Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Domino carbometalation/coupling reactions of N-arylpropiolamides: a novel and promising synthetic strategy toward stereocontrolled preparation of highly substituted 3-methyleneindolinones
E. Vessally
 *a,
R. Hosseinzadeh-Khanmiri
b,
E. Ghorbani-Kalhor
b,
M. Es'haghi
b and
A. Bekhradnia
*c
*a,
R. Hosseinzadeh-Khanmiri
b,
E. Ghorbani-Kalhor
b,
M. Es'haghi
b and
A. Bekhradnia
*c
aDepartment of Chemistry, Payame Noor University, Tehran, Iran. E-mail: vessally@yahoo.com
bDepartment of Chemistry, Islamic Azad University, Tabriz Branch, Tabriz, Iran
cPharmaceutical Sciences Research Center, Department of Medicinal Chemistry, Mazandaran University of Medical Sciences, Sari, Iran. E-mail: reza_bnia@yahoo.com; abekhradnia@mazums.ac.ir
First published on 29th March 2017
Abstract
3-Methyleneindolinone derivatives are important structural motifs found in many compounds of natural occurrence and pharmacological significance. However, despite their wide importance, mild and highly efficient stereoselective synthesis of (E)- and (Z)-3-methyleneindolinones still remains to be a difficult problem. Therefore, the development of new synthetic methods for their stereocontrolled preparation is of prime importance in organic synthesis. In this mini review, we highlight the advances in stereoselective synthesis of mono- and disubstituted-3-methyleneindolinones through metal-catalyzed intramolecular cyclization of N-arylpropiolamides from 1988 to 2017.
1. Indolinone
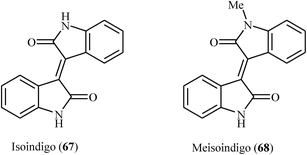
2-Indolinones are privileged structural units not only in natural products1 but also in several other fields such as medicinal,2 agricultural,3 and dye chemistry.4 Fused ring heterocyclic compounds play an important role in the biological system and DNA molecule, antimalarial, anti-inflammatory, quantitative determination of bioorganic compounds and fluorescence properties.5 3-Methyleneindolinones are among the most important structural classes of 2-indolinones, which are present in numerous naturally occurring substances and biologically active compounds.2,5 For example, sunitinib 1 with brand name of sutent is a receptor protein-tyrosine kinase inhibitor that marketed worldwide for the treatment of renal cell carcinoma and imatinib resistant GIST. It is the first anticancer drug to be simultaneously approved (in 2006) for two different indications.6 Toceranib 2, with trade name palladia, is a dog-specific anti-cancer drug. It works by killing tumor cells directly and by cutting off the tumor's blood supply.7 It is interesting that toceranib is the first drug developed specifically for the treatment of cancer in dogs. Nintedanib 3 with brand names of Vargatef and Ofev is a kinase inhibitor that used for the treatment of idiopathic pulmonary fibrosis (IPF) and a type of non-small-cell lung cancer called adenocarcinoma.8 SU4984 4 is an inhibitor of tyrosine kinase activity of fibroblast growth factor receptor 1 (FGFR1) undergoing clinical trials for the treatment of human cancers.9 Alkaloids isatinone A 5 and B 6, which were isolated from ethanolic extracts of the herb Isatis costata, have significant antifungal activity.10 As these examples show, 3-methyleneindolinone derivatives offer very attractive options for drug design of various pharmacological agents. However, despite their wide importance, highly stereoselective synthesis of (E)- and (Z)-3-methyleneindolinones still remains to be a formidable challenge (Fig. 1). | ||
| Fig. 1 Selected examples of 3-methyleneindolinone-based drugs (sunitinib, toceranib, nintedanib, SU4984) and natural products (isotinone A and B). | ||
Over the past several years, N-propargyl-amines and -amides have received significant attention in organic synthesis owing to their easy availability, cost effectiveness, and versatility in the synthesis of a wide variety of nitrogen based heterocycles.11 In this regard, the synthesis of 3-methyleneindolinones from N-arylpropiolamides have undergone very rapid growth in recent years. Cyclization of N-arylpropiolamide derivatives provide one of the most powerful one-step and atom-economic methods for the stereoselective synthesis of highly substituted 3-methyleneindolinones. These reactions were highlighted by Millemaggi and Taylor in their interesting review in 2010.12 In continuation of our works,11 our aim in this review is to try to provide a comprehensive and updated overview of recent developments in the (E)- and (Z)-selective synthesis of 3-methyleneindolinone derivatives from cyclization of N-arylpropiolamides through metal-catalyzed approaches with the emphasis on the mechanistic aspects of the reactions.
2. Synthesis of 3-methyleneindolinones from ortho-halo N-arylpropiolamides
The first example of transformation of N-phenylpropiolamides to 3-methyleneindolinones through a 5-exo-dig fashion was reported by the groups of Bowman, in 1988. They showed that intramolecular radical cyclization of N-(2-iodophenyl)-N-methyl-3-phenylpropiolamide 7 in the presence of Bu3SnH/AIBN system in refluxing toluene gave E/Z-mixture of corresponding 3-alkenyl-indolinone 8 in 37% yield (Scheme 1).13 Twelve years later, Brunton and Jones reinvestigated this reaction employing N-phenylpropiolamides bearing tert-butyldimethylsilyl (TBDMS) groups in the alkyne terminus as substrates. The corresponding indolinones were obtained in moderate yields and good (Z)-selectivity.14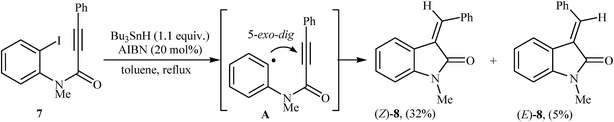 | ||
| Scheme 1 Synthesis of 3-alkenyl-indolinones 8 via stannane mediated intramolecular cyclization of N-(2-iodophenyl)-N-methyl-3-phenylpropiolamide 7. | ||
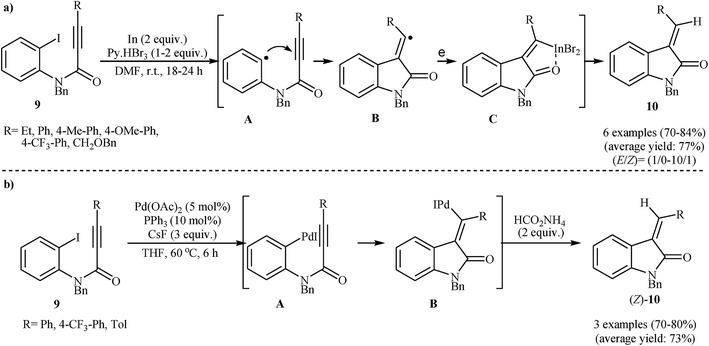
Subsequently, Takemoto and co-workers found that N-protected N-(2-iodophenyl)propiolamides 9 in the presence of In/Py + HBr3/DMF system, underwent a reductive radical cyclization to afford corresponding (E)-3-alkylideneindolinones 10 in good to high yields with excellent stereoselectivity (Scheme 2a).15 The (E)-selectivity of the reaction can be ascribed to the strong coordination of the indium atom to amide carbonyl oxygen of the intermediate C. It is worth noting that the N-(2-iodophenyl)propiolamides bearing an electron-withdrawing groups on the aryl ring failed to enter into the cyclization reactions and the substrates bearing electron-rich aryl rings gave very poor results. The same authors also were able to demonstrate that a range of disubstituted 3-alkylideneoxindoles could be obtained from the corresponding N-phenylpropiolamides through a tandem In-mediated reductive radical cyclization and Pd-catalyzed cross-coupling reaction.16 Later, the same group showed that the treatment of internal aryl substituted N-(2-iodophenyl)propiolamides 9 with a catalytic amount of Pd(OAc)2 and PPh3 in the presence of cesium fluoride and ammonium formate in THF resulted in good yields of corresponding (Z)-3-alkylideneindolinones (Z)-10 through an intramolecular Heck cyclization (Scheme 2b). However, N-phenylpropiolamides having alkyl groups in the alkyne terminus failed to participate in the reaction. The authors claimed that the key step of these reactions is the syn addition of arylpalladium halide A to the triple bond. They also successfully extended this chemistry to synthesis of further functionalized indolinones via palladium-catalyzed Heck/Suzuki–Miyaura, Heck/Heck, and Heck/carbonylation/Suzuki–Miyaura domino reactions using aforementioned catalytic system.17
Alongside this, Player and co-workers presented a palladium-catalyzed tandem Heck cyclization/Suzuki–Miyaura coupling of N–H free N-(2-iodophenyl)propiolamide 11 with arylboronic acids into (E)-3-(diarylmethylene)indolinones 12. Thus, the careful analysis of the optimized reactions revealed that the optimum condition for this reaction was the addition of Pd(PPh3)4 (15 mol%), copper(I) thiophen-2-carboxylate (CuTC) (2.1 equiv.), and arylboronic acid (2.1 equiv.) at room temperature, to a solution of N-(2-iodophenyl)propiolamide in dry THF. Under optimized conditions, the reaction tolerates both electron-rich and electron-poor arylboronic acids and gave the corresponding 3,3-(diarylmethylene)indolinones 12 in moderate to high yields and good to excellent regioselectivity (Scheme 3).18
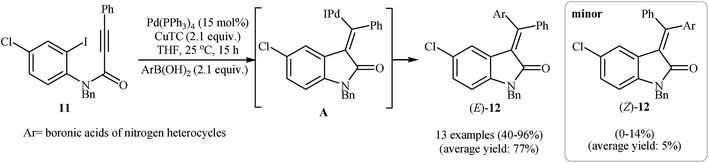 | ||
| Scheme 3 Pd-Catalyzed tandem Heck cyclization/Suzuki–Miyaura coupling of N–H free N-(2-iodophenyl)propiolamide 11 with arylboronic acids. | ||
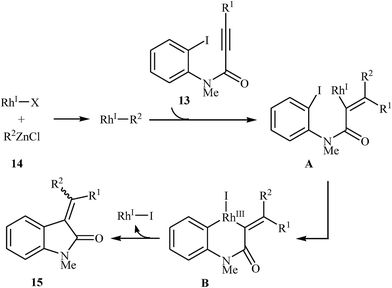
Shortly afterward, Shintani, Yamagami, and Hayashi extended the scope of electrophilic partners to organozinc chlorides 14. They showed that treatment of N-methylated N-(2-iodophenyl)propiolamides 13 with organozinc chlorides 14 in presence of [RhCl(C2H4)2]2/dppf as catalytic system in dioxane resulted in corresponding 3,3-(diarylmethylene)indolinones 15 in good to high yields (Scheme 4). Mechanistically, in contrast to the palladium catalyzed processes, this reaction is believed to proceed through a carborhodation-oxidative addition-reductive elimination sequential process (Scheme 5).19
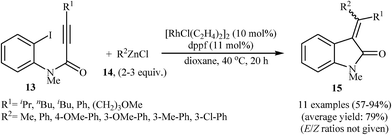 | ||
| Scheme 4 Synthesis of 3,3-(diarylmethylene)indolinones 15 via Rh-catalyzed cascade reaction of N-(2-iodophenyl)propiolamides 13 and organozinc chlorides 14. | ||
In 2011, Balalaie and co-workers developed the stereoselective synthesis of (Z)-3-(aminoarylmethylene)indolinones 22 via a tandem palladium-catalyzed carbopalladative cyclization-Buchwald reaction of Ugi-adducts 20 with amines 21. Thus, in the presence of Pd(OAc)2/P(2-furyl)3/Cs2CO3 as catalytic system in refluxing toluene, N-substituted phenylpropiolamides 20, which were prepared from the reaction of benzaldehydes 16, 2-iodoaniline 17, phenylpropiolicacid 18, and isocyanides 19, were treated with secondary amines 21 to give (Z)-3-(aminoarylmethylene)indolinones 22 in good to high yields (Scheme 6).20 Later, in a closely related investigation, U. Sharma, E. Eycken, and co-workers showed that 3-methyleneindolinones 25 were formed from N-propiolamide 23 and 1,3,4-oxadiazoles 24 (Scheme 7). This transformation requires the use of Pd(OAc)2 as catalyst and PPh3 as ligand under N2 atmosphere. The reaction was run in refluxing toluene and leads to (E)-3-((1,3,4-oxadiazol-2-yl)(aryl)methylene)indolinones 27 through an intermolecular domino carbopalladative cyclization/C–H functionalization sequence of an N-propiolamide 25 and an 1,3,4-oxadiazole 26.21 These results indicated that the nature of ligands have a great impact on the stereoselectivity of products.
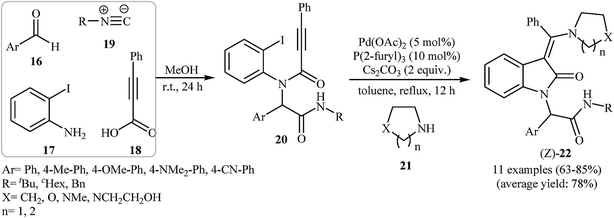 | ||
| Scheme 6 Stereoselective synthesis of (Z)-3-(aminoarylmethylene)indolinones 22 via Ugi-carbopalladative cyclization-Buchwald reaction sequences developed by Balalaie. | ||
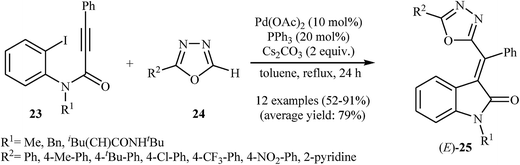 | ||
| Scheme 7 Pd-Catalyzed synthesis of (E)-3-((1,3,4-oxadiazol-2-yl)(aryl)methylene)indolinones 25 from N-propiolamides 23 and 1,3,4-oxadiazoles 24 described by Sharma. | ||
In 2013, a beautiful approach for the synthesis of 3,3-(diarylmethylene)indolinones 27 via a sequential Pd-catalyzed Sonogashira/Heck/Suzuki–Miyaura reactions of terminal N-(2-bromophenyl)propiolamides 26 with aryl iodides and arylboronic acids was demonstrated by Seo and coworkers. After studying a number of catalyst and bases, a combination of Pd(PPh3)4/CuI/NaOAc in DMF was found to be optimum with respect to the yield of product isolated (Scheme 8). The results demonstrated that under this reaction conditions electron poor aryl iodides lead to a strong preference for Z-products and electron rich aryl iodides resulting in a relatively weak preference for E-products.22 Subsequently, the same group reinvestigated this reaction by displacing of N-(2-bromophenyl)propiolamides with 2-(propiolamido)phenyl triflates but lower yields than previously reported examples were observed.23 Recently, the same authors improved the efficiency of this reaction in terms of the yield and reaction time by performing the process under microwave irradiation conditions employing Pd(PPh3)4/PPh3/CuI/NaOAc/Ag3PO4 as catalytic system.24
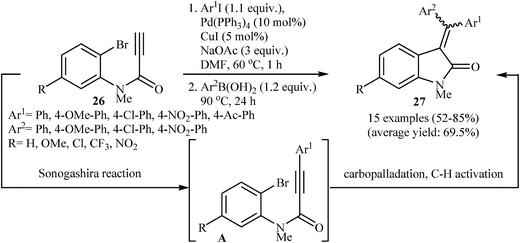 | ||
| Scheme 8 Pd-Catalyzed Sonogashira/Heck/Suzuki–Miyaura combined reaction of N-(2-bromophenyl)propiolamides 26. | ||
In a recent study, the same research team extended their methodology to the highly stereoselective synthesis of (E)- and (Z)-isomers of 3-(1,3-diarylallylidene)indolinones 31, through microwave-assisted three-component tandem Sonogashira/Heck/Suzuki–Miyaura reactions of N-(2-bromophenyl)propiolamide 28, aryl iodides 29, and vinyl boronic acids 30. They found that the nature of the phosphine ligand, reaction temperature, and reaction time had a major impact on the E/Z ratio of products. Thus, reaction with PPh3 at high temperatures and longer reaction times gave E-isomers with excellent stereoselectivity, whereas reaction with t-Bu XPhos at lower temperatures and shorter reaction times lead to Z-isomers with moderate stereoselectivity (Scheme 9).25
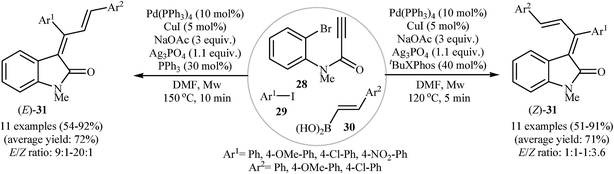 | ||
| Scheme 9 Three-component tandem reactions for the stereoselective synthesis of 3-(1,3-diarylallylidene)indolinones 31. | ||
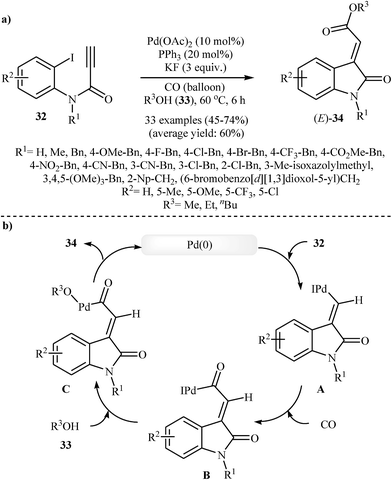
In 2016, Li et al. developed a new methodology toward (E)-oxindolylidene acetates 34 through a Pd-catalyzed tandem Heck and alkoxycarbonylation reactions of N-substituted N-(2-iodophenyl)propiolamides 32 with CO and alcohols 33 (Scheme 10). They tested several catalysts, ligands, and additives, and the system Pd(OAc)2/PPh3/KF was found to be optimal with respect to isolated product yield. The optimized protocol tolerated a variety of sensitive functional groups, such as nitro, cyano, fluoro, chloro, bromo, methoxyl, and ester functionalities on the aryl ring of propiolamides and gave final products in moderate to good yields (Scheme 10a). However, the scope of alcohols are limited to the small primary aliphatic alcohols. The author proposed mechanism for this cyclization, which is outlined in Scheme 10b. It is noted that the resulting products showed a growth inhibitory activity against a variety of human cancer cell lines.26
An interesting domino procedure for the synthesis of spirocyclic indolones 37 from internal N-protected N-(2-iodophenyl)propiolamides 35 and propargyl allyl ethers 36, in which four new C–C bonds are formed, was developed by Müller et al. (Scheme 11). The electron-poor vinylpropargyl allyl ether A, which is generated from Pd-catalyzed coupling of 35 and 36, undergoes a triethylamine-catalyzed propargyl-allene isomerization to give enallene B. A subsequent intramolecular [4 + 2] cycloaddition leads to the observed products 37.27
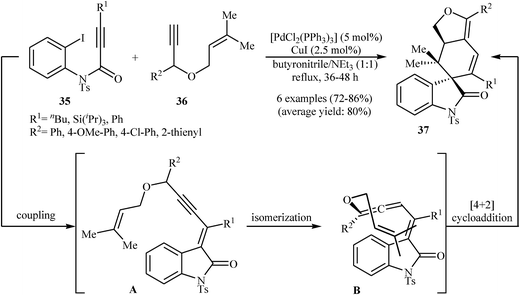 | ||
| Scheme 11 Synthesis of spirocyclic indolones 37 through a Pd-catalyzed insertion/coupling/isomerization/Diels–Alder sequential process. | ||
3. Synthesis of 3-methyleneindolinones from ortho-unsubstituted N-phenylpropiolamides
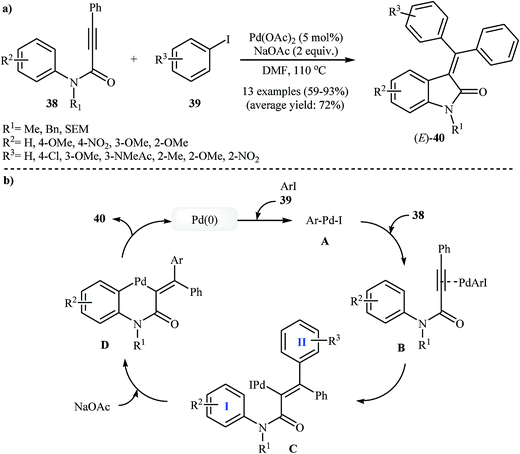
The possibility of cyclization of ortho-unsubstituted N-phenylpropiolamides to indolinones was first realized by Zhu and co-workers, in 2006, who synthesized (E)-3-(diarylmethylene)indolinones 40 from corresponding tertiary N-phenylpropiolamides 38 and aryl iodides 39 using Pd(OAc)2/NaOAc/DMF system (Scheme 12a). This method is compatible with a large range of tertiary N-phenylpropiolamides, but it could not be extended to secondary N-phenylpropiolamides. Depending on the electronic effects of substituents on the aryl iodides, substrates with electron-withdrawing groups gave higher yields than those with electron-donating groups. The mechanism proposed to explain this reaction is shown in Scheme 12b and starts with the formation of arylpalladium(II) species A via oxidative addition of aryl iodide to palladium(0), and then coordination of A to the triple bond of propiolamide to give intermediate B, which undergoes a syn carbopalladation to produce vinylpalladium intermediate C. Subsequently, C–H activation of ring I gives six-membered palladacycle D. Finally, a reductive elimination affords the observed products 40 (Scheme 12b).28 Shortly afterward, the same authors extended their methodology to the stereocontrolled three-component (external N-phenylpropiolamides 41, aryl iodides 42 and 43) synthesis of unsymmetrically substituted 3-(diarylmethylene)indolinones 44 through a domino Sonogashira/carbopalladation/C–H activation/C–C bond-forming sequence (Scheme 13).29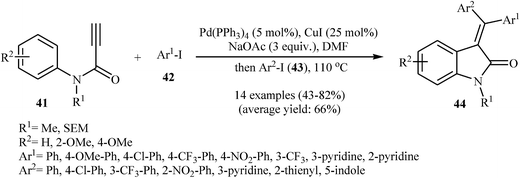 | ||
| Scheme 13 Stereocontrolled three-component synthesis of unsymmetrically substituted 3-(diarylmethylene)indolinones 44 developed by Zhu. | ||
Following these works, the Li group developed an analogous one-pot access to (E)-3-(diarylmethylene)indolinones 47 by the reaction of N-phenylpropiolamides 45 with aryliodonium salts 46 (Scheme 14). Several palladium sources, additives and solvents were tested, and the system Pd(OAc)2/Et3N/THF/MeCN (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) was found to be superior. However, N–H free and terminal propiolamides failed to participate in this reaction.30 To overpass the limitation encountered by N–H free phenylpropiolamides, the same authors investigated the reaction of secondary N-phenylpropiolamides with aryl iodides in the presence of Pd(OAc)2/CuI/N,N′-dimethylethane-1,2-diamine/K2CO3/DMF/MeCN (1
4) was found to be superior. However, N–H free and terminal propiolamides failed to participate in this reaction.30 To overpass the limitation encountered by N–H free phenylpropiolamides, the same authors investigated the reaction of secondary N-phenylpropiolamides with aryl iodides in the presence of Pd(OAc)2/CuI/N,N′-dimethylethane-1,2-diamine/K2CO3/DMF/MeCN (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.4) system and satisfactory results were obtained.31 In a beautiful approach, Pinto, Neuville, and Zhu disclosed a one-pot, three-component version of the same reaction where the requisite N-phenylpropiolamides were prepared in situ from propiolamides and arylbromides.32 With the objective of designing a comprehensive protocol to 3-(diarylmethylene)indolinones, the scope of coupling partners were extended to benzene. The Pd(OAc)2/PPh3/AgOAc combination was found to be optimal for this reaction. Under optimized conditions, the reaction tolerates electron-donating and electron-withdrawing substituents and gave the corresponding (Z)-3-(diarylmethylene)indolinones 49 in moderate yields (Scheme 15). However, N-phenylpropiolamides having alkyl groups in the alkyne terminus failed to participate in the reaction.33
1.4) system and satisfactory results were obtained.31 In a beautiful approach, Pinto, Neuville, and Zhu disclosed a one-pot, three-component version of the same reaction where the requisite N-phenylpropiolamides were prepared in situ from propiolamides and arylbromides.32 With the objective of designing a comprehensive protocol to 3-(diarylmethylene)indolinones, the scope of coupling partners were extended to benzene. The Pd(OAc)2/PPh3/AgOAc combination was found to be optimal for this reaction. Under optimized conditions, the reaction tolerates electron-donating and electron-withdrawing substituents and gave the corresponding (Z)-3-(diarylmethylene)indolinones 49 in moderate yields (Scheme 15). However, N-phenylpropiolamides having alkyl groups in the alkyne terminus failed to participate in the reaction.33
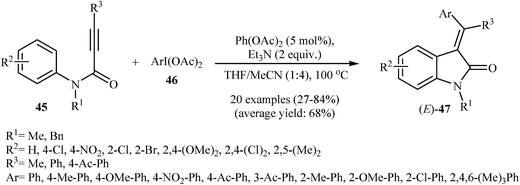 | ||
| Scheme 14 Synthesis of (E)-3-(diarylmethylene)indolinones 47 via Pd-catalyzed C–H functionalization of N-arylpropiolamides 45 with aryliodonium salts 46. | ||
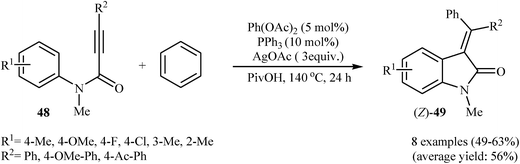 | ||
| Scheme 15 Synthesis of (Z)-3-(diarylmethylene)indolinones 49 through a Pd-catalysed double C–H activation process. | ||
In 2008, Li and co-workers reported an efficient and beautiful protocol for the stereoselective synthesis of biologically important (E)-3-(phthalimido(aryl)methylene)indolinones 52 from the reaction between tertiary N-phenylpropiolamides 50 and phthalimide 51 through a palladium-catalyzed intermolecular aminopalladation/C–H activation strategy. They discovered that the reaction of 1 equiv. of N-phenylpropiolamides 50 with 3 equiv. of phthalimide 51 in DCE at 100 °C in the presence of catalytic amounts of Pd(OAc)2 and over-stoichiometric amounts of PdI(OAc)2 produced desired products 52 in moderate to good yields (Scheme 16a). The reaction, however, appears to be limited to N-phenylpropiolamides containing aryl groups in the alkyne terminus. Extension to alkyl subsumed internal phenylpropiolamides and external phenylpropiolamides were attempted, but the corresponding indolinones were isolated in very poor yields.34 In a subsequent extension of the substrate scope of the methodology, it was shown that both aryl and alkyl-substituted internal N-phenylpropiolamides 50 could be treated with carboxylic acids 53 under the abovementioned reaction conditions to give corresponding (E)-(2-indolinon-3-ylidene)methyl acetates 54 (Scheme 16b). However, N–H free and N-acetylated phenylpropiolamides failed to give any of the desired indolinone derivative.35
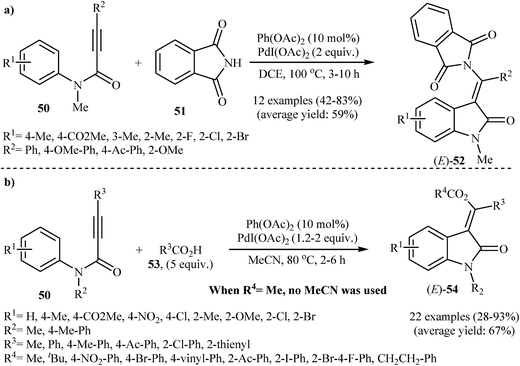 | ||
| Scheme 16 (a) Li's synthesis of (E)-3-(phthalimido(aryl)methylene)indolinones 52; (b) Li's synthesis of (E)-(2-indolinon-3-ylidene)methyl acetates 54. | ||
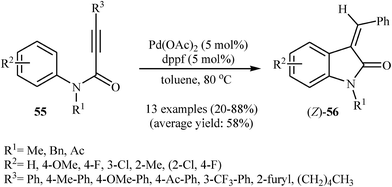
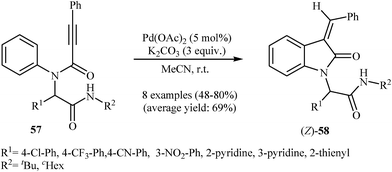
The same authors were also able to demonstrate that a range of (Z)-3-(monosubstituted-methylene)indolinones 56 could be stereoselectively obtained from the palladium-catalyzed intramolecular hydroarylation of corresponding N-arylpropiolamides 55 through a 5-exo-dig fashion employing Pd(OAc)2 as catalyst and dppf (1,1′-bis(diphenyphosphino)ferrocene) as ligand in toluene at 80 °C (Scheme 17). It should be mentioned that the (E)-isomers have been isolated in some cases as the minor products. Interestingly, at higher temperatures than 80 °C the yield of (Z)-isomers is decreased in favor of the (E)-isomers. When the cyclization was performed at 140 °C, the (E)-products were obtained in good to high yields (59–86%) with a mixture of (Z)-products (8–30%).36 Later, Balalaie and co-workers reinvestigated this reaction using Pd(OAc)2/K2CO3 combination as catalytic system. The reactions were run in acetonitrile at room temperature and provided corresponding (Z)-3-(arylmethylene)-indolinones 58 in good to high yields (Scheme 18).37
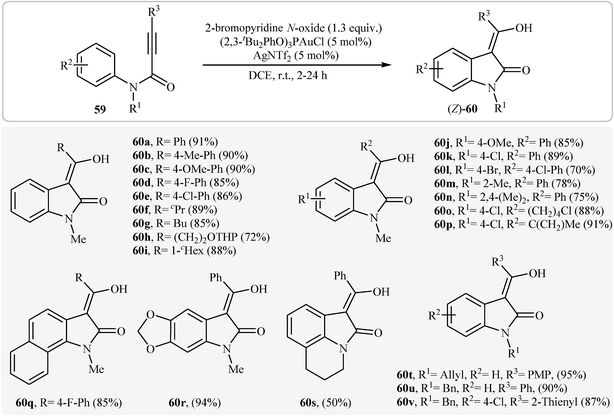
Recently, Qian and Zhang demonstrated an elegant gold-catalyzed oxidation/C–H functionalization reaction of tertiary N-arylpropiolamides 59 into 3-acylindolinone derivatives 60 (Scheme 19). In most cases, the nature of the substituents had no influence on the results of this reaction. However, N-acylated substrates failed to participate in this cyclization.38
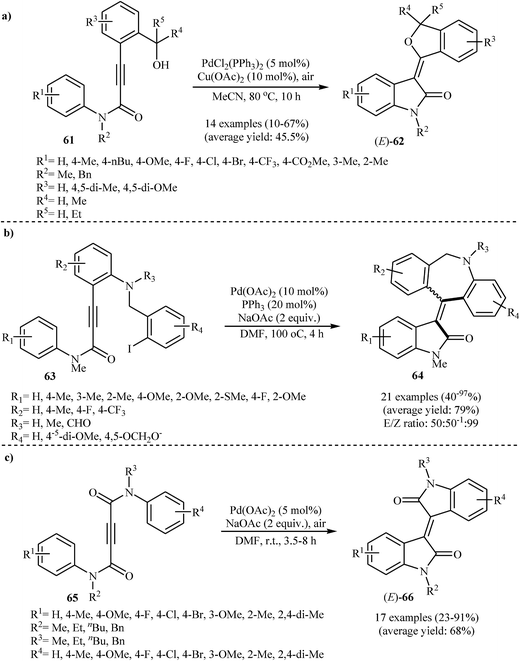
In 2009, Li's group elegantly applied palladium-catalyzed intramolecular C–H activation reactions of 3-(2-(hydroxymethyl)aryl)-N-phenylpropiolamides 61 in the syntheses of (E)-3-(isobenzofuran-3(1H)-ylidene)indolinones 62 (Scheme 20a). Among the various catalysts and oxidants examined, PdCl2(PPh3)2 and Cu(OAc)2/air emerged as the optimal choices for the cyclization. The reaction conditions allowed the use of substrates bearing a variety of important functional groups including chloro, bromo, methoxy, and ester.39 Shortly afterwards, the same group disclosed that a series of potential biological active 3-[5H-dibenzo[b,e]azepin-11(6H)-ylidene]indolinones 64 could be synthesized from the corresponding 3-[2-(2-iodobenzylamino)aryl]-N-arylpropiolamides 63 through a sequential intramolecular arylation of the alkyne moiety with an aryl iodide/annulation of vinyl carbon with an arene C–H bond process (Scheme 20b). The reactions performed in DMF, and Pd(OAc)2/PPh3/NaOAc was used as the catalytic system.40 Alongside this, more recently, Du et al. have reported an innovative approach for the synthesis of (E)-bisindolinones 66 in moderate to good yields via an intramolecular double annulation of N1,N4-diphenylbut-2-ynediamides 65 catalyzed by Pd(OAc)2 under oxygen atmosphere at room temperature (Scheme 20c). They showed the application of this procedure for the high yielding syntheses of antileukemic drugs isoindigo 67 and meisoindigo 68 (Fig. 2).41
4. Summary and outlook
From this mini review, it is clear that metal (specially palladium)-catalyzed domino reactions of N-arylpropiolamides has emerged as a promising synthetic strategy toward stereocontrolled preparation of mono- and disubstituted-3-methyleneindolinones that has been successfully used in the synthesis of many important bioactive compounds, like isoindigo and meisoindigo. The nature of the catalysts, ligands, reaction temperatures and times have great influences on the E/Z ratio of products. We believed that more and more catalytic systems will be developed for the exclusively (E)- and (Z)-selective synthesis of biologically important and complex 3-methyleneindolinone-based natural products in the near future.References
- (a) O. R. Suárez-Castillo, M. Sánchez-Zavala, M. Meléndez-Rodríguez, L. E. Castelán-Duarte, M. S. Morales-Ríos and P. Joseph-Nathan, Tetrahedron, 2006, 62, 3040–3051 CrossRef; (b) A. P. Antonchick, C. Gerding-Reimers, M. Catarinella, M. Schürmann, H. Preut, S. Ziegler, D. Rauh and H. Waldmann, Nat. Chem., 2010, 2, 735–740 CrossRef CAS PubMed; (c) C. V. Galliford and K. A. Scheidt, Angew. Chem., Int. Ed., 2007, 46, 8748–8758 CrossRef CAS PubMed.
- (a) C. R. Prakash and S. Raja, Mini-Rev. Med. Chem., 2012, 12, 98–119 CrossRef CAS PubMed; (b) A. Leoni, A. Locatelli, R. Morigi and M. Rambaldi, Expert Opin. Ther. Pat., 2016, 26, 149–173 CrossRef CAS PubMed.
- K. Wada, T. Gomibuchi, Y. Yoneta, Y. Otsu, K. Shibuya and H. Okuya, WO 2004110149, 2004Chem. Abstr., 2005, 142, 34060.
- A. Iqbal, F. Herren and O. Wallquist, High Performance Pigments, John Wiley, New York, 2001, pp. 231–247 Search PubMed.
- (a) S. Arshadi, A. R. Bekhradnia and A. Ebrahimnejad, Can. J. Chem., 2011, 89, 1403–1409 CrossRef CAS; (b) M. Z. Kassaee and A. R. Bekhradnia, J. Biosci. Bioeng., 2003, 95, 526–529 CrossRef CAS PubMed; (c) A. Bekhradnia, E. Domehri and M. Khosravi, Spectrochim. Acta, Part A, 2016, 152, 18–22 CrossRef CAS PubMed; (d) S. N. Azizi, P. Shakeri, M. J. Chaichi, A. Bekhradnia, M. Taghavi and M. Ghaemy, Spectrochim. Acta, Part A, 2014, 122, 482–488 CrossRef CAS PubMed; (e) C. R. Prakash, P. Theivendren and S. Raja, Pharmacol. Pharm., 2012, 3, 62–71 CrossRef CAS; (f) E. Vessally and M. Abdoli, J. Iran. Chem. Soc., 2016, 13, 1235–1256 CrossRef CAS; (g) E. Vessally, H. Saeidian, A. Hosseinian, L. Edjlali and A. Bekhradnia, Curr. Org. Chem., 2017, 21, 249–271 CrossRef CAS; (h) E. Vessally, A. Hassanpour, R. Hosseinzadeh-Khanmiri, M. Babazadeh and J. Abolhasani, Monatsh. Chem., 2017, 148, 321–326 CrossRef CAS; (i) L. Dinparast, H. Valizadeh, E. Vessally and M. B. Bahadori, J. Mol. Struct., 2016, 1105, 118–127 CrossRef CAS; (j) Z. Asadi, M. B. Asnaashariisfahani, E. Vessally and M. D. Esrafili, Spectrochim. Acta, Part A, 2015, 140, 585–599 CrossRef CAS PubMed; (k) E. Vessally, E. Fereyduni, H. Shabrendi and M. D. Esrafili, Spectrochim. Acta, Part A, 2013, 116, 65–73 CrossRef CAS PubMed; (l) E. Vessally, M. Ghasemisarabbadeih, Z. Ekhteyari, R. Hosseinzadeh-Khanmiri, E. Ghorbani-Kalhor and L. Ejlali, RSC Adv., 2016, 6, 106769–106777 RSC; (m) E. Vessally, R. Hosseinzadeh-Khanmiri, M. Babazadeh, E. Ghorbani-Kalhor and L. Ejlali, Appl. Organomet. Chem., DOI:10.1002/aoc.3603; (n) E. Vessally, R. Hosseinzadeh-Khanmiri, E. Ghorbani-Kalhor, M. Es'haghi and L. Ejlali, Appl. Organomet. Chem., DOI:10.1002/aoc.3729.
- L. Q. Chow and S. G. Eckhardt, J. Clin. Oncol., 2007, 25, 884–896 CrossRef CAS PubMed.
- C. A. London, P. B. Malpas, S. L. Wood-Follis, J. F. Boucher, A. W. Rusk, M. P. Rosenberg, C. J. Henry, K. L. Mitchener, M. K. Klein and J. G. Hintermeister, Clin. Cancer Res., 2009, 15, 3856–3865 CrossRef CAS PubMed.
- L. Richeldi, R. M. du Bois, G. Raghu, A. Azuma, K. K. Brown, U. Costabel, V. Cottin, K. R. Flaherty, D. M. Hansell and Y. Inoue, N. Engl. J. Med., 2014, 370, 2071–2082 CrossRef PubMed.
- M. Mohammadi, G. McMahon, L. Sun, C. Tang, P. Hirth, B. K. Yeh, S. R. Hubbard and J. Schlessinger, Science, 1997, 276, 955–960 CrossRef CAS PubMed.
- I. Fatima, I. Ahmad, I. Anis, A. Malik and N. Afza, Molecules, 2007, 12, 155–162 CrossRef CAS PubMed.
- (a) E. Vessally, RSC Adv., 2016, 6, 18619–18631 RSC; (b) E. Vessally, A. Hosseinian, L. Edjlali, A. Bekhradnia and M. D. Esrafili, RSC Adv., 2016, 6, 71662–71675 RSC; (c) E. Vessally, L. Edjlali, A. Hosseinian, A. Bekhradnia and M. D. Esrafili, RSC Adv., 2016, 6, 49730–49746 RSC; (d) E. Vessally, A. Hosseinian, L. Edjlali, A. Bekhradnia and M. D. Esrafili, RSC Adv., 2016, 6, 99781–99793 RSC; (e) E. Vessally, S. Soleimani-Amiri, A. Hosseinian, L. Edjlali and A. Bekhradnia, RSC Adv., 2017, 7, 7079–7091 RSC; (f) E. Vessally, A. Hosseinian, L. Edjlali, A. Bekhradnia and M. D. Esrafili, Curr. Org. Synth., 2017, 14, 557–567 Search PubMed; (g) E. Vessally, S. Arshadi, L. Edjlali, E. Ghorbani-Kalhor and R. Hosseinzadeh-Khanmir, RSC Adv., 2017, 7, 13198–13211 RSC; (h) E. Vessally, L. Edjlali, E. Ghorbani-Kalhor and R. Hosseinzadeh-Khanmir, J. Co2 Util., 2017, 19, 120–129 CrossRef.
- A. Millemaggi and R. J. Taylor, Eur. J. Org. Chem., 2010, 4527–4547 CrossRef CAS.
- W. R. Bowman, H. Heaney and B. M. Jordan, Tetrahedron Lett., 1988, 29, 6657–6660 CrossRef CAS.
- S. A. Brunton and K. Jones, J. Chem. Soc., Perkin Trans. 1, 2000, 763–768 RSC.
- R. Yanada, S. Obika, M. Oyama and Y. Takemoto, Org. Lett., 2004, 6, 2825–2828 CrossRef CAS PubMed.
- R. Yanada, S. Obika, Y. Kobayashi, T. Inokuma, M. Oyama, K. Yanada and Y. Takemoto, Adv. Synth. Catal., 2005, 347, 1632–1642 CrossRef CAS.
- R. Yanada, S. Obika, T. Inokuma, K. Yanada, M. Yamashita, S. Ohta and Y. Takemoto, J. Org. Chem., 2005, 70, 6972–6975 CrossRef CAS PubMed.
- W. S. Cheung, R. J. Patch and M. R. Player, J. Org. Chem., 2005, 70, 3741–3744 CrossRef CAS PubMed.
- R. Shintani, T. Yamagami and T. Hayashi, Org. Lett., 2006, 8, 4799–4801 CrossRef CAS PubMed.
- M. Bararjanian, S. Hosseinzadeh, S. Balalaie, H. R. Bijanzadeh and E. Wolf, Tetrahedron Lett., 2011, 52, 3329–3332 CrossRef CAS.
- U. K. Sharma, N. Sharma, Y. Kumar, B. K. Singh and E. V. Van der Eycken, Chem.–Eur. J., 2016, 22, 481–485 CrossRef CAS PubMed.
- G. R. Dong, S. Park, D. Lee, K. J. Shin and J. H. Seo, Synlett, 2013, 24, 1993–1997 CrossRef CAS.
- D. Lee, S. Park, Y. Yu, K. J. Shin and J. H. Seo, Molecules, 2015, 20, 14022–14032 CrossRef CAS PubMed.
- S. Park, K. J. Shin and J. H. Seo, Synlett, 2015, 26, 2296–2300 CrossRef CAS.
- Y. Yu, K. J. Shin and J. H. Seo, J. Org. Chem., 2017, 82, 1864–1871 CrossRef CAS PubMed.
- W.-J. Lin, K.-S. Shia, J.-S. Song, M.-H. Wu and W.-T. Li, Org. Biomol. Chem., 2016, 14, 220–228 CAS.
- D. M. D'Souza, F. Rominger and T. J. Müller, Angew. Chem., Int. Ed., 2005, 44, 153–158 CrossRef PubMed.
- A. Pinto, L. Neuville, P. Retailleau and J. Zhu, Org. Lett., 2006, 8, 4927–4930 CrossRef CAS PubMed.
- A. Pinto, L. Neuville and J. Zhu, Angew. Chem., Int. Ed., 2007, 46, 3291–3295 CrossRef CAS PubMed.
- S. Tang, P. Peng, P. Zhong and J.-H. Li, J. Org. Chem., 2008, 73, 5476–5480 CrossRef CAS PubMed.
- R.-J. Song, Y. Liu, R.-J. Li and J.-H. Li, Tetrahedron Lett., 2009, 50, 3912–3916 CrossRef CAS.
- A. Pinto, L. Neuville and J. Zhu, Tetrahedron Lett., 2009, 50, 3602–3605 CrossRef CAS.
- D.-J. Tang, B.-X. Tang and J.-H. Li, J. Org. Chem., 2009, 74, 6749–6755 CrossRef CAS PubMed.
- S. Tang, P. Peng, S.-F. Pi, Y. Liang, N.-X. Wang and J.-H. Li, Org. Lett., 2008, 10, 1179–1182 CrossRef CAS PubMed.
- S. Tang, P. Peng, Z.-Q. Wang, B.-X. Tang, C.-L. Deng, J.-H. Li, P. Zhong and N.-X. Wang, Org. Lett., 2008, 10, 1875–1878 CrossRef CAS PubMed.
- T.-S. Jiang, R.-Y. Tang, X.-G. Zhang, X.-H. Li and J.-H. Li, J. Org. Chem., 2009, 74, 8834–8837 CrossRef CAS PubMed.
- S. Balalaie, H. Motaghedi, M. Bararjanian, D. Tahmassebi and H. R. Bijanzadeh, Tetrahedron, 2011, 67, 9134–9141 CrossRef CAS.
- D. Qian and J. Zhang, Chem. Commun., 2012, 48, 7082–7084 RSC.
- P. Peng, B.-X. Tang, S.-F. Pi, Y. Liang and J.-H. Li, J. Org. Chem., 2009, 74, 3569–3572 CrossRef CAS PubMed.
- T. Zou, X. G. Zhang, J. H. Li, C. L. Deng and R. Y. Tang, Adv. Synth. Catal., 2012, 354, 889–898 CrossRef CAS.
- G. Li, G. Zhou, D. Zhang-Negrerie, Y. Du, J. Huang and K. Zhao, Adv. Synth. Catal., 2016, 358, 3534–3540 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2017 |