. M. Raposo, Tetrahedron, 2012, 68, 8147–8155 CrossRef CAS; (g) M. C. R. Castro, M. Belsley and M. M. M. Raposo, Dyes Pigm., 2016, 128, 89–95 CrossRef CAS; (h) M. C. R. Castro, M. Belsley and M. M. M. Raposo, Dyes Pigm., 2016, 131, 333–335 CrossRef CAS.
- C. T. Walsh and T. A. Wencewicz, Nat. Prod. Rep., 2013, 30, 175–200 RSC.
- V. Nandwana, I. D. W. Samuel, G. Cooke and V. M. Rotello, Acc. Chem. Res., 2013, 46, 1000–1009 CrossRef CAS PubMed.
- B. J. Jordan, M. A. Pollier, Y. Ofir, S. Joubanian, J. G. Mehtala, C. Sinkel, S. T. Caldwell, A. Kennedy, G. Rabani, G. Cooke and V. M. Rotello, Chem. Commun., 2008, 1653–1655 RSC.
- (a) X. Yu, S. Eymur, V. Singh, B. Yang, M. Tonga, A. Bheemaraju, G. Cooke, C. Subramani, D. Venkataraman, R. J. Stanley and V. M. Rotello, Phys. Chem. Chem. Phys., 2012, 14, 6749–6754 RSC; (b) M. Murakami, K. Ohkubo and S. Fukuzumi, Chem.–Eur. J., 2010, 16, 7820–7832 CrossRef CAS PubMed.
- R. F. Pauszek, G. Kodali, S. T. Caldwell, B. Fitzpatrick, N. Y. Zainalabdeen, G. Cooke, V. M. Rotello and R. J. Stanley, J. Phys. Chem. B, 2013, 117, 15684–15694 CrossRef CAS PubMed.
- Y.-M. Legrand, M. Gray, G. Cooke and V. M. Rotello, J. Am. Chem. Soc., 2003, 125, 15789–15795 CrossRef CAS PubMed.
- A. P. Menezes, A. Jayarama and S. W. Ng, J. Mol. Struct., 2015, 1088, 85–94 CrossRef CAS.
- (a) M. M. Oliva, J. Casado, M. M. M. Raposo, A. M. C. Fonseca, H. Hartmann, V. Hernández and J. T. L. Navarrete, J. Org. Chem., 2006, 71, 7509–7520 CrossRef PubMed; (b) S. P. G. Costa, R. M. F. Batista, P. Cardoso, M. Belsley and M. M. M. Raposo, Eur. J. Org. Chem., 2006, 3938–3946 CrossRef CAS; (c) S. Kothavale and N. Sekar, Dyes Pigm., 2017, 136, 31–45 CrossRef CAS.
- C. Coluccini, A. K. Sharma, D. Merli, D. V. Griend, B. Mannucci and D. Pasini, Dalton Trans., 2011, 40, 11719–11725 RSC.
- (a) K. Clays and A. Persoons, Rev. Sci. Instrum., 1992, 63, 3285–3289 CrossRef CAS; (b) C. Herbivo, A. Comel, G. Kirsch, A. M. C. Fonseca, M. Belsley and M. M. M. Raposo, Dyes Pigm., 2010, 86, 217–226 CrossRef CAS.
- C. Hansch, A. Leo and R. W. Taft, Chem. Rev., 1991, 91, 165–195 CrossRef CAS.
- C. Coluccini, A. K. Sharma, M. Caricato, A. Sironi, E. Cariati, S. Righetto, E. Tordin, C. Botta, A. Forni and D. Pasini, Phys. Chem. Chem. Phys., 2013, 15, 1666–1674 RSC.
- (a) J. L. Oudar, J. Chem. Phys., 1977, 67, 446–457 CrossRef CAS; (b) J. L. Oudar and D. S. Chemla, J. Chem. Phys., 1977, 66, 2664–2668 CrossRef CAS; (c) J. Zyss and J. L. Oudar, Phys. Rev. A, 1982, 26, 2016–2027 CrossRef.
- (a) P. Kaatz and D. P. Shelton, J. Chem. Phys., 1996, 105, 3918–3929 CrossRef CAS; (b) H. Reis, J. Chem. Phys., 2006, 125, 014506 CrossRef CAS PubMed.
- R. D. Pyatt and D. P. Shelton, J. Chem. Phys., 2001, 114, 9938–9946 CrossRef CAS.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, G. A. Petersson, H. Nakatsuji, X. Li, M. Caricato, A. Marenich, J. Bloino, B. G. Janesko, R. Gomperts, B. Mennucci, H. P. Hratchian, J. V. Ortiz, A. F. Izmaylov, J. L. Sonnenberg, D. Williams-Young, F. Ding, F. Lipparini, F. Egidi, J. Goings, B. Peng, A. Petrone, T. Henderson, D. Ranasinghe, V. G. Zakrzewski, J. Gao, N. Rega, G. Zheng, W. Liang, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, K. Throssell, J. A. Montgomery Jr, J. E. Peralta, F. Ogliaro, M. Bearpark, J. J. Heyd, E. Brothers, K. N. Kudin, V. N. Staroverov, T. Keith, R. Kobayashi, J. Normand, K. Raghavachari, A. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, J. M. Millam, M. Klene, C. Adamo, R. Cammi, J. W. Ochterski, R. L. Martin, K. Morokuma, O. Farkas, J. B. Foresman and D. J. Fox, Gaussian 09, Revision A.02, Gaussian, Inc., Wallingford CT, 2016 Search PubMed.
- K. S. Thanthiriwatte and K. M. N. de Silva, J. Mol. Struct.: THEOCHEM, 2002, 617, 169–175 CrossRef CAS.
- (a) S.-S. Sun and L. R. Dalton, Introduction to Organic Electronic and Optoelectronic Materials and Devices, CRC Press, 2nd revised edn, 2016 Search PubMed; (b) E. Kang, T. Bu, P. Jin, J. Sun, Y. Yang and J. Shen, Langmuir, 2007, 23, 7594–7601 CrossRef CAS PubMed; (c) Y. Liao, S. Bhattacharjee, K. A. Firestone, B. E. Eichinger, R. Paranji, C. A. Anderson, B. H. Robinson, P. J. Reid and L. R. Dalton, J. Am. Chem. Soc., 2006, 128, 6847–6853 CrossRef CAS PubMed; (d) A. Facchetti, E. Annoni, L. Beverina, M. Morone, P. Zhu, T. J. Marks and G. A. Pagani, Nat. Mater., 2004, 3, 910–917 CrossRef CAS PubMed; (e) J. Yu, Y. Cui, C. Wu, Y. Yang, Z. Wang, M. O'Keefe, B. Chen and Q. Qian, Angew. Chem., Int. Ed., 2012, 51, 10542–10545 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: NMR spectra, electrochemistry, thermal and modelling data for FLA-A–C. See DOI: 10.1039/c7ra03400h |
| ‡ These authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2017 |