Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Generation of cloned adult muscular pigs with myostatin gene mutation by genetic engineering
Jin-Dan Kang†
a,
Seokjoong Kim†*b,
Hai-Ying Zhua,
Long Jina,
Qing Guoa,
Xiao-Chen Lia,
Yu-Chen Zhanga,
Xiao-Xu Xinga,
Mei-Fu Xuana,
Guang-Lei Zhanga,
Qi-Rong Luoa,
Yong Soo Kimc,
Cheng-Du Cuia,
Wen-Xue Lia,
Zheng-Yun Cuia,
Jin-Soo Kimd and
Xi-Jun Yin *a
*a
aJilin Provincial Key Laboratory of Transgenic Animal and Embryo Engineering, Yanbian University, Yanji, 133002, China. E-mail: yinxj33@msn.com
bToolGen, Inc., Byucksan Kyoungin Digital Valley 2-Cha, Geumcheon-Gu, Seoul 153-023, South Korea. E-mail: sj.kim@toolgen.com
cDepartment of Human Nutrition, Food and Animal Sciences, University of Hawaii, 1955 East-West Rd., Honolulu, HI 96822, USA
dCenter for Genome Engineering, Institute for Basic Science, Gwanak-ro 1, Gwanak-gu, Seoul 151-747, South Korea
First published on 21st February 2017
Abstract
Skeletal muscle is the most economically valuable tissue in meat-producing animals and enhancing muscle growth in these species may enhance the efficiency of meat production. Skeletal muscle mass is negatively regulated by myostatin (MSTN), and non-functional mutations of the MSTN gene in various animal species have led to dramatic hypermuscularity. This study was designed to assess the characteristics of male MSTN-knockout (KO) pigs. A transcription activator-like effector nuclease (TALEN) pair targeting exon 1 of the swine MSTN gene was constructed and used to transfect porcine fetal fibroblasts (PFFs). We obtained a cell line consisting of a 2-bp deletion in one allele and a 4-bp deletion in the other allele, and this was used as a donor to generate cloned pigs via SCNT, and delivered 18 live piglets. They developed and grew normally to sexual maturity. These MSTN-KO boars grew normally to adulthood and showed visually-clear hypermuscular characteristics, increased carcass dressing percentage and loin eye size, and decreased backfat thickness. These pigs may show greater meat production, as well as being used in animal models of human diseases.
Introduction
Myostatin (MSTN), also known as growth and differentiation factor 8 (GDF8), regulates skeletal muscle tissue homeostasis by inhibiting skeletal muscle growth.1,2 Homozygous knockout (KO) of MSTN in mice resulted in a marked increase in skeletal muscle mass, yielding the ‘mighty mouse’ phenotype. Naturally-occurring non-functional mutations in the MSTN gene have been reported to cause increased muscling in cattle,3–6 sheep,7 dogs,8 and humans.9Important breeding strategies in commercial pig production include increased lean growth efficiency, reduced back fat, and an increased percentage of prime cuts in the carcass. Recent developments in custom endonuclease techniques, such as zinc-finger nucleases (ZFNs), transcription activator-like effector nuclease (TALENs), and the clustered regularly interspaced short palindromic repeats (CRISPR)/CRISPR-associated protein (Cas) 9 (CRISPR/Cas9) system,10–13 appear useful in generating gene-edited pig breeds with such traits. Because loss of function of the MSTN gene has led to a double muscling phenotype in meat-producing species, including cattle3–5 and sheep,7 the MSTN gene was thought to be an ideal candidate for editing with recently available custom endonuclease techniques to determine the effect of loss of MSTN gene function on lean growth efficiency in pigs. One polymorphism in the MSTN promoter region has been shown to be associated with MSTN expression level and hypermuscularity in Pietrain pigs.14 Since the first report describing MSTN−/− boars in Nature News,15 two groups reported the generation of MSTN-mutant pigs using genome-editing techniques.16,17 These studies resulted in the generation of heterozygous MSTN-mutant pigs and produced homozygous MSTN-mutant pigs by combining natural breeding16 and MSTN-knockout pigs that died within 4 days after birth.17 There is no report regarding the production of sexually matured adult homozygous MSTN-mutant pigs.
This study describes the methods used to produce MSTN−/− male pigs with a genome-editing technology in combination with somatic cell nuclear transfer (SCNT). We have successfully generated 18 live homozygous MSTN-KO boars. Molecular tests proved the functional disruption of MSTN. These MSTN KO pigs exhibited muscle hypertrophy, over grown skeleton muscle and decreased fat mass, which duplicated the double muscling (DM) phenotype.
Experimental
Ethics statement
This study was carried out in strict accordance with the guidelines for the care and use of animals of Yanbian University. All animal experimental procedures were approved by the Committee on the Ethics of Animal Experiments at Yanbian University (Approval ID: 20130310).TALEN design and construction
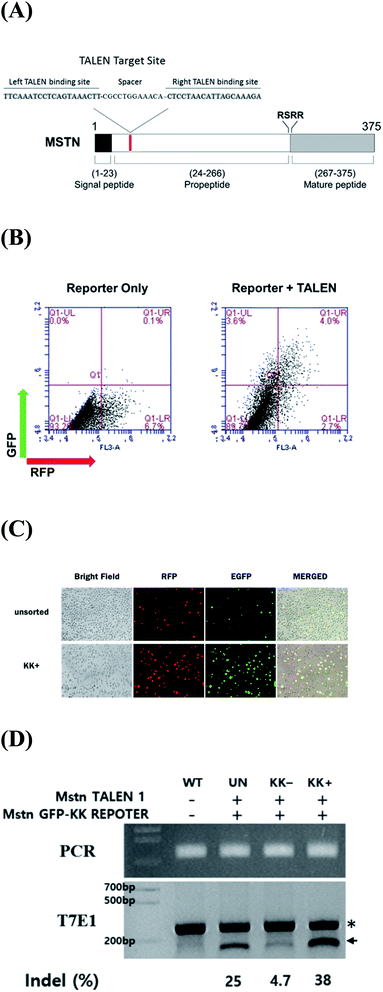
To induce DNA double-strand breaks in the MSTN locus, a potential transcription activator-like effector nuclease targeted site with a preferred configuration of 20-bp (left TALEN binding site) 12-bp (spacer) 20-bp (right TALEN binding site) within exon 1 of the swine MSTN gene was identified (Fig. 1A). TALEN expression vectors composed of TALEN repeat domains and a FokI nuclease domain containing a Sharkey RR or Sharkey DAS heterodimer mutation for the chosen target sequence were prepared via GoldenGate assembly as described.18 Plasmids harboring the MSTN-targeting TALEN (MSTN-TALEN) expression vectors were also prepared as described.18 To select cells containing TALEN-mediated mutations by magnetic separation, an episomal surrogate reporter containing the chosen target sequence was also constructed.18 Plasmids encoding MSTN-TALENs and the surrogate reporter used in this study were obtained from ToolGen (Seoul, South Korea). HEK293 cells were cultured in Dulbecco's modified Eagle medium (DMEM, HyClone) supplemented with 1% nonessential amino acids, 100 U mL−1 penicillin, 100 mg mL−1 streptomycin, and 10% fetal bovine serum (FBS, GIBCO, 10099-141). Surrogate reporter assays were performed by transfecting HEK293 cells and placing them in 48 well plates with plasmids encoding left and right MSTN-TALENs (50 ng each) and a surrogate reporter (100 ng). The expression of fluorescent proteins was assayed 48 h later by flow cytometry.Cell culture, transfection and selection
Porcine fetal fibroblasts (PFF) were established from a day 40 post-conception male fetus of mixed breed, as described.19 PFFs were cultured for four passages in DMEM (HyClone) supplemented with 1% nonessential amino acids, 100 U mL−1 penicillin, 100 mg mL−1 streptomycin, and 15% FBS. Aliquots of 1 × 106 PFF cells were electroporated with 40 μg plasmid DNA encoding the left and right MSTN-TALENs and the surrogate reporter at a weight ratio of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, respectively, according to the manufacturer's protocol. The transfected cells were cultured for 2 days at 37 °C and subjected to magnetic separation. Briefly, trypsinized cell suspensions were incubated with magnetic bead-conjugated antibody against H-2Kk (MACSelect Kk microbeads; Miltenyi Biotech, Cologne, Germany) for 15 min at 4 °C. Labeled cells were separated using a MACS LS column (Miltenyi Biotech), according to the manufacturer's protocol.
1, respectively, according to the manufacturer's protocol. The transfected cells were cultured for 2 days at 37 °C and subjected to magnetic separation. Briefly, trypsinized cell suspensions were incubated with magnetic bead-conjugated antibody against H-2Kk (MACSelect Kk microbeads; Miltenyi Biotech, Cologne, Germany) for 15 min at 4 °C. Labeled cells were separated using a MACS LS column (Miltenyi Biotech), according to the manufacturer's protocol.
Generation of fetuses and offspring by SCNT
Nuclear transfer was performed as described.19 Briefly, mature eggs showing the first polar body were cultured for 1 h in medium supplemented with 0.4 mg mL−1 demecolcine and 0.05 M sucrose, with the sucrose added to enlarge the perivitelline space of the eggs. Treated eggs with a protruding membrane were transferred to medium containing 5 mg mL−1 cytochalasin B (CB) and 0.4 mg mL−1 demecolcine, and the protrusions were removed with a beveled pipette. A single donor PFF cell was injected into the perivitelline space of each egg and the cells were electrically fused using two direct current pulses of 150 V mm−1 for 50 μs each in 0.28 M mannitol supplemented with 0.1 mM MgSO4 and 0.01% PVA. The fused eggs were cultured in NCSU-37 medium for 1 h before electro activation. These cells were subsequently cultured in 5 mg mL−1 of CB-supplemented medium for 4 h, followed by activation of the fused eggs by two direct current pulses of 100 V mm−1 for 20 μs each in 0.28 M mannitol supplemented with 0.1 mM MgSO4 and 0.05 mM CaCl2. Activated eggs were cultured in medium for 7 days in an atmosphere of 5% CO2 and 95% air at 39 °C. Cleavage and blastocyst formation were evaluated on day 2 and 7, respectively. Cloned embryos at the one-cell stage after fusion or at the two- to four-cell stage after a day of culture were transferred into the oviducts of naturally cycling gilts on the first day of standing estrus. Recipient pigs to which 1st SCNT embryos had been transferred were euthanized at day 26–36 of gestation, and the fetuses were collected. These fetuses were used to confirm the MSTN mutations. The MSTN-KO fetuses were used to generate rejuvenated porcine fetal fibroblasts for the 2nd round of SCNT to produce boars. Pregnancy was assessed ultrasonographically on day 25. Cloned piglets were delivered naturally or by inducing labor with intramuscular injections of prostaglandin F2 alpha (Ningbo, China) on day 113 of gestation.T7 endonuclease 1 (T7E1) assays and sequencing
T7E1 assays were performed as described.20 Briefly, genomic DNA was isolated using DNeasy Blood & Tissue Kits (Qiagen, Germany), according to the manufacturer's instructions. The region of DNA containing the nuclease target site was PCR amplified using the primers in Table 1. The amplicons were denatured by heating and annealed to form heteroduplex DNA, which was treated with 5 units of T7E1 (New England Biolabs) for 20 min at 37 °C and electrophoresed on agarose gels. To confirm the mutation introduced by TALEN to a target allele, PCR amplicons spanning the target sites were purified using aGel Extraction Kits (Macherry-Nalgen, Germany) and cloned into the T-Blunt vector using T-Blunt PCR Cloning Kits (SolGent, Daejeon, Korea). The cloned inserts were again amplified using the same primers and sequenced.| Direction | Sequence (5′ to 3′) | |
|---|---|---|
| First | Forward | CTGGTCCCGTGGATCTGAATG |
| Reverse | GATCGTTTCCGTCGTAGCGTG | |
| Second | Forward | GAATGAGAACAGCGAGCAAAAGG |
| Reverse | CATCTTCCAAGGAGCCATCAC |
Western blot assays
Biceps brachii muscle samples were homogenized in RIPA-based lysis buffer (Millipore, USA) containing complete EDTA-free protease and phosphatase inhibitor cocktails (Roche, Germany). Lysed samples were centrifuged at 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000g for 10 min at 4 °C, the supernatants were collected, and their protein concentrations were determined using a Pierce micro protein assay kit (Thermo Fisher Scientific). Proteins were subsequently separated by SDS-PAGE and blotted onto nitrocellulose membranes (Bio-Rad Laboratories, USA), as described.21 The membranes were incubated with primary rabbit anti-MSTN (1
000g for 10 min at 4 °C, the supernatants were collected, and their protein concentrations were determined using a Pierce micro protein assay kit (Thermo Fisher Scientific). Proteins were subsequently separated by SDS-PAGE and blotted onto nitrocellulose membranes (Bio-Rad Laboratories, USA), as described.21 The membranes were incubated with primary rabbit anti-MSTN (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000 dilution; Sigma-Aldrich) and anti-GAPDH (Sigma-Aldrich) antibodies. Band intensities were estimated by densitometry, with MSTN band intensity normalized to GAPDH band intensity in the same sample.
1000 dilution; Sigma-Aldrich) and anti-GAPDH (Sigma-Aldrich) antibodies. Band intensities were estimated by densitometry, with MSTN band intensity normalized to GAPDH band intensity in the same sample.
Immunohistochemical and histochemical staining of muscle
Immediately after slaughter, samples of the longissimus dorsi and biceps femoris muscles were immersed in 4% paraformaldehyde solution for 24 hours and transferred to 70% ethanol. Sections were embedded in paraffin and rehydrated in PBS prior to antigen retrieval in citrate buffer (1.8 mM citric acid, 8.2 mM sodium citrate, pH 6.0). The sections were washed twice with PBS, endogenous peroxidase activity was blocked by incubation with 0.3% hydrogen peroxide for 5 min, and the samples were again washed in PBS. Sections were incubated with monoclonal anti-myosin (clone MY-32, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500, M4276, Sigma-Aldrich) overnight at 4 °C, washed extensively with PBS, and incubated for 1 h with biotin-labeled goat anti-mouse IgG (SA1021, Boster) prior to extensive washing with PBS and visualization with DAB. Basic muscle morphology was assessed by H&E staining of samples according to standard protocols. Gum was used to mount the samples. Digital images were captured at 200× magnification using a Leica DM5000B microscope. Six sections of 50 contiguous myofibers for each muscle were circled, with an average of 300 fibers evaluated for fiber cross-sectional area and diameter. Data were analyzed using Image J software (NIH).
500, M4276, Sigma-Aldrich) overnight at 4 °C, washed extensively with PBS, and incubated for 1 h with biotin-labeled goat anti-mouse IgG (SA1021, Boster) prior to extensive washing with PBS and visualization with DAB. Basic muscle morphology was assessed by H&E staining of samples according to standard protocols. Gum was used to mount the samples. Digital images were captured at 200× magnification using a Leica DM5000B microscope. Six sections of 50 contiguous myofibers for each muscle were circled, with an average of 300 fibers evaluated for fiber cross-sectional area and diameter. Data were analyzed using Image J software (NIH).
Hematoxylin and eosin staining of adipose tissues
Fat pads from wild-type and MSTN−/− pigs were fixed in 10% formalin for 24 h at room temperature and dehydrated in an automated tissue processor. The fat pads were embedded in paraffin, sectioned at 5 μm, deparaffinized, rehydrated, and stained with hematoxyl in for 5 min. The sections were rinsed in running tap water and stained with eosin for 4 min. H&E-stained sections were dehydrated and mounted. Images were captured using a Leica DM5000B microscope.Statistical analysis
Data are presented as the mean ± SD and are derived from at least three independent experiments. Groups were compared using Student's t tests, with P values <0.05 considered statistically significant.Results and discussion
Characterization of MSTN-targeting TALENs and validation of activity
The activity of constructed TALENs was evaluated using a surrogate reporter system. In these assays, the induction of small indel mutations in the TALEN target sequence, inserted in front of the inactive eGFP and H-2Kk cell surface reporter genes, can restore the expression of these genes, while mRFP is constitutively expressed independent of TALEN activity. HEK293 cells were transformed with MSTN-targeting TALENs and surrogate reporter plasmids. Cells expressing eGFP were detected only upon the co-transfection of TALENs and reporter plasmids, validating the activity of MSTN-TALENs in HEK293 cells (Fig. 1B). Incubation of magnetic beads (MACSelect Kk microbeads; Miltenyi Biotech, Germany) conjugated to anti-H-2Kk antibody with HEK293 cells and elution through a MACS LS column (Miltenyi Biotech) led to the separation of H-2Kk-expressing cells.18,22 These H-2Kk-positive cells were enriched with eGFP-expressing cells (Fig. 1C).Additionally, to directly evaluate the extent of TALEN-induced mutations, genomic DNA isolated from sorted and unsorted cell populations were subjected to T7E1 assays. PCR amplified a 257 bp band, whereas PCR products from cells transformed with MSTN-TALEN showed a cleaved band upon T7E1 digestion, confirming the presence of mutations in the transformed cells (Fig. 1D). The MSTN mutation frequency was higher in magnetically sorted than in unsorted cells (38% vs. 25%, Fig. 1D).
Production of MSTN-knockout fetuses by SCNT
PFF cells collected from males were transfected with MSTN-TALENs and reporter plasmids by electroporation, and the transfected cells were enriched magnetically as described. H-2Kk-positive cells expressing both RFP and eGFP (Fig. 2) were cultured for two additional days, and were used as donor cells in SCNT.The competency of reconstructed embryos cultured in vitro was investigated by examining the development of blastocysts. The rates of blastocyst development (20.4% vs. 21.6%, P < 0.05) and the mean numbers of cells (36.7 ± 3.8 vs. 38.1 ± 8.1, P < 0.05) were similar in embryos reconstructed with transformed and non-transformed donor cells (Table 2).
| Donor cell type | No. of embryos | No. of 2–4 cell (%) | No. of blastocysts (%) | Numbers of cells in blastocysts (mean ± SEM) |
|---|---|---|---|---|
| Non-transfected PFF | 259 | 218 (84.2) | 56 (21.6) | 38.1 ± 8.1 |
| Transfected PFF | 260 | 220 (84.6) | 53 (20.4) | 36.7 ± 3.8 |
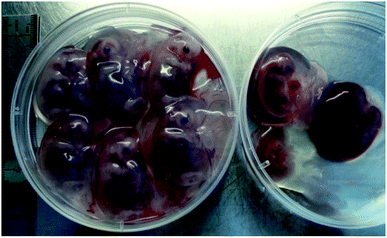
To produce MSTN-mutant fetuses, 428 cloned embryos with H-2Kk-positive PFF cells were transferred into two surrogate mothers (R-19 and R-29). Five fetuses were surgically collected from R-19 after 27 days of gestation, and nine fetuses (Fig. 3) were surgically collected from R-29 after 36 days of gestation (Table 3). Fibroblast cell lines from these fetuses were established, expanded, and cultured, with cells at an early stage of passage preserved in liquid nitrogen. In the current study, the MACS-separated fetal fibroblast cells were used in SCNT, with results indicating that the competence of reconstructed embryos derived from the MACS-separated fetal fibroblast cells did not differ markedly from that of untreated embryos, with a similar rate of blastocyst development and similar numbers of blastocysts. Furthermore, pregnancies were maintained for both surrogates implanted with reconstructed embryos derived from the MACS-separated fetal fibroblast cells, while other studies reported surrogate pregnancy rates of 36–90%.12,16,17,23 Taken together, our results indicate that the surrogate reporter-based MACS is effective in selecting nuclear donor cells with nuclease-mediated gene modification.
| Recipient | No. of embryos transferred | No. of fetuses | Days of gestation | Fetal fibroblast cell lines | Mono-allelic cell lines (%) | Bi-allelic cell lines (%) |
|---|---|---|---|---|---|---|
| R-19 | 232 | 5 | 27 | 19-1, 19-2, 19-3, 19-4, 19-5 | 19-1, 19-4 (40) | 19-3 (20) |
| R-29 | 196 | 9 | 36 | F1, F2, F3, F4, F5, F6, F7, F8, F9 | F3, F5, F9 (33.3) | F1, F2 (22.2) |
Detection of mutations and genotyping of MSTN-mutant fetuses
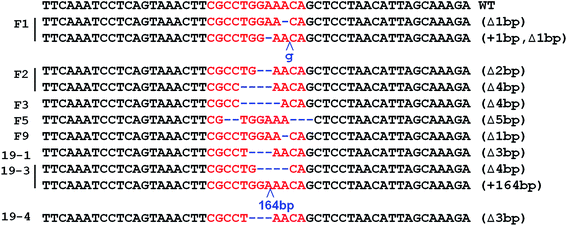
The fibroblast cell lines from the 14 fetuses were analyzed individually by T7E1 assays and by sequencing PCR products covering the target locus. Mutations at the target locus (MSTN gene) were present in 8 of the 14 fetuses (57%; Table 3). Three fetuses (F1, F2, and 19-3) carried biallelic mutations and five (F3, F5, F9, 19-1, and 19-4) carried monoallelic mutations in the MSTN gene. Among the fetuses with biallelic MSTN mutations, fetus F1 had a 1-bp deletion and a 1-bp insertion in one allele, and a 1-bp deletion in the other allele; fetus F2 had 2 and 4-bp deletions in the two alleles; and fetus 19-3 had a 4-bp deletion in one allele and a 164-bp insertion in the other allele (Fig. 4).Generation of biallelic MSTN gene knockout males by SCNT
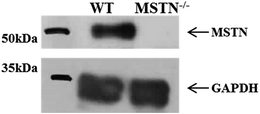
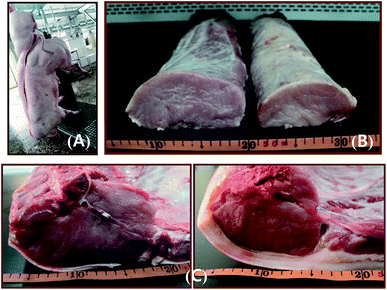
F2 fetus-derived PFF cells carrying biallelic MSTN mutations were used as donor cells to produce MSTN−/− piglets by SCNT. A total of 646 cloned embryos were transferred to three surrogates in estrus, resulting in the delivery of 18 live and two stillborn piglets (Table 4). At birth, these piglets were hardly distinguishable phenotypically from wild-type piglets. However, starting at 2 month of age, they showed visually-clear hypermuscular characteristics (Fig. 5). DM pigs showed some health-associated issues, including early death due to increased susceptibility to stress and umbilical hernia, although it was not clear whether these issues were associated with the MSTN−/− phenotype. Additional studies are required to determine how MSTN−/− in pigs affects their general health and production traits associated with animal physiology. Western blotting of muscle sample showed no expression of MSTN precursor in the mutant piglet from surrogate 3 sacrificed at 1 month (Fig. 6). By contrast, MSTN precursor was present in a muscle sample of 3 wild-type control pigs of the same age.In generating MSTN−/− boars, we used a two-step SCNT method (recloning), in which fetal fibroblast cells derived from the 1st SCNT were the nuclear donors for the 2nd SCNT. Previous studies have shown that this recloning method is very efficient in generating animals, especially livestock, with targeted gene mutations.24,25 Compared with clonally selected transformed fibroblasts, fetal fibroblasts derived from the 1st SCNT have some advantages in the generation of gene-edited animals. Selection of nuclear donor cells with genetic modifications frequently involves drug treatment of cells in long-term culture. These cultures are inevitably at greater risk for cell exhaustion and senescence,26 factors associated with chromosomal aberrations.27
Growth characteristics and histological examination of MSTN-knockout boars
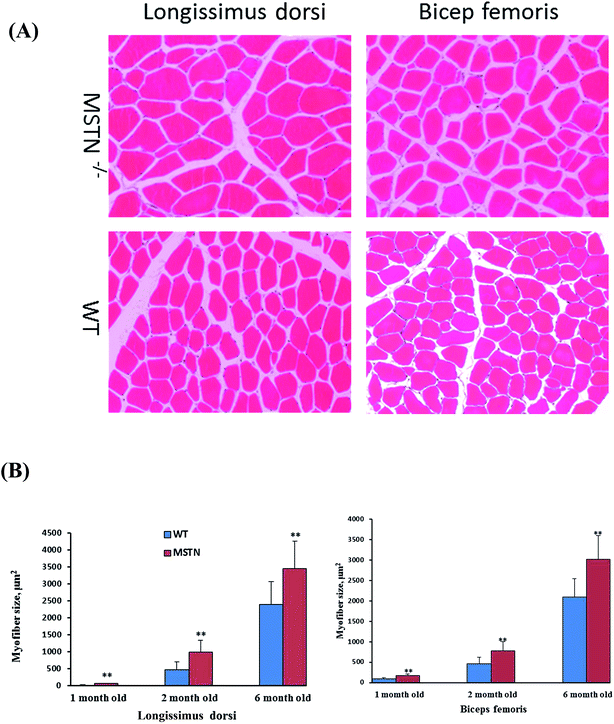
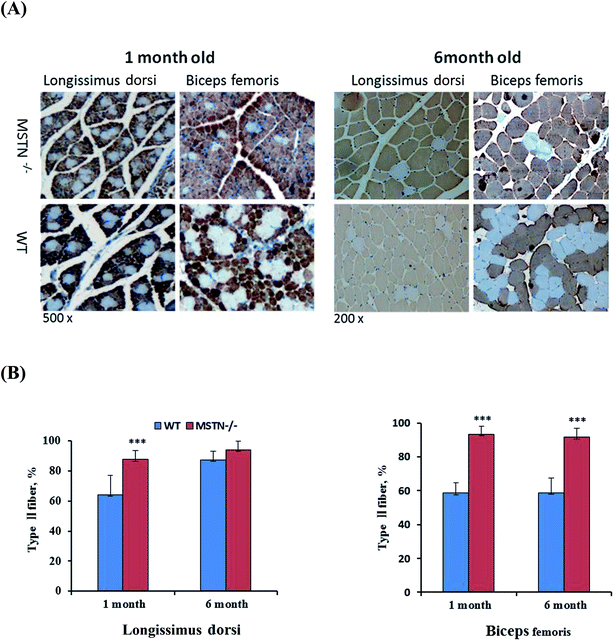
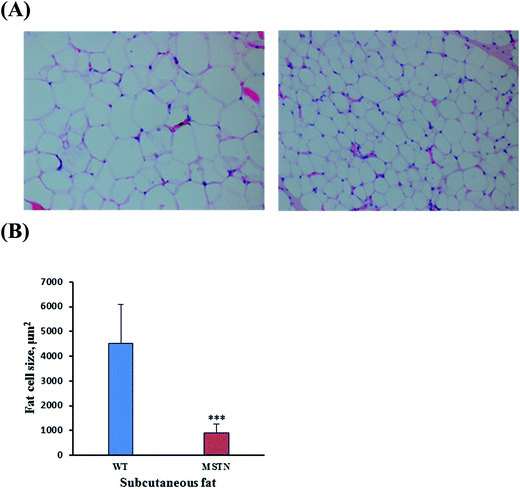
At birth, MSTN−/− pigs were relatively indistinguishable phenotypically from wild-type pigs. At around age 2 months, however, MSTN−/− pigs started to show the hypermuscular phenotype, particularly in the hip area. Examination of four MSTN−/− boars sacrificed at 6 months showed the carcass dressing percentage of 83.7%, a cross-sectional area of the loin eye muscle at the last rib of 46.3 cm2, and a back fat thickness at the last rib of 10.7 mm (Fig. 7). In comparison, wild-type boars of similar weight showed the average carcass dressing percentage of 73.5%, cross-sectional area of the loin eye muscle at the last rib of 35.9 cm2, and back fat thickness at the last rib of 26.4 mm (Fig. 7). The differences observed between MSTN−/− and wild-type boars strongly indicate that the MSTN−/− genotype induces hypermuscular characteristics.Myofiber sizes in the longissimus dorsi and biceps femoris muscles were compared in MSTN−/− and wild-type boars aged 1, 2, and 6 months (Fig. 8A). The average sizes of longissimus myofibers of MSTN−/− pigs aged 1, 2, and 6 months were 66.5 ± 11.7, 989.8 ± 346.0, and 3462.3 ± 810.4 μm2, significantly larger than those of the average myofiber sizes in age-matched wild-type pigs were 22.9 ± 9.4, 483.2 ± 233.6, and 2393.1 ± 680.4 μm2, respectively. Similarly, the average sizes of biceps femoris myofibers in MSTN−/− pigs aged 1, 2, and 6 months were 176.4 ± 42.7, 778.1 ± 222.1, and 3019.9 ± 580.0 μm2, respectively, whereas the average myofiber sizes in age-matched control pigs were 95.2 ± 25.0, 470.1 ± 159.6, and 2097.2 ± 447.2 μm2 (P < 0.01, Fig. 8B), respectively. Type II fiber distribution in the longissimus dorsi and biceps femoris muscles were also compared in 1- and 6-month-old MSTN−/− and wild-type pigs (Fig. 9A). The percentages of type II myofibers in the longissimus dorsi muscles were 87.5 ± 6.0% and 93.8 ± 6.0% in MSTN−/− pigs aged 1 and 6 months, respectively, compared with 64.0 ± 13.1% (P < 0.001) and 87.4 ± 5.8% (P > 0.05), respectively, in age-matched controls. The percentages of type II myofibers in the biceps femoris muscles were 93.3 ± 4.5% and 91.5 ± 5.4% in MSTN−/− pigs aged1 and 6 months, respectively, but were 58.7 ± 5.8% (P < 0.001) and 58.7 ± 8.7% (P < 0.001), significantly higher than those in age-matched wild-type pigs (Fig. 9B). Mean adipocyte size was much lower in 6 month-old MSTN−/− than in 6 month-old wild-type boars (904.9 ± 372.0 vs. 4522.7 ± 154.6 cm2, P < 0.001, Fig. 10A and B), indicating that adipocyte hypertrophy was more suppressed in MSTN−/− than in control boars.
As expected, the MSTN-KO piglets showed characteristics of the DM phenotype at birth, such as prominent muscular protrusion with intermuscular boundaries and clearly visible grooves, mostly in the proximal fore and hind quarter regions. These DM characteristics became more prominent as the piglets grew. One boar sacrificed aged 6 months showed clear evidence of DM characteristics, including increased carcass dressing percentage, cross-sectional area and reduced back fat thickness compared with an age-matched wild-type boar. Nevertheless, the testes of 6 month-old DM boars were found to develop normally, allowing viable sperm to be collected from the caudal epididymis; these sperm were able to fertilize mature oocytes in vitro and artificial inseminated for two estrus recipients and one of whom was pregnant delivered four healthy piglets (unpublished data).
MSTN−/− piglets had higher proportions of fast-glycolytic muscle fiber type, similar to findings in DM cattle28 and MSTN−/− mice.4,29 The increased muscle size in MSTN heterozygous and homozygous knockout mutant mice, relative to wild-type mice, is thought to be due to fiber hyperplasia and hypertrophy of skeletal muscle fibers.2 Hypertrophy of skeletal muscle fibers was also observed in 6 month-old and MSTN−/− piglets, consistent with results in DM cattle showing a 20–25% increase in muscle mass, with the increase apparently resulting from muscle fiber hyperplasia rather than hypertrophy.30–32
Conclusions
In conclusion, using a new genome-editing technology in combination with SCNT, we have successfully generated homozygous MSTN-KO boars. These MSTN−/− boars developed and grew normally to sexual maturity, showing DM phenotypic characteristics observed in cattle and sheep. Homozygous DM pigs may show enhanced meat production, as well as being useful as animal models for human disease.Acknowledgements
This work was supported by the State Key Development Program for Basic Research of China (Grant No. 20150622005JC) and ToolGen, Inc. We thank Hanji and Longxing Pig farms for providing surrogate pig recipients and their enthusiasm and support for this research.Notes and references
- S. J. Lee, Annu. Rev. Cell Dev. Biol., 2004, 20, 61 CrossRef CAS PubMed.
- C. Mcpherron, A. M. Lawler and S. J. Lee, Nature, 1997, 387, 83 CrossRef PubMed.
- L. Grobet, L. J. Martin, D. Poncelet, D. Pirottin, B. Brouwers, J. Riquet, A. Schoeberlein, S. Dunner, F. Menissier, J. Massabanda, R. Fries, R. Hanset and M. Georges, Nat. Genet., 1997, 17, 71 CrossRef CAS PubMed.
- A. Hennebry, C. Berry, V. Siriett, P. O'Callaghan, L. Chau, T. Watson, M. Sharma and R. Kambadur, Am. J. Physiol.: Cell Physiol., 2009, 296, 525 CrossRef PubMed.
- C. Mcpherron and S. J. Lee, Proc. Natl. Acad. Sci. U. S. A., 1997, 94, 12457 CrossRef.
- J. A. Smith, A. M. Lewis, P. Wiener and J. L. Williams, Anim. Genet., 2000, 31, 306 CrossRef CAS PubMed.
- A. Clop, F. Marcq, H. Takeda, D. Pirottin, X. Tordoir, B. Bibe, J. Bouix, F. Caiment, J. M. Elsen, F. Eychenne, C. Larzul, E. Laville, F. Meish, D. Milenkovic, J. Tobin, C. Charlier and M. Georges, Nat. Genet., 2006, 38, 813 CrossRef CAS PubMed.
- D. S. Mosher, P. Quignon, C. D. Bustamante, N. B. Sutter, C. S. Mellersh, H. G. Parker and E. A. Ostrander, PLoS Genet., 2007, 3, 245 Search PubMed.
- M. Schuelke, K. R. Wagner, L. E. Stolz, C. Hubner, T. Riebel, W. Komen, T. Braun, J. F. Tobin and S. J. Lee, N. Engl. J. Med., 2004, 350, 2682 CrossRef CAS PubMed.
- J. C. Miller, S. Tan, G. Qiao, K. A. Barlow, J. Wang, D. F. Xia, X. Meng, D. E. Paschon, E. Leung, S. J. Hinkley, G. P. Dulay, K. L. Hua, I. Ankoudinova, G. J. Cost, F. D. Urnov, H. S. Zhang, M. C. Holmes, L. Zhang, P. D. Gregory and E. J. Rebar, Nat. Biotechnol., 2011, 29, 143 CrossRef CAS PubMed.
- J. Wood, T. W. Lo, B. Zeitler, C. S. Pickle, E. J. Ralston, A. H. Lee, R. Amora, J. C. Miller, E. Leung, X. Meng, L. Zhang, E. J. Rebar, P. D. Gregory, F. D. Urnov and B. J. Meyer, Science, 2011, 307, 1126 Search PubMed.
- D. F. Carlson, W. Tan, S. G. Lillico, D. Stverakova, C. Proudfoot, M. hristan, D. F. Voytas, C. R. Long, C. B. Whitelaw and S. C. Fahrenkrug, Proc. Natl. Acad. Sci. U. S. A., 2012, 109, 17382 CrossRef CAS PubMed.
- L. Cong, F. A. Ran, D. Cox, S. Lin, R. Barretto, N. Habib, P. D. Hsu, X. Wu, W. Jiang, L. A. Marraffini and F. Zhang, Science, 2013, 339, 819 CrossRef CAS PubMed.
- A. Stinckens, T. Luyten, J. Bijttebier, K. Van den Maagdenberg, D. Dieltiens, S. Janssens, S. De Smet, M. Georges and N. Buys, Anim. Genet., 2008, 39, 1365 CrossRef PubMed.
- D. Cyranoski, Nature, 2015, 523, 13 CrossRef CAS PubMed.
- L. Qian, M. Tang, J. Yang, Q. Wang, C. Cai, S. Jiang, H. Li, K. Jiang, P. Gao, D. Ma, Y. Chen, X. An, K. Li and W. Cui, Sci. Rep., 2015, 5, 14435 CrossRef CAS PubMed.
- K. Wang, H. Ouyang, Z. Xie, C. Yao, N. Guo, M. Li, H. Jiao and D. Pang, Sci. Rep., 2015, 5, 16623 CrossRef CAS PubMed.
- H. Kim, M. S. Kim, G. Wee, C. I. Lee, H. Kim and J. S. Kim, PLoS One, 2013, 8, 2235 Search PubMed.
- X. J. Yin, T. Tani, I. Yonemura, M. Kawakami, K. Miyamoto, R. Hasegawa, Y. Kato and Y. Tsunoda, Biol. Reprod., 2002, 67, 442 CrossRef CAS PubMed.
- H. J. Kim, H. H. Lee, H. Kim, S. W. Cho and J. S. Kim, Genome Res., 2009, 19, 1279 CrossRef CAS PubMed.
- S. Hu, N. Wei, W. Sai, Z. Hui, X. Cao and J. Qiao, Biotechnol. Lett., 2011, 33, 1949 CrossRef CAS PubMed.
- H. Kim, E. Um, S. R. Cho, C. Jung, H. Kim and J. S. Kim, Nat. Methods, 2011, 8, 941 CrossRef CAS PubMed.
- J. Hauschild, B. Petersen, Y. Santiago, A. L. Queisser, J. W. Carnwath, A. Lucas-Hahn, L. Zhang, X. Meng, P. D. Gregory, R. Schwinzer, G. J. Cost and H. Niemann, Proc. Natl. Acad. Sci. U. S. A., 2011, 108, 12013 CrossRef CAS PubMed.
- T. Fujimura, Y. Takahagi, T. Shigehisa, H. Nagashima, S. Miyagawa, R. Shirakura and H. Murakami, Mol. Reprod. Dev., 2008, 75, 1372 CrossRef CAS PubMed.
- K. T. Suzuki, Y. Isoyama, K. Kashiwagi, T. Sakuma, H. Ochiai, N. Sakamoto, N. Furuno, A. Kashiwagi and T. Yamamoto, Biol. Open, 2013, 2, 448 CrossRef CAS PubMed.
- L. Hayflick and P. S. Moorhead, Exp. Cell Res., 1961, 25, 585 CrossRef CAS PubMed.
- R. A. DePinho, Nature, 2000, 408, 248 CrossRef CAS PubMed.
- D. Stavaux, T. Art, K. McEntee, M. Reznick and P. Lekeux, Zentralbl Veterinarmed A, 1994, 41, 229 CrossRef CAS PubMed.
- S. Girgenrath, K. Song and L. A. W. hittemore, Muscle Nerve, 2005, 31, 34 CrossRef CAS PubMed.
- E. Casas, J. W. Keele, S. D. Shackelford, M. Koohmaraie, T. S. Sonstegard, T. P. Smith, S. M. Kappes and R. T. Stone, J. Anim. Sci., 1998, 76, 468 CrossRef CAS PubMed.
- M. Manceau, J. Gros, K. Savage, V. Thomé, A. Mcpherron, B. Paterson and C. Marcelle, Genes Dev., 2008, 22, 668 CrossRef CAS PubMed.
- Y. S. Lee and S. J. Lee, Proc. Natl. Acad. Sci. U. S. A., 2013, 110, 3713 CrossRef PubMed.
Footnote |
| † Both authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2017 |