Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Development of a compact MnO2 filter for removal of SO2 from diesel vehicle emissions
Xuecheng Liuab,
Yugo Osakac,
Hongyu Huang *a,
Jun Li*d,
Zhaohong Hea,
Xixian Yanga,
Huhetaolia,
Shijie Lia and
Noriyuki Kobayashid
*a,
Jun Li*d,
Zhaohong Hea,
Xixian Yanga,
Huhetaolia,
Shijie Lia and
Noriyuki Kobayashid
aKey Laboratory of Renewable Energy, Guangzhou Institute of Energy Conversion, Chinese Academy of Sciences, No. 2 Nengyuan Rd. Wushan, Tianhe District, Guangzhou 510640, P. R. China. E-mail: huanghy@ms.giec.ac.cn; Fax: +86-20-37210762; Tel: +86-20-37210762
bUniversity of Chinese Academy of Sciences, Beijing 100049, PR China
cKanazawa University, Kakuma, Kanazawa, Ishikawa 920-1192, Japan
dNagoya University, Furo-cho, Chikusa-ku, Nagoya 464-8603, Japan. E-mail: junli@energy.gr.jp; Fax: +81-52-7895428; Tel: +81-52-7895486
First published on 27th March 2017
Abstract
Increasing concern about sulfur dioxide (SO2) from diesel vehicle exhausts causing detrimental effects on NOx removal catalysts has resulted in the development of dry desulfurization filters for complete removal of SO2. In this study, a compact MnO2 filter was developed for diesel emission control. The SO2-capture behavior of the compact MnO2 filter was investigated by using a volumetric device in a low temperature range (200–400 °C) and low SO2 pressure conditions. The maximal capacity of the MnO2 filter was 304.1 mgSO2 per gMnO2 at 400 °C. Based on the experimental results, the required volume of the MnO2 filter was estimated as only 0.6 L for a diesel car with 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 km distance traveled per year. The thickness of the MnO2 filter had significant influence on its SO2-capture performance. The sulfate reaction mechanism was also discussed by using a grain model under four reaction temperature conditions for improving the efficiency of the design of the desulfurization filter. The sulfate process can be divided into two control stages (the chemical reaction control stage and the solid diffusion control stage) and the prediction models fit the experimental data well for both control stages, indicating that the two-stage grain model is suitable for the sulfate reaction between the MnO2 filter and SO2. The calculated apparent activation energy of 18.8 kJ mol−1 indicates that the MnO2 filter exhibits high activity for SO2 adsorption in a pure SO2 atmosphere.
000 km distance traveled per year. The thickness of the MnO2 filter had significant influence on its SO2-capture performance. The sulfate reaction mechanism was also discussed by using a grain model under four reaction temperature conditions for improving the efficiency of the design of the desulfurization filter. The sulfate process can be divided into two control stages (the chemical reaction control stage and the solid diffusion control stage) and the prediction models fit the experimental data well for both control stages, indicating that the two-stage grain model is suitable for the sulfate reaction between the MnO2 filter and SO2. The calculated apparent activation energy of 18.8 kJ mol−1 indicates that the MnO2 filter exhibits high activity for SO2 adsorption in a pure SO2 atmosphere.
Introduction
The removal of sulfur dioxide (SO2) from vehicle exhausts is of great interest for the reason that SO2 has detrimental effects on human health and the environment.1,2 Besides, SO2 from diesel vehicle exhaust gas greatly deactivates the NOx removal catalysts because sulfates are more stable than nitrates.3 Compared with the flue gas from large power plants, diesel exhaust gas is quite different because of the low emission amounts, wide temperature range, high space velocity and low sulfur concentration (the sulfur content of diesel varying from tens of ppm to hundreds of ppm). Therefore the traditional wet flue gas desulfurization technologies for large power plants are not suitable for diesel exhaust systems. To solve the issues caused by SO2 emissions from diesel exhausts, compact SO2 traps of dry desulfurization technologies have been proposed for complete capture of SO2 from diesel vehicle exhausts.4–7The traditional dry desulfurization materials, such as calcined limestone8 and CaCO3 materials,9 have good sulfate reactivity over 650 °C with high SO2 concentration over 1000 ppm. However, SO2 capture capacity of these materials declines sharply below 400 °C because the sulfate reaction activity is limited by the decarbonation.9 With continuous development of the diesel engine technology, the maximum temperature of diesel exhaust will be decreased to 400 °C. Therefore the development of low-temperature activation and downsizing of the compact SO2 trap is needed for mounting on mobile diesel vehicle.
To enhance the low-temperature SO2-capture performance of desulfurization materials, Rubio and Izquierdo10 studied the SO2 removal performance of coal fly ash based carbons at flue gas conditions and the amount of SO2 removed is 13 mg g−1 by the Lada activated sample at 100 °C and 1000 ppmv SO2 gas conditions. The SO2 oxidation activity of copper oxide supported on the activated carbon could be improved at low temperature at 250 °C.11,12 The SO2 capture capacity of CeO2 based materials was investigated at low temperature conditions.13–15 Manganese dioxide with a simple sulfate reaction path (MnO2 + SO2 → MnSO4) showed high SO2-capture capacity at low temperature conditions.16 The MnO2 with high specific surface area had the potential to be more compact for disposable SO2 traps on account of high desulfurization SO2-capture performance at low temperature conditions. Li et al.17 investigated the SO2 capture capacity of manganese oxide at 325 °C and found that cryptomelane could be application as a replaceable absorbent for the removal of SO2 from diesel exhaust.
Traditionally, the materials were wash-coated on ceramic carrier (Fig. 1a) by using the binder for final application in the diesel vehicle exhaust system.18–20 The SO2 capture capacity per unit volume of desulfurization filter greatly decreased because the ceramic carrier with low SO2-capture performance occupied too much volume of the filter. Moreover, as the sulfur concentration of diesel is very small (a few hundred ppm), the mutual diffusion resistance could be large, which will greatly lower the diffusion rate of SO2 reaction gas from the external air to the sites of the reactant.21 Therefore, in this study, the compact dry desulfurization filter without ceramic carrier (MnO2 filter) was developed to downsize the desulfurization filter, as shown in Fig. 1b, and the volumetric method was proposed to exclude the mutual diffusion resistance to obtain the real SO2-capture behavior of compact MnO2 filter. The effects of the reaction temperature and thickness of MnO2 filter were investigated at low SO2 pressure condition. The sulfate reaction mechanism between the MnO2 filter and pure SO2 was also discussed under four reaction temperature conditions for improving the efficiency of designing MnO2 filter.
Experimental
MnO2 filter preparation
50 mg MnO2 was blended with 5 mg Carboxymethyl Cellulose (CMC) in the beaker. Exactly 2 mL of silica-sol (3.4 w% in H2O) was added dropwise and mixed the blender by glass rod. Then, the blender was extruded through the metal mold and calcined at 400 °C to form a rounded compact MnO2 filter. The MnO2 used for experiments was supplied by Japan Material and Chemical Co., Ltd. These materials were obtained by the acid treatment of raw material. The chemicals, CMC and silica sol (34 w% suspension in H2O), were purchased from Aladdin Co., Ltd., People's Republic of China and were of analytical reagent grade.Characterization
Morphology of the sample was analyzed by using transmission electron microscope (TEM). In this study, specific surface area, average pore diameter and pore volume were conducted to assess the physical characteristics of the target sample. The specific surface area was measured by the Brunauer–Emmett–Teller with the nitrogen adsorption uptake at the boiling point of nitrogen of 77 K using a capacitive measurement method. The average pore diameter and pore volume were measured by nitrogen physisorption under normal relative pressure of 0.1–1.0 using the Barrett–Joyner–Halenda (BJH) method. The particle size distribution of the sample was analyzed by Malvern laser particle size analyzer.Volumetric measurements
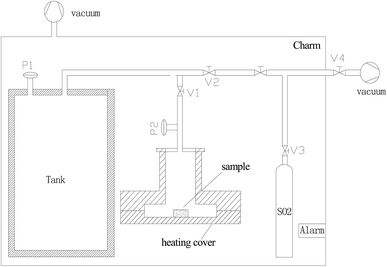
The volumetric test-rig consists mainly of two compartments, as depicted in Fig. 2. The first is sample cell, in which a sample of 0.1 g rounded absorbent is placed. The reaction temperature of sample cell can be adjusted and controlled by using the heater cover. The second compartment is a large constant volume tank. The temperature of this tank is controlled by using a thermostatic chamber.The volumetric test-rig was used in this study to measure the SO2 capture capacity of the prepared absorbent. Table 1 presents the experimental conditions used in the present study. The MnO2 filter was placed into the sample cell and slowly heated (5 K min−1) to the target reaction temperature. At the same time, the tank and sample was evacuated for 1 h by using the vacuum 1 with the valves V1–V3 opened. After the pressure was below 10−2 Pa, the valves V1–V3 were closed. The valves V4 & V2 were slowly opened to put the SO2 gas into the tank and then closed when the pressure of the SO2 gas was up to 40 Pa (equivalent to the partial pressure of 400 ppm SO2 in the exhaust). The temperature of thermostatic chamber was kept at 35 °C. The sulfate reaction process started once the valve V1 was opened, leading to decreasing the pressure in the tank. The pressure variation was measured to determine the amount of the SO2 gas on the sorbent sample by using the high resolution ceramic capacitance manometer P2. The SO2 capture capacity per unit mass of absorbent C and the conversion of MnO2 X are expressed by the following equations:
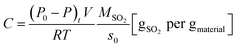 | (1) |
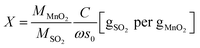 | (2) |
| Variables | Conditions |
|---|---|
| Temperature of the thermostatic chamber (K) | 308 |
| Temperature of the sample (K) | 473–673 |
| Pressure of tank (Pa) | 40 |
| Mass of the sample (g) | 0.05–0.15 |
| Thickness of the absorbent sample (mm) | 0.5–2 |
Results and discussion
Material characterization
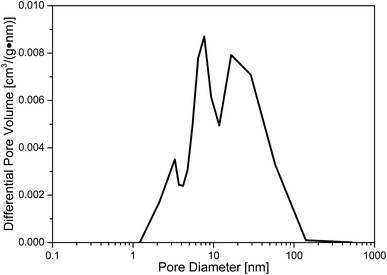
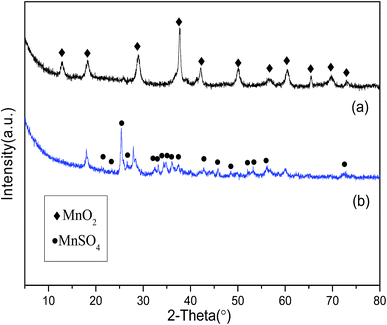
The distribution of pore diameter of the prepared sample is displayed in Fig. 3. From the results of Fig. 3, the relatively large pore diameter of the sample is about 7 nm. The specific surface area, average pore diameter and pore volume of the prepared sample are 275 m2 g−1, 7.5 nm and 0.5 cm3 g−1, respectively. The relatively large particle diameter of the sample is about 1.6 μm. XRD analysis (Fig. 4) is carried out to study the crystal structure of MnO2 filter before and after desulfurization. From the results of Fig. 4, the diffraction peaks of MnO2 filter are similar to those of tetragonal phase MnO2 (JCPDS 00-044-0141), and after desulfurization the diffraction peaks of the sample are changed to the orthorhombic phase MnSO4 (JCPDS 00-011-0088).Basic SO2-capture performance of MnO2 filter by using volumetric device
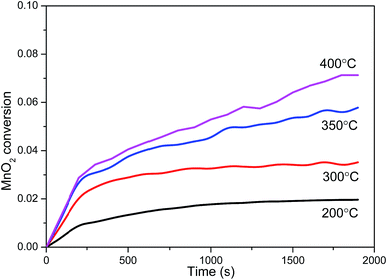
Fig. 5 shows the effect of desulfurization temperature on SO2 capture capacity of MnO2 filter. The SO2 capture capacity of MnO2 filter was investigated at 200 °C, 300 °C, 350 °C and 400 °C. From the results shown in Fig. 5, the SO2 capture capacity of MnO2 filter increases with the experimental temperature. At these experimental conditions, the trap achieves the highest SO2 capture capacity and obtained about 76.3 mgSO2 per gMnO2 after 1 h at 400 °C. At 200 °C, the SO2 trap capacity decreased to about 20% of its capacity at 400 °C and obtained 15.0 mgSO2 per gMnO2. SO2 capture capacity (mgSO2 per gMnO2) of MnO2 filter at 40 Pa pure SO2 under different sulfate reaction temperature conditions has been listed in Table 2. As seen in the results of Table 2, the maximal capacity of MnO2 filter was 304.1 mgSO2 per gMnO2 at 400 °C, significantly higher than the traditional desulfurization materials,14,17,22 as shown in Table 3.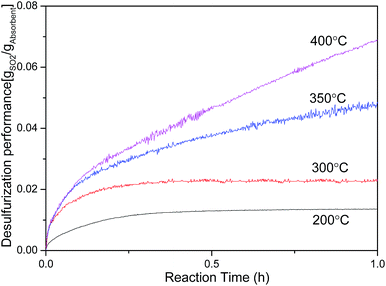 | ||
| Fig. 5 Influence of temperature on SO2 capture performance of MnO2 filter at 200 °C, 300 °C, 350 °C and 400 °C. | ||
| Reaction temperature | 200 °C | 300 °C | 350 °C | 400 °C |
|---|---|---|---|---|
| After adsorption 1 h | 15.0 | 25.7 | 52.7 | 76.3 |
| Maximal capacity | 15.0 | 25.7 | 71.7 | 304.1 |
| Materials | SO2 capture capacity | Reaction conditions | References |
|---|---|---|---|
| MnO2 filter | 304.1 | 400 °C, 40 Pa pure SO2 | In present work |
| CuO–CeO2 | 24 | 400 °C, 3600 ppm SO2 | Rodas-Grapaín et al.14 |
| TO-AC | 161 | 400 °C, 2000 ppm SO2 | Guo et al.22 |
| Pt–Pd–Al2O3 | 11 | 600 °C, 30 ppm SO2 | Limousy et al.4 |
| CaO | 36 | 325 °C, 250 ppm SO2 | Li et al.17 |
| Ca(OH)2 | 32 | 325 °C, 250 ppm SO2 | Li et al.17 |
| MgO | 20 | 325 °C, 250 ppm SO2 | Li et al.17 |
| ZrO2 | 16 | 325 °C, 250 ppm SO2 | Li et al.17 |
| CuO-AC | 30 | 250 °C, 200 ppm SO2 | Tseng et al.12 |
| Coal fly ash | 13 | 100 °C, 1000 ppm SO2 | Rubio and Izquierdo10 |
Based on the experimental results, the optimum volume of the MnO2 filter required for a diesel engine was estimated. A diesel car (traveled distance: about 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 km of one year) is considered in which the concentrations of sulfur in the diesel oil and in lubricating oil are both 30 ppm. It is assumed that the SO2-capture capacity is not affected by the initial sulfur concentration in this evaluation. Fuel consumption of the diesel car is 7 liter per hundred kilometers. The specific gravity of diesel oil is 0.83 kg L−1. The amount of discharged SO2 for one year is 104.6 g. Based on the evaluation that the SO2 capture capacity at a reaction temperature of 400 °C is 182.5 gSO2 per LMnO2 filter (SO2 capture performance is 304.1 mgSO2 per gMnO2 at 400 °C and the density of MnO2 filter is 0.6 g cm−3), this means that a 1.0 L MnO2 filter can capture 182.5 g of SO2. Therefore, the required volume of the MnO2 filter was calculated as only 0.6 L for a diesel vehicle with 30
000 km of one year) is considered in which the concentrations of sulfur in the diesel oil and in lubricating oil are both 30 ppm. It is assumed that the SO2-capture capacity is not affected by the initial sulfur concentration in this evaluation. Fuel consumption of the diesel car is 7 liter per hundred kilometers. The specific gravity of diesel oil is 0.83 kg L−1. The amount of discharged SO2 for one year is 104.6 g. Based on the evaluation that the SO2 capture capacity at a reaction temperature of 400 °C is 182.5 gSO2 per LMnO2 filter (SO2 capture performance is 304.1 mgSO2 per gMnO2 at 400 °C and the density of MnO2 filter is 0.6 g cm−3), this means that a 1.0 L MnO2 filter can capture 182.5 g of SO2. Therefore, the required volume of the MnO2 filter was calculated as only 0.6 L for a diesel vehicle with 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 km traveled distance per one year, remarkably smaller than the traditional wash-coated desulfurization filter.7 Morever, as a part of the storage sites on MnO2 after regeneration are non-renewable16 and the desulfurization product (MnSO4) is always in great demand for the industrial and agricultural raw material, MnO2 filter could be suitable for the disposable desulfurization filter without being recycled every year for economy.23
000 km traveled distance per one year, remarkably smaller than the traditional wash-coated desulfurization filter.7 Morever, as a part of the storage sites on MnO2 after regeneration are non-renewable16 and the desulfurization product (MnSO4) is always in great demand for the industrial and agricultural raw material, MnO2 filter could be suitable for the disposable desulfurization filter without being recycled every year for economy.23
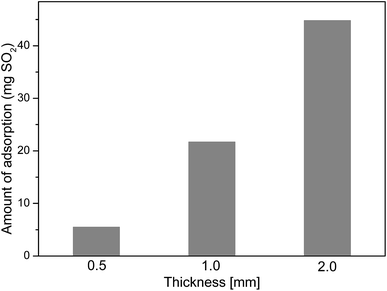
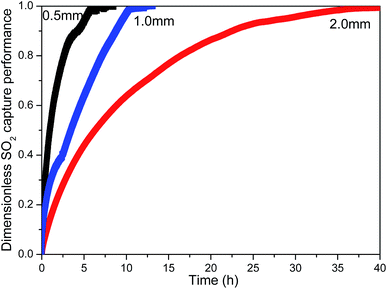
The effect on SO2 capture capacity of MnO2 filter with different thickness
In the traditional SO2 traps,4 the amount of SO2 capture was dependent on the quantity of sorbents in the desulfurization trap. In this study, similar experimental results were obtained. The amount of SO2 adsorption was proportional to the thickness of MnO2 filter, as shown in Fig. 6. The thickness of materials had much influence on the removal performance of diesel exhaust purifier.24 The dimensionless SO2 capture performance per unit mass (mgSO2 per gMnO2)–time (t) curve of MnO2 filter with different thickness was obtained by using the volumetric test-rig. As shown in Fig. 7, the shape of the curve is different when the packing height is changed. The SO2 capture rate of MnO2 filter decreased with the thickness. The 0.5 mm-thick MnO2 filter showed the highest SO2 capture rate than 2 mm-thick MnO2 filter. The reason is that the mass transfer resistance of gas diffusion in the thicker layer is larger than that in the thinner layer in the adsorbent.7Sulfate reaction mechanism
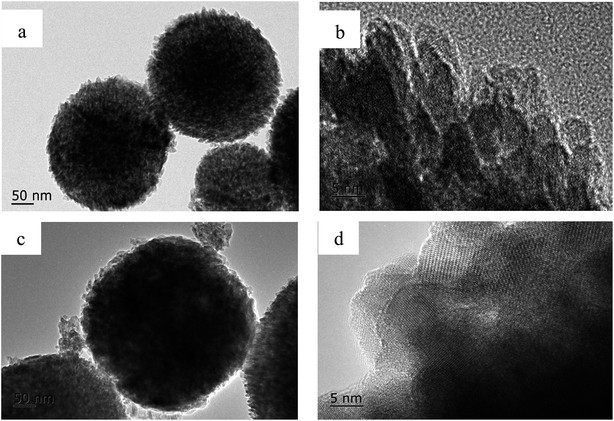
The sulfate reaction mechanism is very important for improving the efficiency of designing the desulfurization filter. In this study, in order to investigate the sulfate reaction mechanism between MnO2 filter and sulfur dioxide, the MnO2 filter with thin layer and low density was prepared to minimize the gas diffusion resistance in the traps. Moreover, a series of experiments were carried out at four reaction temperature conditions (200 °C, 300 °C, 350 °C and 400 °C) with low SO2 pressure of 40 Pa. Fig. 8 shows the conversion-time profiles of MnO2 filter for different temperature at low SO2 pressure of 40 Pa. As this figure shows, the reaction rate increases considerably at higher temperatures. The sulfate degree in the experimental conditions increased steeply at the beginning of the reaction, and then the slopes of sulfate degree curves become gradual after ∼5 min.The sulfate reaction between manganese dioxide and sulfur dioxide with a simple reaction path (MnO2 + SO2 → MnSO4) is a solid–gas non-catalytic reaction. Various models had been discussed to describe the mechanism of the solid–gas non-catalytic reaction.25 Among these models, the grain model was a suitable mathematical representation of gas–solid reactions in chemical and metallurgical industries consist of solid pellets produced from small particles or grains, which assumes that the reaction was accomplished on the surface of the fine and non-porous grains.26 TEM were employed to obtain surface features of the samples before and after desulfurization, the results as shown in the Fig. 9. Based on the TEM images in Fig. 9, the prepared MnO2 filter was formed with a series of smooth spherical grains and these grains are non-porous. Therefore, the grain model should be suitable for the solid–gas reaction between MnO2 filter and SO2.
In the grain model, the sulfate rate is controlled by four steps: external diffusion, gas reactant diffusion to the external surfaces of the solid particles from the bulk gas phase; surface reaction, gas reactant being adsorbed on the solid reactant surface; solid diffusion, gas reactant diffusion through the product layers; internal diffusion, gas reactant intra-particle transport through pores. For the whole systems, the controlling regime changes with the reaction time and was continuously consumed because the solid is a reactant.27 Most solid–gas non-catalytic reactions always presented two consecutive stages: the initial stage with the fast sulfate rate was controlled by the surface chemical reaction; the second stage with the slower sulfate rate was controlled by the product layer diffusion.28,29 According to the grain model, the relationship between the solid conversion equation X and the reaction time t at the chemical reaction control stage for the spherical particle and grain can be expressed by the following equation:
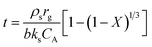 | (3) |
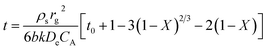 | (4) |
The grain model prediction of chemical reaction control, expressed in eqn (3), was applied to curve-fitting for the beginning of the sulfate process (the initial stage); and the grain model prediction of solid diffusion control, expressed in eqn (4), was applied to fit experimental data for the increase reaction time of sulfate process (the second stage). Fig. 10 shows the lines of the model predictions of chemical reaction control and solid diffusion control under the different reaction temperature conditions. From the results shown in Fig. 10, it can be seen that the prediction models fit the experimental data well for both control stages at 200 °C and 300 °C. At 350 °C and 400 °C, the prediction model of chemical reaction control fit the experimental data well at the initial stage of sulfate process. At higher temperature, the sulfate rate was controlled the zone of sulfate reaction between porous solid and gas became a relative narrow with the sulfate reaction proceeding and the surface reaction rate and diffusion rate were of the same order of magnitude.30 Therefore, the sulfate rate could be controlled by the chemical reaction and solid diffusion at the second stage at 350 °C and 400 °C. Generally, it can be safely concluded that the grain model is suitable for the solid–gas reaction between MnO2 filter and SO2 and the sulfate process could be divided into two control stages (the chemical reaction control stage and the solid diffusion control stage) under the experimental conditions.
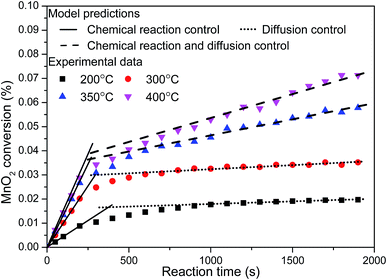 | ||
| Fig. 10 Experimental data and model predictions of MnO2 conversion under four reaction temperature conditions. | ||
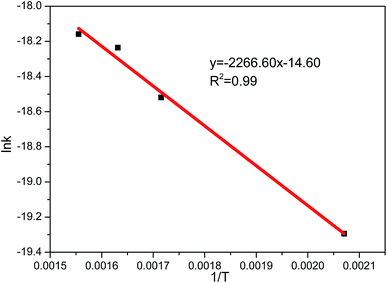
In order to determine the activation energy of the sulfate reaction between the MnO2 filter and pure SO2, the sulfate reaction rate constant of the chemical reaction control stage was measured at 200 °C, 300 °C, 350 °C and 400 °C. The corresponding Arrhenius plot is shown in Fig. 11. The apparent activation energy of 18.8 kJ mol−1 was calculated by using Arrhenius equation under the experimental conditions. Compared with the value of sulfate reaction of manganese oxide (33.5 kJ mol−1) reported by Tikhomirov,31 it can be concluded that in the pure SO2 atmosphere the MnO2 filter exhibits high activity for SO2 adsorption.
Conclusions
MnO2 filter is used for compact SO2 trap to protect the NOx removal catalyst against SO2 poisoning in diesel exhaust system. The SO2-capture performance of MnO2 filter without mutual diffusion resistance was measured by volumetric method under low temperature and low SO2 pressure conditions. MnO2 filters with different thickness and packing density were prepared, and the influences of reaction temperature, thickness of MnO2 filter and recycling characteristic of MnO2 filter on SO2 capture capacity were investigated under low temperature range from 200 °C to 400 °C and low SO2 pressure of 40 Pa conditions. The results of the present work clearly reveal that:• The maximal capacity of MnO2 filter was 304.1 mgSO2 per gMnO2 at 400 °C. The required volume of the MnO2 filter was estimated as only 0.6 L for a diesel car with 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 km traveled distance per one year and MnO2 filter could be suitable for the disposable desulfurization filter.
000 km traveled distance per one year and MnO2 filter could be suitable for the disposable desulfurization filter.
• The 0.5 mm-thick MnO2 filter showed the higher SO2 capture rate than 2 mm-thick MnO2 traps because the mass transfer resistance in the thicker layer was larger than that in the thinner layer.
• The grain model is suitable for the sulfate reaction, and the sulfate process can be divided into two control stages (the chemical reaction control stage and the solid diffusion control stage). The prediction models fit the experimental data well for both control stages. The apparent activation energy of 18.8 kJ mol−1 was calculated by using Arrhenius equation under the experimental conditions, which exhibits high sulfate activity between the MnO2 filter and pure SO2.
Acknowledgements
This research was supported by National Natural Science Foundation of China (NSFC) through International (Regional) Cooperation and Exchange Projects (Grant No. 21550110494) and Chinese Academy of Sciences President's International Fellowship Initiative (Grant No. 2016VTC068).References
- J. Ding, Q. Zhong and S. Zhang, Simultaneous removal of NOx and SO2 with H2O2 over Fe based catalysts at low temperature, RSC Adv., 2014, 4(11), 5394–5398 RSC.
- H. Moshiri, B. Nasernejad, H. Ale Ebrahim and M. Taheri, Solution of coupled partial differential equations of a packed bed reactor for SO2 removal by lime using the finite element method, RSC Adv., 2015, 5(23), 18116–18127 RSC.
- R. Y. Jiang, H. H. Shan, Q. Zhang, C. Y. Li and C. H. Yang, The influence of surface area of De-SOx catalyst on its performance, Sep. Purif. Technol., 2012, 95, 144–148 CrossRef CAS.
- L. Limousy, H. Mahzoul, J. F. Brilhac, P. Gilot, F. Garin and G. Maire, SO2 sorption on fresh and aged SOx traps, Appl. Catal., B, 2003, 42(3), 237–249 CrossRef CAS.
- X. C. Liu, Y. Osaka and H. Y. Huang, et al., Development of low-temperature desulfurization performance of a MnO2/AC composite for a combined SO2 trap for diesel exhaust, RSC Adv., 2016, 6(98), 96367–96375 RSC.
- Y. Osaka, T. Kito, N. Kobayashi, S. Kurahara, H. Huang, H. Yuan and Z. He, Removal of sulfur dioxide from diesel exhaust gases by using dry desulfurization MnO2 filter, Sep. Purif. Technol., 2015, 150, 80–85 CrossRef CAS.
- Y. Osaka, Y. Kohei, T. Tsujiguchi, A. Kodama, H. Huang and Z. He, Study on the Optimized Design of DeSOx Filter Operating at Low Temperature in Diesel Exhaust, J. Chem. Eng. Jpn., 2014, 47, 555–560 CrossRef CAS.
- R. H. Borgwardt, Kinetics of the reaction of sulfur dioxide with calcined limestone, Environ. Sci. Technol., 1970, 4(1), 59–63 CrossRef.
- Y. Osaka, S. Kurahara, N. Kobayashi, M. Hasatani and A. Matsuyama, Study on SO2-Absorption Behavior of Composite Materials for DeSO(x) Filter From Diesel Exhaust, Heat Transfer Eng., 2015, 36(3), 325–332 CrossRef CAS.
- B. Rubio and M. T. Izquierdo, Coal fly ash based carbons for SO2 removal from flue gases, Waste Manage., 2010, 30(7), 1341–1347 CrossRef CAS PubMed.
- H. H. Tseng and M. Y. Wey, Study of SO2 adsorption and thermal regeneration over activated carbon-supported copper oxide catalysts, Carbon, 2004, 42(11), 2269–2278 CrossRef CAS.
- H. H. Tseng, M. Y. Wey and C. H. Fu, Carbon materials as catalyst supports for SO2 oxidation: catalytic activity of CuO-AC, Carbon, 2003, 41(1), 139–149 CrossRef CAS.
- K. Tikhomirov, O. Kröcher, M. Elsener and A. Wokaun, MnOx–CeO2 mixed oxides for the low-temperature oxidation of diesel soot, Appl. Catal., B, 2006, 64(1–2), 72–78 CrossRef CAS.
- A. Rodas-Grapaín, J. Arenas-Alatorre, A. Gómez-Cortés and G. Díaz, Catalytic properties of a CuO–CeO2 sorbent-catalyst for De-SOx reaction, Catal. Today, 2005, 107–108, 168–174 CrossRef.
- Z. Yan, J. P. Wang, R. Q. Zou, L. L. Liu, Z. T. Zhang and X. D. Wang, Hydrothermal Synthesis of CeO2 Nanoparticles on Activated Carbon with Enhanced Desulfurization Activity, Energy Fuels, 2012, 26(9), 5879–5886 CrossRef CAS.
- X. Liu, Y. Osaka, H. Huang, A. Kodama, Z. He, Huhetaoli, X. Yang and Y. Chen, Development of high-performance SO2 trap materials in the low-temperature region for diesel exhaust emission control, Sep. Purif. Technol., 2016, 162, 127–133 CrossRef CAS.
- L. Y. Li and D. L. King, High-capacity sulfur dioxide absorbents for diesel emissions control, Ind. Eng. Chem. Res., 2005, 44(1), 168–177 CrossRef CAS.
- E. Tronconi and A. Beretta, The role of inter- and intra-phase mass transfer in the SCR-DeNOx reaction over catalysts of different shapes, Catal. Today, 1999, 52(2–3), 249–258 CrossRef CAS.
- C. Orsenigo, A. Beretta, P. Forzatti, J. Svachula, E. Tronconi, F. Bregani and A. Baldacci, Theoretical and experimental study of the interaction between NOx reduction and SO2 oxidation over DeNOx-SCR catalysts, Catal. Today, 1996, 27(1), 15–21 CrossRef CAS.
- J. Blanco, P. Avila, A. Bahamonde, M. Yates, J. L. Belinchón, E. Medina and A. Cuevas, Influence of the operation time on the performance of a new SCR monolithic catalyst, Catal. Today, 1996, 27(1), 9–13 CrossRef CAS.
- O. L. Lange and J. D. Tenhunen, Moisture content and CO2 exchange of lichens. II. Depression of net photosynthesis in Ramalina maciformis at high water content is caused by increased thallus carbon dioxide diffusion resistance, Oecologia, 1981, 51(3), 426–429 CrossRef CAS PubMed.
- J. X. Guo, L. Fan, J. F. Peng, J. Chen, H. Q. Yin and W. J. Jiang, Desulfurization activity of metal oxides blended into walnut shell based activated carbons, J. Chem. Technol. Biotechnol., 2014, 89(10), 1565–1575 CrossRef CAS.
- G. Centi and S. Perathoner, Dynamics of SO2 adsorption–oxidation in SOx traps for the protection of NOx adsorbers in diesel engine emissions, Catal. Today, 2006, 112(1–4), 174–179 CrossRef CAS.
- I. Binder-Begsteiger, Improved emission control due to a new generation of high-void-fraction SCR-DeNOx catalysts, Catal. Today, 1996, 27(1), 3–8 CrossRef CAS.
- A. Gómez-Barea and P. Ollero, An approximate method for solving gas–solid non-catalytic reactions, Chem. Eng. Sci., 2006, 61(11), 3725–3735 CrossRef.
- A. A. Ebrahimi, H. A. Ebrahim, M. Hatam and E. Jamshidi, Finite element solution for gas–solid reactions: application to the moving boundary problems, Chem. Eng. J., 2008, 144(1), 110–118 CrossRef CAS.
- F. Fang, Z. Li, N. Cai, X. Tang and H. Yang, AFM investigation of solid product layers of MgSO4 generated on MgO surfaces for the reaction of MgO with SO2 and O2, Chem. Eng. Sci., 2011, 66(6), 1142–1149 CrossRef CAS.
- P. Sun, J. R. Grace, C. J. Lim and E. J. Anthony, The effect of CaO sintering on cyclic CO2 capture in energy systems, AIChE J., 2007, 53(9), 2432–2442 CrossRef CAS.
- J. S. Dennis and R. Pacciani, The rate and extent of uptake of CO2 by a synthetic, CaO-containing sorbent, Chem. Eng. Sci., 2009, 64(9), 2147–2157 CrossRef CAS.
- C. Y. Wen and M. Ishida, Reaction rate of sulfur dioxide with particles containing calcium oxide, Environ. Sci. Technol., 1973, 7(8), 703–708 CrossRef CAS PubMed.
- K. Tikhomirov, O. Krocher, M. Elsener, M. Widmer and A. Wokaun, Manganese based materials for diesel exhaust SO2 traps, Appl. Catal., B, 2006, 67(3–4), 160–167 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2017 |