 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Review on the mass transfer performance of CO2 absorption by amine-based solvents in low- and high-pressure absorption packed columns
Morteza Afkhamipour
 and
Masoud Mofarahi
and
Masoud Mofarahi
 *
*
Department of Chemical Engineering, Faculty of Engineering, Persian Gulf University, P.O. Box 75169-13798, Bushehr, Iran. E-mail: mofarahi@pgu.ac.ir; Fax: +98 773 344 1495
First published on 23rd March 2017
Abstract
The gas-phase volumetric overall mass transfer coefficient (KGaV) plays a key role in the assessment of an absorption packed column's performance since it determines the height of an absorber column. The effective and useable data provided by KGaV is necessary for designing and scaling up absorption packed columns. This study provides the first comprehensive review of mass transfer performance in terms of KGaV for CO2 (KGCO2aV) absorption into amine solutions for absorber columns with random and structured packing. To date, researchers associated with the KGCO2aV parameter have focused on two main fields: experimental works and developing empirical correlations. For experimental works, KGCO2aV has been evaluated in the literature for a large number of conventional and improved amines over a range of operating parameters in laboratory-scale packed columns. In addition, researchers have developed empirical correlations for KGCO2aV based on operating parameters affecting KGCO2aV and physical properties. The details of research determining the KGCO2aV have been reviewed for low- and high-pressure absorption packed columns. Finally, directions for future research of the mass transfer performance for absorber packed columns in the CO2 capture process have been discussed.
1. Introduction
Today, fossil fuels play a major role in the production and supply of energy in the world. With global economic development and population growth, the utilization of these fuels has increased extensively. As a result, the consumption of fossil fuels is causing a sharp increase of CO2 emissions into the atmosphere.1,2 CO2 is the main greenhouse gas responsible for global warming and climate change. Although CO2 is corrosive to exposed equipment and has a low heating value, it can be used in various industries after treatment.3 Research has become essential in achieving an effective process of CO2 removal from industrial exhaust gas streams.4 CO2 is usually produced in different concentrations by three main processes including pre-combustion, post-combustion, and oxyfuel combustion.5,6 Currently, the only CO2 separation process implemented at a fully commercial scale is the post-combustion CO2 capture process. The foremost instances are post-combustion CO2 capture in the TMC Mongstad in Norway, which can capture 300![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 tons of CO2 per year and BD3 Sask Power in Canada, which can capture 1 million tons of CO2 per year.6 Depending on the conditions of the post-combustion capture process, different CO2 separation technologies can be used such as absorption, adsorption, and membrane and cryogenic separation.7,8 One of the most widespread and cost-effective processes in the capture of impurities from various gas streams is the absorption method using chemical solvents. Among chemical absorption processes, the amine processes are among the most important. They have been applied in numerous refineries worldwide for eliminating CO2 and hydrogen sulfide from ammonia-synthesis gas, natural gas, crude hydrogen, fossil-fuel fired power plants, petroleum industry, and town gas streams.9 Over the past decades, different separator devices for CO2 capture processes, including columns with tray, spray, packing types, and membrane contactors have been extensively studied and have received significant attention.10–13 Among these separators, packed columns (random or structured) are well known to have good mass transfer performance features. To accurately estimate the exact size of packed column height necessary for the mass transfer of CO2 from the gas phase to the liquid phase in case of absorption with a chemical reaction, the accurate prediction of the mass transfer coefficients is very important.11 To raise the designer's confidence and provide the best data for scaling up and designing packed columns in CO2 capture plants, a good understanding of the basics of modeling and designing is fundamental.11 Currently, CO2 absorption into amine solutions in packed columns is considered to be one of the most complicated systems because of relationships between the mass/heat transfer, hydromechanics and thermodynamics.14 To calculate the mass transfer rate in an absorber column, whether random or structured, empirical or semi-empirical correlations have been developed by researchers in terms of mass transfer coefficients in gas and liquid phases and interfacial areas. However, these developed correlations can be inaccurate in some case and sometimes they cannot be applied to specific systems such as CO2–amine systems. A review on several mass transfer correlations in packed columns has been performed in the literature.15,16 Razi, et al.17 have assessed these correlations in a rate-based model for CO2 capture with a monoethanolamine (MEA) solution to show the uncertainty associated with using correlations in large-scale CO2 capture plants. They concluded that the interactions among the transport parameters, mass transfer coefficients, effective interfacial areas and kinetics are too complex for the mass transfer correlations to be applied with a sufficient level of confidence.17 So far, none of these reviews has provided a review of mass transfer performance in terms of the KGCO2aV parameter for CO2 absorption into amine solutions in packed columns. In existing studies related to the KGCO2aV for CO2 absorption at a laboratory scale, packed columns have been experimentally investigated for a large number of amine-based solvents across a range of operating parameters. The base of researchers' studies was the prediction of KGCO2aV, the investigation of effects of operating parameters on it, and the correlation between KGCO2aV and operating process parameters. This study presents a review of studies of KGCO2aV parameter at low- and high-pressure packed columns for CO2 absorption into amine solutions in packed columns. The advantage of directly using KGCO2aV when calculating the mass transfer rate is that it makes it unnecessary to calculate the individual mass transfer coefficients in gas and liquid phases and other parameters such as the enhancement factor and Henry's law solubility constant. In addition, the KGCO2aV can be applied to specific systems; for example, Ziaii, et al.18 have used such developed empirical correlation in a CO2 capture simulation with MEA solution for an absorber packed column.
000 tons of CO2 per year and BD3 Sask Power in Canada, which can capture 1 million tons of CO2 per year.6 Depending on the conditions of the post-combustion capture process, different CO2 separation technologies can be used such as absorption, adsorption, and membrane and cryogenic separation.7,8 One of the most widespread and cost-effective processes in the capture of impurities from various gas streams is the absorption method using chemical solvents. Among chemical absorption processes, the amine processes are among the most important. They have been applied in numerous refineries worldwide for eliminating CO2 and hydrogen sulfide from ammonia-synthesis gas, natural gas, crude hydrogen, fossil-fuel fired power plants, petroleum industry, and town gas streams.9 Over the past decades, different separator devices for CO2 capture processes, including columns with tray, spray, packing types, and membrane contactors have been extensively studied and have received significant attention.10–13 Among these separators, packed columns (random or structured) are well known to have good mass transfer performance features. To accurately estimate the exact size of packed column height necessary for the mass transfer of CO2 from the gas phase to the liquid phase in case of absorption with a chemical reaction, the accurate prediction of the mass transfer coefficients is very important.11 To raise the designer's confidence and provide the best data for scaling up and designing packed columns in CO2 capture plants, a good understanding of the basics of modeling and designing is fundamental.11 Currently, CO2 absorption into amine solutions in packed columns is considered to be one of the most complicated systems because of relationships between the mass/heat transfer, hydromechanics and thermodynamics.14 To calculate the mass transfer rate in an absorber column, whether random or structured, empirical or semi-empirical correlations have been developed by researchers in terms of mass transfer coefficients in gas and liquid phases and interfacial areas. However, these developed correlations can be inaccurate in some case and sometimes they cannot be applied to specific systems such as CO2–amine systems. A review on several mass transfer correlations in packed columns has been performed in the literature.15,16 Razi, et al.17 have assessed these correlations in a rate-based model for CO2 capture with a monoethanolamine (MEA) solution to show the uncertainty associated with using correlations in large-scale CO2 capture plants. They concluded that the interactions among the transport parameters, mass transfer coefficients, effective interfacial areas and kinetics are too complex for the mass transfer correlations to be applied with a sufficient level of confidence.17 So far, none of these reviews has provided a review of mass transfer performance in terms of the KGCO2aV parameter for CO2 absorption into amine solutions in packed columns. In existing studies related to the KGCO2aV for CO2 absorption at a laboratory scale, packed columns have been experimentally investigated for a large number of amine-based solvents across a range of operating parameters. The base of researchers' studies was the prediction of KGCO2aV, the investigation of effects of operating parameters on it, and the correlation between KGCO2aV and operating process parameters. This study presents a review of studies of KGCO2aV parameter at low- and high-pressure packed columns for CO2 absorption into amine solutions in packed columns. The advantage of directly using KGCO2aV when calculating the mass transfer rate is that it makes it unnecessary to calculate the individual mass transfer coefficients in gas and liquid phases and other parameters such as the enhancement factor and Henry's law solubility constant. In addition, the KGCO2aV can be applied to specific systems; for example, Ziaii, et al.18 have used such developed empirical correlation in a CO2 capture simulation with MEA solution for an absorber packed column.
2. Overview of amine solvents used in the analysis of KGCO2aV
2.1. Conventional amines
Notable development in CO2 capture processes using reactive solvents has been seen in the past decade due to their ability to offer near-full absorption and desorption of CO2. Among different reactive solvents such as amines, potassium carbonate, and ammonia, that have been studied for CO2 capture processes, amines are considerably well-developed.19MEA is the most well-known amine, with a high reaction rate with CO2 and a low cost.20 These advantages of MEA can decrease the height of an absorber column and facilitate large-scale operations.14,21 However, MEA has some disadvantages such as high-energy consumption for its regeneration, low absorption capacity, and degradation and corrosion problems.7,22 To overcome these disadvantages, a number of important amine solvents have been commercially utilized such as diethanolamine (DEA), N-methyldiethanolamine (MDEA), 2-amino-2-methyl-1-propanol (AMP), and piperazine (PZ).23
As can be seen from Table 1, the aforementioned amines each have their advantages and disadvantages. Clearly, there is no particular amine solvent with all ideal characteristics for CO2 capture processes. Studies have so far focused on the improvement of amine solvents in order to reach a high CO2 capture performance and a low cost of operation.24 In recent years, mixing of conventional amines has shown a considerable improvement of absorption and desorption in CO2 capture processes.25 The higher absorption capacity, faster kinetics and lower energy consumption for stripping of CO2 are good characteristics of mixed amines. Research of mixed amines, such as MEA–MDEA and AMP–PZ, has demonstrated a great enhancement of the kinetics, thermodynamics, mass transfer, as well as energy consumption for regeneration.26–28
| Chemical name | MEA | DEA | MDEA | AMP | PZ, anhydrous | PZ, 65% | 1DMA2P | DETA | DEEA |
|---|---|---|---|---|---|---|---|---|---|
| Molecular formula | C2H7NO | C4H11NO2 | C5H13NO2 | C4H11NO | C4H10N2 | C4H10N2 | C5H13NO | C4H13N3 | C6H15NO |
| Formula weight | 61.08 | 105.14 | 119.16 | 89.14 | 86.13 | 86.13 | 103.16 | 103.17 | 117.19 |
| Freezing point °C | 10.3 | 28 | −21.0 | 26.0 | 110 | 41 | −85.0 | −39.0 | −70 |
| Boling point °C | 170 | 268 | 247.1 | 165 | 146 | 116 | 96.0 | 207 | 161 |
| Density at 25 °C | 1.01 | 1.092 | 1.03 | 0.934 | 0.877 | 1.03 | 0.913 | 0.952 | 0.884 |
| Vapor pressure (at 20 °C) mm Hg | 0.48 | <0.01 | 0.01 | <1 | 0.1 | 6.28 | 8 | 0.08 | 1 |
| Water solubility | Miscible | Miscible | Miscible | Miscible | 14 wt% | Miscible | Miscible | Miscible | Miscible |
| Absorption rate | High | Medium | Low | Medium | High | High | Low | Medium | Low |
| Absorption capacity | Medium | Medium | High | High | High | High | High | High | Medium |
| Heat of absorption | High | High | Medium | High | High | High | Medium | High | Medium |
2.2. Newly developed amines
Recently, the focus of studies has turned to new and promising amines, such as N,N-diethylethanolamine (DEEA), 4-diethylamino-2-butanol (DEAB), diethylenetriamine (DETA), and 1-dimethylamino-2-propanol (1DMA2P).23,29–33 DEEA is a tertiary-type amine, which is a potential candidate for CO2 bulk removal.34 This amine can be made from low-priced resources such as agricultural products and residues.33 Kim and Savage,35 Benitez-Garcia, et al.36 and Li, et al.37 have reported data of CO2 absorption rates by DEEA at different temperatures and concentrations. Li, et al.37 have concluded that the rate of absorption of CO2 in a DEEA solution is higher than in an MDEA solution. Also, Vaidya and Kenig38 have shown that the absorption capacity of a DEEA solution, in terms of CO2 loading, approached a value of 1 mol CO2/mol DEEA. DEAB is an amino alcohol solvent which was synthesized based on a normal molecular design approach.39 Sema, et al.40 have studied and compared the CO2 absorption capacity of DEAB with conventional amines such as MEA, DEA, AMP, and MDEA, and their results showed that the absorption of CO2 in a DEAB solution requires a lower DEAB concentration for the same CO2 removal efficiency as conventional amines. They also concluded that the rate of absorption of CO2 in a DEAB solution is higher than in an MDEA solution and lower than in an MEA solution. DETA, a polyamine comprising two primary and one secondary amine groups, has shown to have a faster reaction rate and a higher absorption capacity compared with conventional amines.41,42 Recently, Chowdhury, et al.43 and Liang, et al.44 have shown that a new tertiary amine, 1-dimethylamino-2-propanol (1DMA2P), has good potential for CO2 absorption because of its superior performance. Kadiwala, et al.30 have shown that 1DMA2P has a faster reaction rate than MDEA but slower than MEA. Chowdhury, et al.43 have reported that the CO2 loading of 1DMA2P (at low CO2 partial pressures) is about twice as high as that of an MDEA solution. Table 1 shows a summary of conventional and newly developed amines' properties45 used in analyses of KGCO2aV in packed columns.3. Mass transfer theory
At a particular point of an absorber column, mass transfer occurs because of a chemical potential gradient between gas and liquid phases. The mass transfer ends when equilibrium is reached. In other words, when the net mass transfer becomes zero.46 Nevertheless, the question is at what rate can the mass be transferred? This problem can be associated with the mass transfer coefficient.47 The mass transfer coefficient is an important parameter in designing absorber columns.11,14 Knowledge of this parameter can help a designer accurately calculate the height of an absorber column. In an absorber packed column in a post-combustion CO2 capture plant, the removal efficiency of CO2 absorption by amine solutions can be determined by the gas–liquid contact degree, physicochemical properties and hydrodynamics of the absorber column, amine reactivity degree and operating parameters related to the gas and amine solution.17 Chemical absorption of CO2 into an amine solution can be described by the two-film theory.48 This theory proposes that there are two thin films near the gas and liquid phase interfaces, which separate them from the liquid and gas bulk phases. This theory assumes that bulk phases are in equilibrium and all resistances of mass and heat transfer exist in the two films.48 In most cases, when CO2 moves from the gas to the liquid phase, a chemical reaction between CO2 and the amine solution can take place in the liquid film or liquid bulk.49 According to Fig. 1 and based on the two-film theory, the reaction between CO2 and the amine solution can be characterized as infinitely fast rate or very slow rate.48 Depending on the relative values of the reaction rate constants, mass transfer coefficients of gas and liquid phases, concentration ratio of reactants and CO2 equilibrium solubility, reactions occur in a narrow zone within the film or through the film and bulk of the liquid. The two-film theory is prevalently used in rate-based models.49–55 A significant number of them consider that the reaction takes place within the liquid film, when the reaction is assumed to be infinitely fast (i.e., CO2 absorption into the MEA solution). | ||
| Fig. 1 Location of the chemical reaction between CO2 and an amine solution based on the two-film theory. | ||
3.1. Determination of KGCO2aV in an absorption packed column
At steady-state conditions, the absorbed mass flux of CO2 (NCO2) across the gas–liquid interface can be represented in terms of KG and the difference between the CO2 partial pressure in the gas bulk (PyCO2) and the CO2 partial pressure at the gas–liquid interface , as shown in eqn (1).56,57
, as shown in eqn (1).56,57
 | (1) |
It is obvious from eqn (1) that NCO2 is greatest when  approaches zero and PyCO2 is at a maximum value. In the same way, NCO2 is zero when PyCO2 is equal to
approaches zero and PyCO2 is at a maximum value. In the same way, NCO2 is zero when PyCO2 is equal to  .The significance of KG can be seen from eqn (1)—for a given driving force, a greater KG can give greater NCO2 into the amine solution.58 Since the driving force of mass transfer occurs at a small distance from the film, the concentration in the interface and, subsequently, KG are difficult to measure in an absorption packed column because of the variations in the interfacial area with varying gas and liquid flow rates.59 Therefore, it is more convenient and useful to express NCO2 based on the unit volume of the absorption packed column rather than the interfacial area unit, as follows:59
.The significance of KG can be seen from eqn (1)—for a given driving force, a greater KG can give greater NCO2 into the amine solution.58 Since the driving force of mass transfer occurs at a small distance from the film, the concentration in the interface and, subsequently, KG are difficult to measure in an absorption packed column because of the variations in the interfacial area with varying gas and liquid flow rates.59 Therefore, it is more convenient and useful to express NCO2 based on the unit volume of the absorption packed column rather than the interfacial area unit, as follows:59
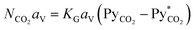 | (2) |
In eqn (2), NCO2aV can be obtained from KGCO2aV and the difference between PyCO2 and the CO2 partial pressure in the gas phase in equilibrium with the CO2 concentration in the liquid bulk  . To calculate NCO2aV, the mass balance according to the rate-based model, considering a small differential height of packing (dZ) of the absorption packed column (Fig. 2), can be written as follow:59
. To calculate NCO2aV, the mass balance according to the rate-based model, considering a small differential height of packing (dZ) of the absorption packed column (Fig. 2), can be written as follow:59
 | (3) |
By substituting eqn (3) into eqn (2), KGCO2aV can be determined using eqn (4):
 | (4) |
Most researchers have used eqn (4) to determine KGCO2aV from experiments on absorption packed columns.32,33,58–61 In eqn (4), the gas flow rate (G) and cross-section area of the column (A) as well as the packed column pressure (P) are known, and only two terms—the driving force and the derivative of the CO2 molar ratio—have to be determined. The term  in eqn (4) can be obtained from the equilibrium solubility data of CO2 into the amine solution. Often,
in eqn (4) can be obtained from the equilibrium solubility data of CO2 into the amine solution. Often,  is assumed to be zero due to a fast reaction between CO2 and the amine solution.14,32,49,58,60–65 The derivative of the CO2 molar ratio
is assumed to be zero due to a fast reaction between CO2 and the amine solution.14,32,49,58,60–65 The derivative of the CO2 molar ratio  can be determined by measuring the CO2 concentration (molar fraction) profile in the gas phase along the height of the absorber packed column. By converting molar fraction values to molar ratio values of CO2, the term
can be determined by measuring the CO2 concentration (molar fraction) profile in the gas phase along the height of the absorber packed column. By converting molar fraction values to molar ratio values of CO2, the term  is calculated by plotting YCO2 against the packing height of the absorber column (Z), as shown in Fig. 3.59
is calculated by plotting YCO2 against the packing height of the absorber column (Z), as shown in Fig. 3.59
 | ||
| Fig. 3 Determination of the molar ratio slopes by measuring the CO2 concentration profile in the gas phase along the height of an absorber column. | ||
When the CO2 concentration is measured at the inlet and outlet of an absorber packed column, the average values of KGCO2aV can be obtained from eqn (5) suggested by Dey and Aroonwilas.66
 | (5) |
The advantage of using directly KGCO2aV when simulating the CO2 absorption process is avoiding the need to calculate the enhancement factor and individual mass transfer coefficients in liquid and gas phases. The correlations between individual mass transfer coefficients are dependent on dimensionless numbers such as the Remolds and Schmidt numbers, as well as some hydrodynamic properties of the absorber column; mostly however, they were not developed to use in specific systems (i.e., amine system and packing type). In our previous work,51 we have applied mass transfer correlations from literature, which were not developed for amine systems, in the simulation of CO2 absorption in amine solutions. We have performed sensitivity analyses of individual mass transfer coefficients and kinetics constants of the CO2 reaction in amine solutions (the kinetic constant is used for calculating the enhancement factor). We concluded that two mass transfer correlations had the best prediction for an absorber column profiles compared with other applied mass transfer correlations from the literature. Therefore, when KGCO2aV is used directly in modeling CO2 absorption into amine solutions, there is no need to evaluate the sensitivity of the absorber model.
4. Experimental studies determining KGCO2aV in low-pressure absorber packed columns
Several experimental works for obtaining KGCO2aV for conventional and newly developed amines have been conducted by researchers for absorption columns packed with random and structured packing. The reason for determining the mass transfer coefficient based on the gas phase is easy and hassle-free measurement of the CO2 concentration along the height of the packed column. In most of these experimental studies, first KGCO2aV was determined and then the effects of operating parameters on it. In addition, the empirical correlations for KGCO2aV have been developed based on the effects of operating parameters in absorber columns. Table 2 provides a list of published works related to the determining of KGCO2aV in absorber packed columns operated in low-pressure conditions. For each list, detailed information about the solvent type, packing type, the height and diameter of packed columns, and operating parameters is given. In the following, the review of the mass transfer performance in terms of KGCO2aV by amine-based solvents operating in low-pressure absorber packed columns has been carried out.| Solvent | Packing type | Packing height (m) | Column diameter (D) | Inlet CO2 concentration (kPa) | Gas flow rate | Amine flow rate | Inlet liquid temperature (°C) | Inlet gas temperature (°C) | Amine concentration (mol L−1) | Inlet CO2 loading (mol CO2/mol amine) | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|
| MEA and AMP | Ceramic Berl saddles | 6.55 | 0.1 | 11.5–19.5 | 11.1–14.8 (mol m−2 s−1) | 9.5–13.5 (m3 m−2 h−1) | N/A | 14–20 | 1.2–3.8 | 0.0–0.37 | (Tontiwachwuthikul et al., 1992) |
| AMP | EX | 1.1 | 0.019 | 4.7–15.15 | 12.82 (mol m−2 s−1) | 9.73 (m3 m−2 h−1) | N/A | N/A | 1.14 | 0.027 | (Aroonwilas and Tontiwachwuthikul, 1997) |
| AMP | EX | 1.1 | 0.019 | 4–10 | 46.2–96.8 (kmol m−2 h−1) | 6.1–14.8 (m3 m−2 h−1) | N/A | N/A | 1.1–3 | 0.027–0.439 | (Aroonwilas and Tontiwachwuthikul, 1998) |
| MEA | 16 mm Pall rings | 2.4 | 0.1 | 14.78–15.23 | 119.5–199.1 (L min−1) | 0.971–2.093 (L min−1) | 24.1–26 | 24.8–26.3 | 6–7 | 0.195–0.225 | (Demontigny et al., 2001) |
| MEA | IMTP-15 | 2.4 | 0.1 | 5–19.9 | 94.7–249.2 (L min−1) | 0.867–3.134 (L min−1) | 23.5–28 | 23.4–31.2 | 2.98–9.02 | 0.077–0.354 | (Demontigny et al., 2001) |
| MEA | Gempak 4A | 2.4 | 0.1 | 14.90–20.30 | 119.7–191.9 (L min−1) | 0.948–2.968 (L min−1) | 22.5–28.7 | 24.4–28 | 3–7 | 0.12–0.227 | (Demontigny et al., 2001) |
| MEA and AMP | EX | 1.1 | 0.019 | Up to 15 | 30–97.5 (kmol m−2 h−1) | 4.9–29.3 (m3 m−2 h−1) | 20–37 | N/A | 1.1–5.2 | N/A | (Aroonwilas et al., 1999) |
| MEA and AMP | Gempak 4A | 0.98–2.21 | 0.1 | Up to 15 | 30–97.5 (kmol m−2 h−1) | 4.9–29.3 (m3 m−2 h−1) | 20–37 | N/A | 1.1–5.2 | N/A | (Aroonwilas et al., 1999) |
| MEA and AMP | BX | 1.02 | 0.25 | Up to 15 | 30–97.5 (kmol m−2 h−1) | 4.9–29.3 (m3 m−2 h−1) | 20–37 | N/A | 1.1–5.2 | N/A | (Aroonwilas et al., 1999) |
| MEA, AMP, DEA, MDEA and DIPA | DX | 2 | 0.02 | 10 | 48.2 (m3 m−2 h−1) | 4.8–10 (m3 m−2 h−1) | N/A | N/A | 3 | 0–0.4 | (Aroonwilas and Veawab, 2004) |
| MEA and MEA–MDEA | DX | 0.165–0.825 | 0.02 | 5–15 | 7.26–10.13 (L min−1) | 4.8–15.3 (m3 m−2 h−1) | 30–50 | N/A | 3–7 | 0–0.29 | (Setameteekul et al., 2006) |
| MEA–MeOH | DX | 0.4 | 0.034 | 15 | 5 (L min−1) | 0.02–0.1 (L min−1) | N/A | N/A | 5 | 0–0.5 | (Usubharatana et al., 2006b) |
| MEA–AMP | DX | 0.5 | 0.02 | 5–15 | 100 (kmol m−2 h−1) | 2.5–5 (m3 m−2 h−1) | N/A | N/A | 3–5 | 0–0.59 | (Dey and Aroonwilas, 2009) |
| MEA, MEA–MDEA, DEAB and DEAB–MEA | DX | 2.15 | 0.0275 | 14.4–14.9 | 4.5–5.7 (L min−1) | 0.025–0.118 (L min−1) | 20–41 | 19.6–30.3 | 1.1–7.17 | 0–0.3 | (Naami et al., 2012) |
| DETA | Dixon rings | 1.14 | 0.028 | 14.8–15.8 | 28.78–46.62 (kmol m−2 h−1) | 2.65–7.56 (m3 m−2 h−1) | 30–50 | N/A | 1–4 | 0.05–0.819 | (Fu et al., 2012) |
| DETA | DX | 1.2 | 0.028 | 8.8–14.1 | 22.2–40.4 (kmol m−2 h−1) | 1.95–4.87 (m3 m−2 h−1) | 30–50 | N/A | 1–4 | 0.184–0.826 | (Fu et al., 2013) |
| MEA–MeOH | DX | 1.25 | 0.028 | 6.7–13.8 | 24.37–63.54 (kmol m−2 h−1) | 2.92–16.09 (m3 m−2 h−1) | 10 | 11–17 | 2.5–5 | 0–0.373 | (Fu et al., 2015) |
| 1DMA2P | DX | 1.4 | 0.028 | 13.2–14.5 | 28.02 (kmol m−2 h−1) | 4.07 (m3 m−2 h−1) | 40 | N/A | 2 | 0.2–0.42 | (Liang et al., 2015a) |
| 1DMA2P | Dixon rings | 1.4 | 0.028 | 13.3–14.5 | 28.75–46.62 (kmol m−2 h−1) | 2.65–7.56 (m3 m−2 h−1) | 30–60 | N/A | 1–3 | 0–0.373 | (Wen et al., 2015) |
| NH3 | N/A | 2 | 0.015 | 2.8–8 | 1400–2300 (m3 m−2 h−1) | 20–30 (m3 min−1) | 27 | N/A | 0.27–0.72 | N/A | (Li et al., 2014) |
| MEA–MeOH | Sulzer BX 500, Mellapale y 500 and Pall rings | 3 | 0.15 | 15 | 1.7–6.5 (m3 h−1) | 20–50 (L h−1) | 20–50 | N/A | 5 | 0.2–0.4 | (Gao et al., 2016) |
| DEEA | DX and Dixon rings | 1.7 | 0.028 | 3–15 | 30.5–43.52 (kmol m−2 h−1) | 3.9–11.7 (m3 m−2 h−1) | 27–60 | N/A | 1–4 | 0.05–0.02 | (Xu et al., 2016) |
4.1. KGCO2aV of conventional amines
Tontiwachwuthikul, et al.67 have reported the mass transfer performance of CO2 absorption into MEA and AMP by measuring the temperature and concentration along the height of the absorption packed column. They performed several experiments in a laboratory-scale absorber packed column with 12.7 mm ceramic Berl saddles packing type. The column was made of six packed bed sections with a total packing height of 6.55 m and a 0.1 m diameter. The profiles along the height of the packed column were obtained for different liquid to gas ratios, inlet CO2 concentration in feed of flue gas, and amine concentrations. They did not determine the KGCO2aV values directly; rather, they modeled the packed column for CO2 absorption with an MEA solution by applying a rate-based model. Aroonwilas and Tontiwachwuthikul68 have studied experimentally KGCO2aV for CO2 absorption into AMP solution. They performed their experiments on a laboratory-scale absorption column of 1.1 m packing height and a 0.019 m diameter, packed with EX-type structured packing with a specific surface area of approximately 1700 m2 m−3. Their experimental results showed that the values of KGCO2aV at fixed operating parameters were unaffected by the CO2 partial pressure over a range of 3–15 kPa. They also compared the values of KGCO2aV for two cases—columns packed with ceramic Berl saddles packing and EX-type structured packing at the same operating conditions. They found that the value of KGCO2aV for EX-type structured packing was six times greater than for the ceramic Berl saddles packing. Afterwards, Aroonwilas and Tontiwachwuthikul63 have studied experimentally the KGCO2aV for CO2 absorption into AMP solution under operating conditions different from their previous work.68 They found that the effect of the CO2 partial pressure on the values of KGCO2aV changed slightly at pressures above 6 kPa and the values of KGCO2aV reduced from 1 to 6 kPa. For flow rates in the absorber column, the KGCO2aV was unaffected by the gas flow rate but the liquid flow rate had a pronounced effect on KGCO2aV, which increased the values of KGCO2aV by increasing the liquid flow rate in the range of 6.1–14.8 m3 m−2 h−1. In addition, by increasing the CO2 loading of the AMP solution, the KGCO2aV values decreased. Demontigny, et al.58 have reported experimental data of CO2 absorption into ultra-highly concentrated MEA solutions (up to 9 kmol m−3) and investigated the effects of process parameters on KGCO2aV in three pilot-scale absorption columns packed with random (16 mm Pall Ring and IMTP-15) and structured packing (Gempak 4A). The diameter and height of the absorption packed columns were 0.1 and 2.4 m, respectively. Their results showed that the values of KGCO2aV increased with increasing liquid flow rates and were unaffected by the gas flow rate. By increasing the CO2 partial pressure and CO2 loading, the values of KGCO2aV decreased. In relation to the MEA concentration, which was one of their important works, by increasing the MEA concentration up to 4 kmol m−3, the values of KGCO2aV decreased with a mild slope but increased in the range of 4–9 kmol m−3. They also studied the effect of the packing type on the KGCO2aV values, and found that structured packing (Gempak 4A) had a better performance compared with random packing (16 mm Pall Ring and IMTP-15). When comparing 16 mm Pall Ring packing with IMTP-15 packing, the IMTP-15 had greater KGCO2aV values. Aroonwilas, et al.69 have performed experiments on the performance of three types of structured packing (laboratory-scale (EX), pilot-scale (Gempak 4A), and industrial-scale (SulzerBX)) in terms of the KGCO2aV coefficient. The experimental data was reported for CO2 absorption into sodium hydroxide (NaOH), MEA and AMP solutions. The laboratory-scale absorption packed column was packed with 20 packing elements of EX and had a total packing height of 1.1 m and a 0.019 m diameter. The pilot-scale absorption packed column was packed with Gempak 4A stainless steel and the packing height varied between 0.98 and 2.21 m, and the absorber had a 0.1 m diameter. The third case was an industrial-scale absorption–desorption unit in which an absorber column was packed with six elements of Sulzer BX gauze structured packing, and the column had a total packing height of 1.02 m and a 0.25 m. Their results indicated that the values of KGCO2aV increased with an increasing liquid flow rate and liquid concentration and were unaffected by the gas flow rate. The values of KGCO2aV decreased with increasing CO2 concentrations up to 15%. The values of KGCO2aV increased with solvent temperature from 20 °C to 37 °C and decreased with temperatures from 40 °C to 65 °C. When comparing structured packing (Gempak 4A) and IMTP-25 packing, the Gempak 4A provided two times greater KGCO2aV values. Aroonwilas and Veawab65 have comprehensively investigated the performance of conventional amines such as MEA, DEA, DIPA (diisopropanolamine), MDEA, and AMP; in addition, they have investigated blends including MEA–MDEA, DEA–MDEA, MEA–AMP, and DEA–AMP. They performed the experiments in a laboratory-scale absorption column with a 2 m packing height and a 0.02 m diameter with 36 DX-type elements of structured packing. Their results were presented based on the CO2 removal efficiency, absorber height requirement, effective mass-transfer area, and KGCO2aV, under identical conditions for the liquid flow rate and CO2 loading. Their result showed that the CO2 removal efficiency in a CO2 loading of zero was in the order MEA > DEA > AMP > DIPA > MDEA. The value of 100% of CO2 removal efficiency was obtained for MEA, DEA, and AMP, requiring a 0.75, 1.75, and 2.0 m height of the packed column, respectively. Therefore, MEA showed a better performance in comparison with other studied amines. For blended amines, the value of 100% of CO2 removal efficiency was obtained for MEA–AMP, DEA–AMP, MEA–MDEA, and DEA–MDEA, requiring a 1.2, 2.3, 3.3, and 5.4 m height of the packed column, respectively. The authors also assessed the performance in terms of the effective mass-transfer area under identical processing parameters and found that MEA provided the highest mass-transfer area among the tested amines, including DEA, DIPA, and MDEA. They also showed that the values of KGCO2aV at different CO2 loadings for MEA were higher compared with other tested amines such as DEA, AMP, DIPA, and MDEA. Setameteekul, et al.70 studied the mass transfer performance for CO2 absorption in a MEA and MDEA blended amine. The experiments were performed based on the factorial experimental design method (a statistical method), and conducted more than 106 tests with three replications in an absorption column packed with a DX-type packing. The packing height varied between 0.165 and 0.825 m, and the absorber had a 0.02 m diameter. The results of the work by Setameteekul, et al.70 indicated that the solvent temperature and solvent concentration have the largest effects on the KGCO2aV values and the other process parameters have smaller effects. Dey and Aroonwilas66 used the blended MEA–AMP amine to determine KGCO2aV by using only two data sampling points of CO2 concentrations at the bottom and top of an absorber column. In fact, they obtained the average values of KGCO2aV for an absorber column packed with a DX-type structured packing. Their results showed that the KGCO2aV values increased with an increasing liquid flow rate, temperature, and total amine concentrations, and decreased with increasing CO2 partial pressure of the feed gas and CO2 loading of the amines. The addition of higher concentrations of MEA in the mixed MEA–AMP amine led to an increase in the KGCO2aV values, except at high CO2 loadings. This was because of a lower reaction rate of CO2 with AMP compared with MEA.Jeon, et al.71 have studied the mass transfer performance and effect of adding ammonia (NH3) to AMP and MDEA. They determined the KGCO2aV values in an absorption packed column with a 1.5 m packing height and a 0.05 m diameter by testing two packing types including 6 mm ceramic Raschig rings, and a wire gauze laboratory-structured packing. They showed that the KGCO2aV values at a CO2 partial pressure of 15 kPa increased for both mentioned systems by using structured packing, and increased even more by adding NH3 from 1 wt% to 3 wt%. They also showed that the KGCO2aV values increased at lower CO2 partial pressure and higher liquid-to-gas ratios. The overall conclusion of their work was that adding NH3 to AMP and MDEA and using structured packing produced higher KGCO2aV values. Li, et al.72 have performed experiments for CO2 absorption using an NH3 solution to determine the KGCO2aV values in an absorber column packed with a novel structured packing with diversion windows type. The height of the absorber column and column diameter were 2.4 m (packing height: 2 m) and 0.15 m, respectively. Their results showed that KGCO2aV was enhanced by increasing the liquid flow rate and its concentration. However, the KGCO2aV values decreased when the CO2 partial pressure increased to 8 kPa, and were unaffected by the gas flow rate. Kang, et al.73 have tested various packing types including ceramic Raschig rings, Berl saddles, a structured gauze packing and a hybrid of Raschig rings and a structured packing in different ratios to investigate the mass transfer performance of a CO2–MEA–AMP system. Their results showed that CO2 removal efficiencies of Raschig rings, Berl saddles, and the structured packing materials provided higher values for the MEA than the AMP solution, and that the structured packing had a greater efficiency than the random packing. They improved the performance of single random and structured packing materials by mixing them in ratios of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 2
1, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, and 1
1, and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2. The optimal performance was obtained for the 2
2. The optimal performance was obtained for the 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio (structured packing/Raschig rings). The KGCO2aV parameter decreased in the order 2
1 ratio (structured packing/Raschig rings). The KGCO2aV parameter decreased in the order 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 hybrid packing > structured packing > Raschig rings > Berl saddles.
1 hybrid packing > structured packing > Raschig rings > Berl saddles.
4.2. KGCO2aV of hybrid amines
Usubharatana, et al.74 have studied KGCO2aV for CO2 absorption by using a hybrid solution containing MEA and methanol. Their experiments were carried out on a laboratory-scale absorption column with a 0.4 m packing height and a 0.034 m diameter, packed with DX-type structured packing with a specific surface area of around 900 m2 m−3. They showed that by increasing the methanol concentration in the MEA solution, KGCO2aV increased. By increasing the liquid flow rate, KGCO2aV increased and led to a decrease of the methanol carryover because of its vaporization to the top of the absorber column. Fu, et al.60 have obtained experimentally KGCO2aV by using a hybrid solution containing MEA and methanol. They performed the experiment on a structured absorber column (packing height 1.25 m; column diameter 0.028 m) packed with a DX packing. They reported the CO2 concentration and temperature data along the height of the packed column (seven points along column) for 33 tested runs. The effects of key process parameters including the MEA concentration, CO2 loading, amine flow rate and gas flow rate were investigated, and it was found that KGCO2aV decreased with increasing CO2 loading, gas flow rate (in higher concentrations of the MEA solution), and CO2 concentration in the inlet feed gas. The authors suggested using a high liquid-to-gas ratio and a low temperature of the feed gas and amine in the absorber column in order to prevent the vaporization of methanol at the top of the absorber column. Gao, et al.75 have studied the effects of different process parameters on the KGCO2aV values using a hybrid solvent: MEA–methanol. The experiments performed on an absorber column (packing height 3 m; column diameter 0.15 m) packed with three different packing types including Sulzer BX500, Mellapale Y500, and Pall rings 16 × 16. Their results indicated that the Sulzer BX500 had higher KGCO2aV values than the Mellapale Y500. The reason for this was the good uniform distribution of gas and liquid on the packing surface. In addition, their results showed that (1) the KGCO2aV values increased as the CO2 lean loading decreased and the hybrid solution temperature, hybrid solution flow rate and gas flow rate increased, (2) the optimal temperature for reducing methanol evaporation was 20 °C, and (3) operating at a high liquid-to-gas ratio led to a reduction of methanol evaporation as suggested by Fu, et al.60 study.4.3. KGCO2aV of new amines
Naami, et al.59 have studied experimentally KGCO2aV for CO2 absorption using a DEAB solution. They also investigated absorption using MEA, MDEA, DEAB, and blended solutions included MDEA–MEA and DEAB–MEA. The authors performed their experiments on an absorber column packed with DX-type structured packing (height 2.15 m; diameter 0.0275 m). It was concluded that the presence of MEA in the DEAB solution increased the KGCO2aV values. The authors also investigated the effect of the liquid flow rate on the KGCO2aV values, and found that by increasing it in a narrow range, KGCO2aV values increased rapidly. Nevertheless, the KGCO2aV values were unaffected by the gas flow rate for the above-mentioned amines. The absorption capacity and cyclic capacity between DEAB and MDEA were also compared in the study, and it was found that the new amine, DEAB, has a higher CO2 absorption and cyclic capacity than MDEA, which causes a reduction in the amine circulation rate and energy consumption for amine regeneration. Fu, et al.61 have determined experimentally KGCO2aV for CO2 absorption into a DETA solution. They performed the experiment on a random absorber column (packing height 1.14 m; column diameter 0.024 m) packed with Dixon rings which have a specific surface area of around 2400 m2 m−3. Their results showed that the KGCO2aV values for DETA were higher compared with MEA, and the KGCO2aV values increased as the DETA flow rate, DETA concentration, and inlet temperature increased. However, the KGCO2aV values decreased when the CO2 loading of DETA increased. Fu, et al.76 have investigated the KGCO2aV parameter using a DETA solution. They performed the experiment on a structured absorber column (packing height 1.7 m; column diameter 0.028 m) packed with a DX packing which has a specific surface area of around 900 m2 m−3. They compared the KGCO2aV values between DETA–CO2 and MEA–CO2 systems, and found that the KGCO2aV values of DETA were higher compared with MEA. In addition, they showed that by increasing the DETA flow rate, concentration, and inlet temperature, the KGCO2aV values increased. Nevertheless, the KGCO2aV values decreased as the CO2 loading of DETA increased. Liang, et al.44 have investigated the mass transfer performance of CO2 absorption by a 1DMA2P solution. They obtained KGCO2aV for a structured absorber column (packing height 1.4 m; column diameter 0.028 m) packed with DX packing. The authors compared the KGCO2aV values for 1DMA2P–CO2 with those for MEA–CO2 and MDEA–CO2 systems, and found that the KGCO2aV values of the MEA solution were higher compared with 1DMA2P and MDEA solutions. They ranked them as MEA > 1DMA2P > MDEA. In addition, they showed that by increasing the CO2 loading for the three mentioned systems, the KGCO2aV values decreased. Following the study of Liang, et al.,44 Wen, et al.77 obtained the KGCO2aV values in an absorber column packed with Dixon rings for 1DMA2P–CO2 system. They compared the KGCO2aV values for 1DMA2P–CO2 with those for the MDEA–CO2 system, and found that the KGCO2aV values of the new amine solution were higher compared with the MDEA solution. In addition, the effects of the gas flow rate, amine concentration, amine flow rate, CO2 loading, and amine temperature on the KGCO2aV values were investigated and the results indicated that the KGCO2aV values increased with increasing amine concentrations and amine flow rates, but decreased with increasing CO2 loading. The inert gas flow rate had little effect on the KGCO2aV values. For the amine temperature, the KGCO2aV values increased up to 323 K in temperature range of 303–333 K, and decreased as amine temperature went above 323 K. Xu, et al.33 have conducted experiments for CO2 absorption in a DEEA solution. The experiments were carried out for both random and structured packing columns, namely DX and Dixon rings. The authors obtained the KGCO2aV values in an absorber column with a 1.7 m packing height and a 0.028 m diameter. The effects of important process parameters on the KGCO2aV values were investigated. The results showed that the DEEA concentration, CO2 loading, and liquid flow rate had the most pronounced effect on KGCO2aV, whilst the gas flow rate had a negligible effect. The authors compared the KGCO2aV values between MDEA–CO2 and DEEA–CO2 systems, and showed that the KGCO2aV values for DEEA were higher compared with the MDEA solution. They also showed that the DX-type structured packing enhanced the KGCO2aV values and cyclic capacity compared with the Dixon rings packing. Chen, et al.78 have used the experimental design method including the Taguchi method to select blended amines (MEA–DEAE, MEA–MDEA, MEA–DIPA, and MEA + AMP) as promising solvents for CO2 capture in a packed column (packing height 1.2 m; column diameter 0.05 m). The optimum points and effects of three key process parameters including the liquid flow rate, gas flow rate and amine concentration on the absorption rate and KGCO2aV were studied. Sixteen runs were carried out by the Taguchi method at four levels for four factors to obtain the response values (absorption efficiency, absorption rate, scrubbing factor, and KGCO2aV). In case of KGCO2aV, the values were obtained using a two-film model. The results of the study indicated that the gas flow rate and amine flow rate are significant parameters, while the type of amine and amine concentration showed little effect on the KGCO2aV values.5. Experimental studies determining KGCO2aV in high-pressure absorber packed columns
In the previous section, we reviewed the experimental studies for determining KGCO2aV in low-pressure absorber packed columns. The evaluation of the KGCO2aV coefficient under high pressure is essential for removing CO2 from natural gas streams. Few studies are available in the literature for determining KGCO2aV in high-pressure absorber packed columns.Abdul Halim, et al.62 performed experiments in order to remove CO2 from a mixture of CO2 and methane using an MEA solution under a pressure of 50 bar. The experiments were performed in a packed column (packing height 2.04 m; column diameter 0.046 m) with a packing type of Sulzer gauze, which has a surface area of around 500 m2 m−3. The aim of their work was to determine KGCO2aV and the CO2 removal efficiency under high pressure. The KGCO2aV values were obtained at a fixed CO2 concentration of 20% mol, an amine concentration range of 1–4 kmol m−3, an amine flow rate range of 4.51–8.1 m3 m−2 h−1, a gas flow rate range of 18.89–35.08 kmol m−2 h−1, and feed temperature range of 27 °C to 45 °C. They validated their experiments' reliability by the work of Maneeintr, et al.79 under low-pressure conditions. Their results indicated that high pressure, amine flow rate, and amine concentration had a significant effect on KGCO2aV, and that by increasing these parameters, the KGCO2aV values would increase. They also showed that the KGCO2aV values were unaffected by the gas flow rate and that the optimal point for the temperature of the inlet amine to the absorption column was found to be 40 °C. Halim, et al.80 performed their experiments for removing CO2 from a mixture of CO2 and methane using an AMP–PZ solution in a packed column (packing height 2.04 m; column diameter 0.046 m) with a packing type of Sulzer gauze. The aim of their work was to determine the KGCO2aV values under high pressures (10–40 bar). The KGCO2aV values were obtained at a fixed CO2 concentration of 40% mole, a total amine concentration of 30% wt (PZ with 7 wt% and AMP with 23 wt%), an amine flow rate range of 2.89–3.97 m3 m−2 h−1, a gas flow rate range of 33–51 kmol m−2 h−1, and a feed temperature range of 30 °C to 35 °C. Their results indicated that high pressure, amine flow rate, and amine concentration had a large effect on KGCO2aV, and; the KGCO2aV values were unaffected by the gas flow rate. Following the work of Halim, et al.,80 Hairul, et al.81 performed experiments under different conditions of operating parameters. Their setup for an absorber column was similar the one in the work of Halim, et al.80 They determined KGCO2aV under different operating conditions over a pressure range of 10–50 bar, a CO2 concentration range of 30% to 50% mol, an amine concentration range of 3–9 wt% for PZ and 23–30 wt% for AMP, an amine flow rate range of 2.89–4.33 m3 m−2 h−1, a gas flow rate range of 33–40 kmol m−2 h−1, and feed temperature range of 30 °C to 35 °C. Their results showed that by increasing pressure above 20 bar, KGCO2aV increased, and by increasing the CO2 concentration in the feed gas, KGCO2aV decreased. In addition, the performance of AMP in removing CO2 from natural gas was compared with the AMP–PZ solution, and results showed the AMP–PZ system to be superior in terms of CO2 removal efficiency.
6. General investigation of operating parameters affecting KGCO2aV
In the above-mentioned reviewed works on KGCO2aV, initially, the KGCO2aV values were obtained experimentally using the concentration profile of CO2 in the gas phase and the mass balance equation, and then the effects of process parameters on it were investigated. In previous sections, we have shown how are the response values affected by increasing and decreasing one operating parameter. An understanding of how KGCO2aV of different amine-based solvents changes with different operating parameters and configurations of pilot-plant is significant in evaluating and optimizing CO2 removal processes.14,82 The variables affecting KGCO2aV in the above-mentioned reviewed works have been described in detail as follows.6.1. CO2 partial pressure
Increasing the CO2 partial pressure in the gas feed to the absorber column has two effects on KGCO2aV. First, increasing the CO2 partial pressure can lead to an increase of the partial pressure gradient83 because of the consummation of more actively free-amine molecules and, as a result, a decrease of KGCO2aV can occur (according to eqn (2)). Second, increasing the CO2 partial pressure can intensify the gas flow turbulence in the absorber column and, as a result, increase KGCO2aV.84 In the above-mentioned reviewed works, researchers showed that by increasing the CO2 partial pressure in the gas feed, KGCO2aV decreased. This effect shows that amine solutions, especially those which have higher reaction kinetic constant values (such as MEA or PZ), have good performance under lower partial pressure than under higher partial pressure.85,86 This effect indicates that amine solutions have a high CO2 removal efficiency in a lower partial pressure of CO2 in the gas feed. This occurrence shows that the liquid phase resistance dominates the mass transfer performance of absorption into amine solutions.876.2. Gas flow rate
The turbulence increases in the gas phase because an increase in the gas flow rate can lead to an increase of KGCO2aV.60,77,78 However, experiments by many researchers, which were pointed out in previous sections, differed from the above-mentioned prediction by Fu, et al.60 and Chen, et al.78 works. They showed that the liquid film could control the process of CO2 absorption in an amine solution and, as a result, KGCO2aV in such a system is unaffected by the gas flow rate.6.3. Liquid flow rate
One of the key parameters, which can affect the mass transfer performance, is the liquid flow rate. Many researchers showed that when the liquid flow rate increased, KGCO2aV increased as well. The reasons for this effect are (1) an increase in the amine flow rate can cause an increase in free active molecules of amines for high CO2 absorption, (2) an increase in the amine flow rate has the greatest effect on the surface of the packing, increasing the wet surface area between the amine and gas phases, and (3) an increase in the mass transfer coefficient in the liquid phase decreases the mass transfer resistance in the liquid phase and, as a result, increases the mass transfer coefficient in the gas phase.50,63,65,66,68 However, increasing this factor above the optimum point can lead to a loss of amines and to high-energy consumption for amine regeneration.6.4. Liquid concentration
In general, increasing the amine concentration causes an increase of the KGCO2aV values. This is because of the availability of an extra amount of amine molecules for CO2 absorption at the interface of the gas and liquid, and this increases the possibility for CO2 to react with amines over a larger active surface area.33,60,76 As mentioned before, the absorption of CO2 in amine solutions is a process, which is controlled by the liquid phase. As a result, this phenomenon decreases the resistance in the liquid phase and increases the mass transfer coefficient in the gas phase. However, increasing the concentration leads to an increase of the viscosity, which can hinder the diffusion of CO2 into amines. Under these conditions, the balance between increasing KGCO2aV and the cost involved should be considered by increasing the amine concentration.6.5. Liquid temperature
Another other key parameter is liquid temperature, which can have an effect on KGCO2aV, the reaction kinetic, and equilibrium solubility. According to the Arrhenius equation,88 the reaction kinetic constant for the reaction between CO2 and an amine solution is temperature dependent, and by increasing the temperature a higher reaction rate constant is achieved.89 Consequently, the enhancement factor can be increased and consequently, KGCO2aV can be increased as well.90 Under absorption conditions, this effect can be reversed at higher temperatures when the reaction between CO2 and amines becomes reversible (it approaches desorption conditions), and it can decrease KGCO2aV. In addition, the higher temperature can lead to an increase in the vapor pressure of CO2 above the amine solution's and this can cause an increase in the Henry's law solubility constant; as result, a decrease of KGCO2aV and the solubility of CO2 in amine solutions can occur.91 The balance between the above-mentioned parameters should be considered for increasing KGCO2aV.6.6. CO2 loading
An increase of CO2 loading in amine solutions leads to a decrease in the existing active amine concentration, which consequently decreases KGCO2aV.33,71,74,76,77 This is obvious when the amount of CO2 loading in the lean amine solution is high, the mass transfer driving force from the gas phase to the liquid phase will decrease and, in these cases, increasing the liquid flow rate to compensate the low absorption rate is not an effective method. The optimum way for decreasing the CO2 loading in amine solutions is heating the amine solution to increase KGCO2aV.6.7. Absorption pressure
Increase of pressure in an absorber column can lead to a decrease of CO2 concentration in equilibrium with the amine solution and, as a result, the driving force for mass transfer can increase. Halim, et al.80 showed that the absorption of CO2 (from natural gas) in an AMP–PZ solution under a pressure range of 20–40 bar increased KGCO2aV. However, the increasing absorber pressure in CO2 capture post-combustion processes will increase the cost of operation.92 Increasing KGCO2aV in CO2 capture post-combustion processes by increasing pressure is not cost-effective.6.8. Packing type
Packing is helpful in making more time for gas–liquid contact throughout the CO2 absorption process, so it can increase the surface area and KGCO2aV in the packed column. Recently, researchers showed that using a structured packing in the absorption column could create higher surface are as compared with random packing.93 This is because of greatly higher geometric wet surface areas per structured packing volume unit. However, the packing surface area should not be the only selection criteria for creating a higher KGCO2aV in the packed column.94 The other parameters affecting packing, such as packing arrangement pattern, angle of corrugation, void fraction, and height of crimp, should be also considered when designing the absorption packed column, to minimize the pressure drop and capacity of the liquid entrainment.7. Empirical correlations for KGCO2aV in packed columns
Several mass-transfer coefficient correlations are available for absorber columns packed with random and structured packing.12,95–98 These developed correlations differ in their accuracy and system-specific applicability. By having these correlations and calculating the enhancement factor, KGCO2aV can be obtained. However, these correlations can increase the error in calculations, for example in rate-based models, and one needs to perform a sensitive analysis for the mentioned correlations, which may not be applicable to CO2–amine systems.The review on these correlations was done by Wang, et al.16 for random and structured packing columns. Herein, we have reviewed the empirical correlations of KGCO2aV in absorber packed columns, which were obtained experimentally, mainly from analyzing the effects of operating parameters on KGCO2aV in CO2–amine systems. The reviewed correlations in this study are presented in Table 3 and the corresponding values of operating parameters for developed correlations have been listed in Table 2.
Kohl and Riesenfeld99 have developed an empirical correlation of KGCO2aV for CO2 absorption in an MEA solution. The correlation applies to an absorption column packed with random packing. In their study, the KGCO2aV correlation is as function of the amine flow rate (L), amine loading (α), equilibrium loading (αe), amine concentration (M), viscosity (μ), CO2 partial pressure, and amine temperature. Their empirical correlation is
 | (6) |
In the above equation, the F value is the packing factor, and Kohl and Riesenfeld99 have reported F values for some random packing types. For example, the F values have been reported as 0.0021 and 0.003 for 1 inch ceramic saddles and 3/8 inch ceramic saddles, respectively. Demontigny, et al.58 have developed an empirical correlation of KGCO2aV for a CO2–MEA system based on the work of Kohl and Riesenfeld.99 The correlation is as function of the same parameters developed by Kohl and Riesenfeld99 except for the viscosity term as this term was not considered in the work of Demontigny, et al.58 The developed equation in their work is
 | (7) |
Demontigny, et al.58 have reported that the eqn (7) had errors when predicting results using experimental data and is valid only up to a concentration of 3 kmol m−3. They reported that the errors arose from the CO2 loading data. Despite of variations of CO2 loading along the height of the packed column, the CO2 loading value was assumed in its saturation point (0.5 mol CO2/mol amine). Aroonwilas and Tontiwachwuthikul63 have developed an empirical correlation of KGCO2aV for a CO2–AMP system based on the work of Demontigny, et al.58 The correlation obtained for an absorber column packed with a laboratory DX-type packing. The correlation is as function of the same parameters developed in the Demontigny, et al.58 study. The developed correlation of KGCO2aV for a CO2–AMP system byAroonwilas and Tontiwachwuthikul63 is
 | (8) |
They showed that the error between experimental data of KGCO2aV and results predicted by the above equation was 16.5%. Setameteekul, et al.70 have developed a KGCO2aV correlation based on the experimental design factorial method. The KGCO2aV correlation was obtained for two systems including CO2–MEA and CO2–MEA–MDEA as a function of the amine flow rate, amine loading, amine concentration, amine temperature, and CO2 partial pressure. The correlation is based on the results of interaction of operating parameters, and for the CO2–MEA system, it is obtained from the equation below.
| KGae = 4.106 − 0.370A − 0.077B + 0.044C − 0.001758D − 4.74E + 0.00215A2 + 0.004B2 − 0.00162C2 + 6.105E2 − 0.02AC +… | (9) |
It should be noted that the above equation was obtained after truncation of insignificant parameters. Insignificant parameters were those, which had less probable values in the analysis of variance results. The results obtained by the factorial method had an error of 18.39% for the CO2–MEA system and a high error reported for the CO2–MEA–MDEA system. Dey and Aroonwilas66 have developed the KGCO2aV correlation for CO2 absorption into a MEA–AMP solution in an absorber column packed with a laboratory DX-type packing as
 | (10) |
In eqn (10), AMP/MEA is the molar ratio of the amine mixture, α is the CO2 loading, xCO2 is the molar fraction of CO2 in liquid, Cs is the amine concentration, L1 is the amine flow rate and T is the amine temperature. The regressed coefficients (K, A, B, C, D, E, and F) were obtained for different AMP/MEA molar ratios of the amine mixture, and the errors were reported as 6.75%, 10.05%, 11.72% and 12.2% for MEA, MEA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) AMP = 1
AMP = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, MEA
2, MEA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) AMP = 1
AMP = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, and MEA
1, and MEA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) AMP = 2
AMP = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, respectively. Sema, et al.32 have developed the KGCO2aV correlation for CO2 absorption into a DEAB solution. They have reported that the correlation is valid for an absorber column packed with a DX-type packing. Their results showed that the reported error for the developed correlation (eqn (11)) against experimental data was 14.6%.
1, respectively. Sema, et al.32 have developed the KGCO2aV correlation for CO2 absorption into a DEAB solution. They have reported that the correlation is valid for an absorber column packed with a DX-type packing. Their results showed that the reported error for the developed correlation (eqn (11)) against experimental data was 14.6%.
 | (11) |
Fu, et al.61 have developed a predictive correlation for KGCO2aV in a CO2–DETA system. This correlation is valid for an absorber column packed with Dixon rings. The developed correlation was obtained based on the work of Demontigny, et al.58 The correlation was validated the KGCO2aV; first, by developing the correlation according to the work of Kohl and Riesenfeld99 for a CO2–MEA system, and then, the correlation was validated for a CO2–DETA system. These correlations are, for the CO2–MEA (eqn (12)) and CO2–DETA (eqn (13)) systems:
 | (12) |
 | (13) |
The predicted results by the correlations above showed errors of 16% and 14%, for CO2–DETA and CO2–MEA systems, respectively, against experimental results. Fu, et al.76 have applied an artificial neural network (ANN) in order to estimate of KGCO2aV values for absorption of CO2 into a DETA solution in an absorber column packed with laboratory Ex-type packing. In the ANN model, 8 parameters such as gas flow rate, CO2 partial pressure, liquid flow rate, amine concentration, amine density, amine viscosity, diffusion of CO2 in an amine solution, and the cycling loading of amines are defined as input parameters and KGCO2aV as the output parameter. To develop the ANN model, 75% and 25% of the dataset were used for training and testing, respectively. Their results showed that the ANN model could predict experimental data very well, with an error of 7.6% for the CO2–DETA system in a packed column. Naami, et al.100 have developed a correlation for the KGCO2aV in a CO2–MEA–MDEA system in an absorber packed with a DX-type packing over MDEA–MEA concentrations of 27/3, 25/5, and 23/7 wt%. The developed correlation was based on the work of Dey and Aroonwilas66 (eqn (10)), and is
 | (14) |
In eqn (14), MDEA/MEA is the molar ratio of the amine mixture, α is the CO2 loading of the amine, xCO2 is the molar fraction of CO2 in the liquid, Cs is the amine concentration, L1 is the amine flow rate and T is the amine temperature. The regression coefficients (K, A, B, C, D, E, and F) were obtained for different MDEA/MEA molar ratios of the amine mixture, and the errors were reported as 20.9%, 21.7%, and 22.8% for MEA/MDEA = 3/27 wt%, MEA/MDEA = 5/25 wt%, and MEA/MDEA = 7/23 wt%, respectively. Wen, et al.77 have correlated the KGCO2aV data in an absorber column packed with Dixon rings for a 1DMA2P–CO2 system. Their developed correlation was based on the Demontigny, et al.58 study:
 | (15) |
Their results showed that the developed correlation had an error of 9.8% when predicting experimental data. Li, et al.72 have developed the KGCO2aV correlation in an absorber column packing with diversion windows type in a NH3–CO2 system. The correlation is based on important parameters such as the liquid flow rate (L), NH3 concentration and CO2 partial pressure:
 | (16) |
In addition, the modeling and simulation of absorption of CO2 in a NH3 solution was carried out using the computational mass transfer model with a developed correlation of KGCO2aV (eqn (16)). The authors did not report the error values between the developed correlation and experimental data, but trends of the developed correlation showed that it had a small error in comparison with the experimental data. Xu, et al.33 have developed two correlations of KGCO2aV for absorber columns packed with Dixon rings and DX-type packing materials. They developed the correlation for a CO2–DEEA system based on the work of Demontigny, et al.58 as follows:
 | (17) |
 | (18) |
Their results showed that the errors obtained by eqn (17) (Dixon rings packing) and eqn (18) (DX-type packing) were 3% and 8%, respectively.
8. Conclusions
In this study, a review has been provided on KGCO2aV associated with amine-based solvents in absorption packed columns. As a first step, we have reviewed the experimental determination of KGCO2aV, previously done by researchers. With measuring CO2 concentrations in the gas phase along the height of absorber columns and using the two-film theory, KGCO2aV was obtained by researchers for columns packed with random and structured packing materials. Details of pilot-plant data related to the determination of KGCO2aV have been reported, and the determination of KGCO2aV has been reviewed for various amine-based solvents (conventional amines, hybrid amines, newly developed amines) over a range of operating parameters of low- and high-pressure absorber columns. Second, we have reviewed and described the effects of operating parameters on the KGCO2aV data in absorber packed columns. In most studies, authors showed that the KGCO2aV values are unaffected by the gas flow rate, and by increasing the liquid flow rate, amine concentration, and column pressure, the KGCO2aV values increased. Increasing the CO2 loading of amines and the partial pressure of CO2 in the gas feed lead to a KGCO2aV decrease. Third, we have reviewed the developed empirical correlations of KGCO2aV for absorber low-pressure columns. The most developed correlations are functions of operating parameters of the absorber column and somewhat depend on the physical properties such as viscosity. It should be noted that developed correlations, which were reviewed in this study, are only based on specific systems (such as packing type and amine solvent). The advantages of these correlations are that one does not need to calculate the enhancement factor and perform a sensitivity analysis of mass transfer coefficients in both liquid and gas phases.9. Prospects
In recent years, CO2 removal using amine solutions has attracted extensive consideration by many researchers. One of the most important subjects is to evaluate the mass transfer performance in absorption packed columns in terms of mass transfer coefficients. As we have discussed in this study, the determination of the KGCO2aV term can help the designer gain a deeper understanding of such a system because of the simplicity of using CO2 concentration measurements in the gas phase for absorber columns. However, some attempts are needed to gain a better understanding of the mass transfer performance in terms of KGCO2aV. Following are the possible future directions concerning the analysis and evolution of KGCO2aV in absorption packed columns:• Determination of KGCO2aV for a pilot-plant and plant data.
• Determination of KGCO2aV for a stripper column under high temperature.
• More analyses are needed of KGCO2aV parameter in high-pressure conditions.
• Sophisticated equipment is required for high quality measuring of CO2 along the packed column which is used for determination of KGCO2aV.
• Considering other gases in the flue gas feed to the absorber when determining KGCO2aV.
• Simultaneous effects of operating parameters on KGCO2aV need to be investigated, for example using statistical methods.
• Determination of KGCO2aV using a optimization technique such as the work of Ji, et al.101 who did this for the mass transfer coefficient in the liquid phase.
• More research on mixed amines is needed; for example, the effects of activators on the amine solutions for the determination of KGCO2aV.
• More research on the packing type for determination of KGCO2aV is needed, especially for plant data.
• The empirically developed correlations should not depend only on the operating parameters but should also depend on physical properties, while for the CO2–amines systems reaction kinetics are also important.
• Applying the empirically developed correlation of KGCO2aV directly in the rate-based model and considering the errors.
• Modeling and optimization KGCO2aV are needed to find the optimum operating parameters effecting KGCO2aV.
Nomenclature
References
- A. J. McMichael, R. E. Woodruff and S. Hales, Lancet, 2006, 367, 859–869 CrossRef.
- N. Panwar, S. Kaushik and S. Kothari, Renewable Sustainable Energy Rev., 2011, 15, 1513–1524 CrossRef.
- S. A. Rice, Environments, 2014, 3, 7–15 Search PubMed.
- E. S. Rubin, H. Mantripragada, A. Marks, P. Versteeg and J. Kitchin, Progr. Energy Combust. Sci., 2012, 38, 630–671 CrossRef CAS.
- T. N. G. Borhani, A. Azarpour, V. Akbari, S. R. W. Alwi and Z. A. Manan, Int. J. Greenhouse Gas Control, 2015, 41, 142–162 CrossRef CAS.
- Z. H. Liang, W. Rongwong, H. Liu, K. Fu, H. Gao, F. Cao, R. Zhang, T. Sema, A. Henni and K. Sumon, Int. J. Greenhouse Gas Control, 2015, 40, 26–54 CrossRef CAS.
- M. Mofarahi, Y. Khojasteh, H. Khaledi and A. Farahnak, Energy, 2008, 33, 1311–1319 CrossRef CAS.
- P. Usubharatana, D. McMartin, A. Veawab and P. Tontiwachwuthikul, Ind. Eng. Chem. Res., 2006, 45, 2558–2568 CrossRef CAS.
- A. L. Kohl and R. Nielsen, Gas purification, Gulf Professional Publishing, 1997 Search PubMed.
- J. Kuntz and A. Aroonwilas, Ind. Eng. Chem. Res., 2008, 47, 145–153 CrossRef CAS.
- Z. H. Liang, T. Sanpasertparnich, P. P. Tontiwachwuthikul, D. Gelowitz and R. Idem, Carbon Manage., 2011, 2, 265–288 CrossRef CAS.
- R. F. Strigle, Random Packings and Packed Towers, 2nd edn, Gulf Publishing Company, Houston, TX, 1987 Search PubMed.
- P. Tontiwachwuthikul and A. Chakma, J. Membr. Sci., 2006, 277, 99–107 CrossRef.
- K. Fu, G. Chen, Z. Liang, T. Sema, R. Idem and P. Tontiwachwuthikul, Ind. Eng. Chem. Res., 2014, 53, 4413–4423 CrossRef CAS.
- N. Razi, O. Bolland and H. Svendsen, Int. J. Greenhouse Gas Control, 2012, 9, 193–219 CrossRef CAS.
- G. Wang, X. Yuan and K. Yu, Ind. Eng. Chem. Res., 2005, 44, 8715–8729 CrossRef CAS.
- N. Razi, H. F. Svendsen and O. Bolland, Int. J. Greenhouse Gas Control, 2014, 26, 93–108 CrossRef CAS.
- S. Ziaii, S. Cohen, G. T. Rochelle, T. F. Edgar and M. E. Webber, Energy Procedia, 2009, 1, 4047–4053 CrossRef CAS.
- J. Salazar, U. Diwekar, K. Joback, A. H. Berger and A. S. Bhown, Energy Procedia, 2013, 37, 257–264 CrossRef.
- A. Bandyopadhyay, Clean Technol. Environ. Policy, 2011, 13, 269–294 CrossRef CAS.
- M. Iijima, T. Nagayasu, T. Kamijyo and S. Nakatani, Mitsubishi Heavy Ind. Tech. Rev., 2011, 48, 26 Search PubMed.
- F. Y. Jou, A. E. Mather and F. D. Otto, Can. J. Chem. Eng., 1995, 73, 140–147 CrossRef CAS.
- K. Maneeintr, R. O. Idem, P. Tontiwachwuthikul and A. G. H. Wee, Energy Procedia, 2009, 1, 1327–1334 CrossRef CAS.
- G. Puxty, R. Rowland, A. Allport, Q. Yang, M. Bown, R. Burns, M. Maeder and M. Attalla, Environ. Sci. Technol., 2009, 43, 6427–6433 CrossRef CAS PubMed.
- G. Puxty and R. Rowland, Environ. Sci. Technol., 2011, 45, 2398–2405 CrossRef CAS PubMed.
- S. Bishnoi and G. T. Rochelle, Chem. Eng. Sci., 2000, 55, 5531–5543 CrossRef CAS.
- S. Ma'mun, H. F. Svendsen, K. A. Hoff and O. Juliussen, Energy Convers. Manage., 2007, 48, 251–258 CrossRef.
- B. Mandal, M. Guha, A. Biswas and S. Bandyopadhyay, Chem. Eng. Sci., 2001, 56, 6217–6224 CrossRef CAS.
- Y.-C. Chang, R. B. Leron and M.-H. Li, J. Chem. Thermodyn., 2013, 64, 106–113 CrossRef CAS.
- S. Kadiwala, A. V. Rayer and A. Henni, Chem. Eng. J., 2012, 179, 262–271 CrossRef CAS.
- X. Luo, N. Chen, S. Liu, W. Rongwong, R. O. Idem, P. Tontiwachwuthikul and Z. Liang, Int. J. Greenhouse Gas Control, 2016, 53, 160–168 CrossRef CAS.
- T. Sema, A. Naami, K. Fu, G. Chen, Z. Liang, R. Idem and P. Tontiwachwuthikul, Chem. Eng. Sci., 2013, 100, 183–194 CrossRef CAS.
- B. Xu, H. Gao, X. Luo, H. Liao and Z. Liang, Int. J. Greenhouse Gas Control, 2016, 51, 11–17 CrossRef CAS.
- D. D. D. Pinto, J. G. M. S. Monteiro, B. Johnsen, H. F. Svendsen and H. Knuutila, Int. J. Greenhouse Gas Control, 2014, 25, 173–185 CrossRef CAS.
- C. Kim and D. Savage, Chem. Eng. Sci., 1987, 42, 1481–1487 CrossRef CAS.
- J. Benitez-Garcia, G. Ruiz-Ibanez, H. A. Al-Ghawas and O. C. Sandall, Chem. Eng. Sci., 1991, 46, 2927–2931 CrossRef CAS.
- J. Li, A. Henni and P. Tontiwachwuthikul, Ind. Eng. Chem. Res., 2007, 46, 4426–4434 CrossRef CAS.
- P. D. Vaidya and E. Y. Kenig, Chem. Eng. Technol., 2009, 32, 556–563 CrossRef CAS.
- P. Tontiwachwuthikul, A. G. Wee, R. Idem, K. Maneeintr, G.-j. Fan, A. Veawab, A. Henni, A. Aroonwilas and A. Chakma, US Pat., US7910078 B2, Grant Application number US 11/843,958, 2011.
- T. Sema, A. Naami, R. Idem and P. Tontiwachwuthikul, Ind. Eng. Chem. Res., 2011, 50, 14008–14015 CrossRef CAS.
- A. Hartono, K. A. Hoff, T. Mejdell and H. F. Svendsen, Energy Procedia, 2011, 4, 179–186 CrossRef CAS.
- A. Hartono and H. F. Svendsen, Energy Procedia, 2009, 1, 853–859 CrossRef CAS.
- F. A. Chowdhury, H. Yamada, Y. Matsuzaki, K. Goto, T. Higashii and M. Onoda, Energy Procedia, 2014, 63, 572–579 CrossRef CAS.
- Y. Liang, H. Liu, W. Rongwong, Z. Liang, R. Idem and P. Tontiwachwuthikul, Fuel, 2015, 144, 121–129 CrossRef CAS.
- All physical properties listed in Table 1 were taken from MSDS (Material Safety Data Sheet), http://ScienceLab.com.
- E. L. Cussler, Diffusion: mass transfer in fluid systems, Cambridge university press, 2009 Search PubMed.
- R. Taylor and R. Krishna, Multicomponent mass transfer, John Wiley & Sons, 1993 Search PubMed.
- O. Levenspiel, Ind. Eng. Chem. Res., 1999, 38, 4140–4143 CrossRef CAS.
- F. Khan, V. Krishnamoorthi and T. Mahmud, Chem. Eng. Res. Des., 2011, 89, 1600–1608 CrossRef CAS.
- M. Afkhamipour and M. Mofarahi, Int. J. Greenhouse Gas Control, 2013, 15, 186–199 CrossRef CAS.
- M. Afkhamipour and M. Mofarahi, Int. J. Greenhouse Gas Control, 2014, 25, 9–22 CrossRef CAS.
- T. N. G. Borhani, M. Afkhamipour, A. Azarpour, V. Akbari, S. H. Emadi and Z. A. Manan, J. Ind. Eng. Chem., 2016, 34, 344–355 CrossRef CAS.
- T. N. G. Borhani, V. Akbari, M. K. A. Hamid and Z. A. Manan, J. Ind. Eng. Chem., 2015, 22, 306–316 CrossRef CAS.
- J. Gabrielsen, H. F. Svendsen, M. L. Michelsen, E. H. Stenby and G. M. Kontogeorgis, Chem. Eng. Sci., 2007, 62, 2397–2413 CrossRef CAS.
- P. Mores, N. Scenna and S. Mussati, Int. J. Greenhouse Gas Control, 2012, 6, 21–36 CrossRef CAS.
- R. B. Bird, Appl. Mech. Rev., 2002, 55, R1–R4 CrossRef.
- W. M. Deen, Analysis of transport phenomena (topics in chemical engineering), Oxford University Press, New York, 1998 Search PubMed.
- D. Demontigny, P. Tontiwachwuthikul and A. Chakma, Can. J. Chem. Eng., 2001, 79, 137–142 CrossRef CAS.
- A. Naami, M. Edali, T. Sema, R. Idem and P. Tontiwachwuthikul, Ind. Eng. Chem. Res., 2012, 51, 6470–6479 CrossRef CAS.
- K. Fu, W. Rongwong, Z. Liang, Y. Na, R. Idem and P. Tontiwachwuthikul, Chem. Eng. J., 2015, 260, 11–19 CrossRef CAS.
- K. Fu, T. Sema, Z. Liang, H. Liu, Y. Na, H. Shi, R. Idem and P. Tontiwachwuthikul, Ind. Eng. Chem. Res., 2012, 51, 12058–12064 CrossRef CAS.
- H. N. Abdul Halim, A. M. Shariff, L. S. Tan and M. A. Bustam, Ind. Eng. Chem. Res., 2015, 54, 1675–1680 CrossRef CAS.
- A. Aroonwilas and P. Tontiwachwuthikul, Ind. Eng. Chem. Res., 1998, 37, 569–575 CrossRef CAS.
- A. Aroonwilas, P. Tontiwachwuthikul and A. Chakma, Sep. Purif. Technol., 2001, 24, 403–411 CrossRef CAS.
- A. Aroonwilas and A. Veawab, Ind. Eng. Chem. Res., 2004, 43, 2228–2237 CrossRef CAS.
- A. Dey and A. Aroonwilas, Energy Procedia, 2009, 1, 211–215 CrossRef CAS.
- P. Tontiwachwuthikul, A. Meisen and C. J. Lim, Chem. Eng. Sci., 1992, 47, 381–390 CrossRef CAS.
- A. Aroonwilas and P. Tontiwachwuthikul, Sep. Purif. Technol., 1997, 12, 67–79 CrossRef CAS.
- A. Aroonwilas, A. Veawab and P. Tontiwachwuthikul, Ind. Eng. Chem. Res., 1999, 38, 2044–2050 CrossRef CAS.
- A. Setameteekul, A. Veawab and A. Aroonwilas, 2006 IEEE EIC Climate Change Conference, 2006 Search PubMed.
- S.-B. Jeon, H.-D. Lee, M.-K. Kang, J.-H. Cho, J.-B. Seo and K.-J. Oh, J. Taiwan Inst. Chem. Eng., 2013, 44, 1003–1009 CrossRef CAS.
- W. Li, X. Zhao, B. Liu and Z. Tang, Ind. Eng. Chem. Res., 2014, 53, 6185–6196 CrossRef CAS.
- M.-K. Kang, I.-D. Kim, B.-J. Kim, J.-S. Kang and K.-J. Oh, Ind. Eng. Chem. Res., 2015, 54, 5853–5861 CrossRef CAS.
- P. Usubharatana, A. Veawab, A. Aroonwilas and P. Tontiwachwuthikul, 2006 IEEE EIC Climate Change Conference, 2006 Search PubMed.
- J. Gao, J. Yin, F. Zhu, X. Chen, M. Tong, W. Kang, Y. Zhou and J. Lu, Sep. Purif. Technol., 2016, 163, 23–29 CrossRef CAS.
- K. Fu, G. Chen, T. Sema, X. Zhang, Z. Liang, R. Idem and P. Tontiwachwuthikul, Chem. Eng. Sci., 2013, 100, 195–202 CrossRef CAS.
- L. Wen, H. Liu, W. Rongwong, Z. Liang, K. Fu, R. Idem and P. Tontiwachwuthikul, Chem. Eng. Technol., 2015, 38, 1435–1443 CrossRef CAS.
- P.-C. Chen, M.-W. Yang, C.-H. Wei and S. Z. Lin, Int. J. Greenhouse Gas Control, 2016, 45, 245–252 CrossRef CAS.
- K. Maneeintr, R. O. Idem, P. Tontiwachwuthikul and A. G. Wee, Ind. Eng. Chem. Res., 2010, 49, 2857–2863 CrossRef CAS.
- H. Halim, A. Shariff and M. Bustam, Sep. Purif. Technol., 2015, 152, 87–93 CrossRef CAS.
- N. Hairul, A. Shariff and M. Bustam, Int. J. Greenhouse Gas Control, 2016, 49, 121–127 CrossRef CAS.
- M. Afkhamipour and M. Mofarahi, Int. J. Greenhouse Gas Control, 2016, 49, 24–33 CrossRef CAS.
- D. Bailey and P. Feron, Oil Gas Sci. Technol., 2005, 60, 461–474 CAS.
- B. Xu, H. Gao, X. Luo, H. Liao and Z. Liang, Int. J. Greenhouse Gas Control, 2016, 51, 11–17 CrossRef CAS.
- Y. K. Salkuyeh and M. Mofarahi, Int. J. Energy Res., 2012, 36, 259–268 CrossRef CAS.
- Y. K. Salkuyeh and M. Mofarahi, Int. J. Energy Res., 2013, 37, 973–981 CrossRef CAS.
- A. Aroonwilas, A. Chakma, P. Tontiwachwuthikul and A. Veawab, Chem. Eng. Sci., 2003, 58, 4037–4053 CrossRef CAS.
- H. S. Fogler, Elements of chemical reaction engineering, 3rd edn, Prentice-Hall international, New Jersey, 1999 Search PubMed.
- J. E. Crooks and J. P. Donnellan, J. Chem. Soc., Perkin Trans. 2, 1989, 331–333 RSC.
- C. Kale, A. Górak and H. Schoenmakers, Int. J. Greenhouse Gas Control, 2013, 17, 294–308 CrossRef CAS.
- J. A. Rocha, J. L. Bravo and J. R. Fair, Ind. Eng. Chem. Res., 1996, 35(5), 1660–1667 CrossRef CAS.
- P. Mores, N. Rodríguez, N. Scenna and S. Mussati, Int. J. Greenhouse Gas Control, 2012, 10, 148–163 CrossRef.
- J. A. Kean, H. Turner and B. Price, Oil Gas J., 1991, 89, 41–47 CAS.
- Y. Taitel, D. Bornea and A. Dukler, AIChE J., 1980, 26, 345–354 CrossRef CAS.
- R. Billet and M. Schultes, Chem. Eng. Res. Des., 1999, 77, 498–504 CrossRef CAS.
- J. Gualito, F. Cerino, J. Cardenas and J. Rocha, Ind. Eng. Chem. Res., 1997, 36, 1747–1757 CrossRef CAS.
- B. Hanley and C. C. Chen, AIChE J., 2012, 58, 132–152 CrossRef CAS.
- K. Onda, H. Takeuchi and Y. Okumoto, J. Chem. Eng. Jpn., 1968, 1, 56–62 CrossRef CAS.
- A. L. Kohl and F. Riesenfeld, Gas Purification, Gulf Publishing Company, Houston Texas, USA, 1985 Search PubMed.
- A. Naami, T. Sema, M. Edali, Z. Liang, R. Idem and P. Tontiwachwuthikul, Int. J. Greenhouse Gas Control, 2013, 19, 3–12 CrossRef CAS.
- X. Ji, W. Kritpiphat, A. Aboudheir and P. Tontiwachwuthikul, Can. J. Chem. Eng., 1999, 77, 69–73 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2017 |

















