Open Access Article
Open Access ArticleInhibitory effect of a genistein derivative on pigmentation of guinea pig skin
Quancheng Zhouab,
Chuanxing Fengb and
Zheng Ruan *a
*a
aSchool of Food Science and Technology, Nanchang University, Nanchang, 330031, China. E-mail: ruanzheng@ncu.edu.cn; Fax: +86-791-88304402
bSchool of Agricultural Engineering and Food Science, Shandong University of Technology, Zibo, 255049, China
First published on 23rd January 2017
Abstract
In this study, to improve the properties of genistein, a genistein derivative (GD) was designed and synthesized by combining it with 1-benzylpiperazine. Then, the effects of GD on skin pigmentation in guinea pigs induced by ultraviolet B (UVB) radiation were studied. The guinea pigs were divided into a model group and a GD group. The GD group was coated with different doses of GD for 30 days. Biopsy specimens were taken from the treated skin. Tyrosinase (TYR), malondialdehyde (MDA), superoxide dismutase (SOD) and mammalian target of rapamycin (mTOR), as well as AMP-activated protein kinase (AMPK), were detected according to the commercial kits’ protocols. The distribution and secretion of melanin were assayed by hematoxylin-eosin (HE) stain and Fontana-Masson stain methods. The positive staining index of cyclooxygenase-2 (COX-2) and AMPK was detected using an immunohistochemical technique (IHC). UVB radiation resulted in a marked increase in the content of TYR, mTOR and MDA, and the expression of COX-2, and promoted pigmentation in guinea pig skins, but decreased the SOD and AMPK levels. The effect of GD was similar to that of arbutin and could reverse these changes and the effect of GD was the best when its concentration was 2%. The SOD and AMPK levels were increased by approximately 46% and 40%, meanwhile, the TYR, mTOR and MDA levels and COX-2 expression were reduced by approximately 30%, 34%, 29% and 30%, respectively. This study shows that GD can be used in the treatment of UVB-induced pigmentation in guinea pigs.
Introduction
With aging and environmental factors, skin will develop a variety of pigmentations, such as chloasma, senile plaque, and hyperpigmentation.1 Meanwhile, overexposure to UVB radiation can lead to hyperpigmentation.2 So, in the past few years, many melanin inhibitors have been isolated from natural sources, including flavonoids, quercetin, arbutin, Centella asiatica Urb., and Morus alba roots. Asiaticoside is isolated from Centella asiatica Urb., and it can remarkably decrease the tyrosinase (TYR) and malondialdehyde (MDA) content and increase superoxide dismutase (SOD) activity.3 Arbutin has been widely used as a tyrosinase inhibitor. It exhibits dose-dependent inhibitory effects on TYR activity in cells, and blocks the formation of melanin. Arbutin can combine with TYR directly to accelerate melanin decomposition and excretion, thereby reducing the accumulation of pigment in the skin.4Genistein is the most common type of flavonoid in nature. Recent experimental evidence indicates that it has many pharmacological effects, such as antioxidant, anticancer, prevention of osteoporosis, prevention of cardiovascular disease, estrogen and so on.5–7 1-Benzylpiperazine has antioxidant and anti-inflammatory activity, and the piperazine moiety has a synergistic effect.8 Genistein and resveratrol (1 mmol L−1) effectively abolish melanin synthesis, and convert to orobol and piceatannol, respectively. These results support the strong potential of tyrosinase as a monooxygenase for regio-selective o-hydroxylation of various monophenol compounds.9 Genistein can reduce the quantity of melanin and the degree of erythema in direct proportion to the number of days of treatment.10 Genistein targets TYR directly or indirectly and also induces NRF2 (nuclear factor erythroid 2 [NF-E2]-related factor 2) in different cellular backgrounds for invoking a protective antioxidant mechanism.11
Despite the important roles attributed to genistein in nutrition, the effect and mechanism of genistein supplementation on pigmentation or melanin synthesis has received little attention. The objective of the present study is to clarify the mechanisms of genistein derivative (GD) on skin pigmentation in a guinea pig model.
Materials and methods
Chemicals
Genistein, 1-benzylpiperazine, 95% ethanol, 37% formaldehyde, ethyl acetate, chloroform, dimethyl sulfoxide (DMSO), sodium sulfide and arbutin were purchased from Tianjin Zhiyuan Chemical Reagent Co. Ltd (Tianjin, China). Guinea pig MDA ELISA Kit, Guinea pig SOD ELISA Kit, Guinea pig TYR ELISA Kit, Guinea pig mTOR ELISA Kit and Guinea pig AMPK ELISA Kit were purchased from Shanghai Lanpai Biological Technology Co. Ltd.Synthesis of GD
1 mmol of genistein (270 mg), 25 mL of 95% ethanol, and 0.08 mL of 37% formaldehyde were added to a 50 mL round-bottom flask. The reaction solution was heated to about 55 °C, and after the reaction solution was clarified, 1 mmol of 1-benzyl-piperazine (174 µL) was added, then the reaction solution was stirred at room temperature for about 48 h. When the solid separated out, the solution was filtered and dried to get a solid powder. The solid powder was collected and purified by recrystallization (chloroform![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 95% ethanol = 2
95% ethanol = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1).
1).
The yield of GD obtained from the reaction is 58%. Its melting range is 166.4 °C to 167.5 °C, as measured by the melting point apparatus, and its structure (Fig. 1Ad) was characterized using elemental analysis and 1H nuclear magnetic resonance spectrometry (1H-NMR). The result of the 1H-NMR (DMSO-d6, 400 MHz) (Fig. 1B and C) is 1.25–1.35 (s, 1H), 3.55–3.65 (m, 3H), 3.75–3.85 (d, 2H), 3.91–3.98 (d, 2H), 6.31–6.39 (s, 1H), 6.89–6.95 (d, 2H), 7.33–7.45 (m, 6H), 7.83–7.89 (s, 1H), and 12.85–13.31 (s, 1H). The molecular formula of GD is C27H28N2O5 (C, 70.42; H, 6.13; N, 6.08), and the molecular weight is 460.52.
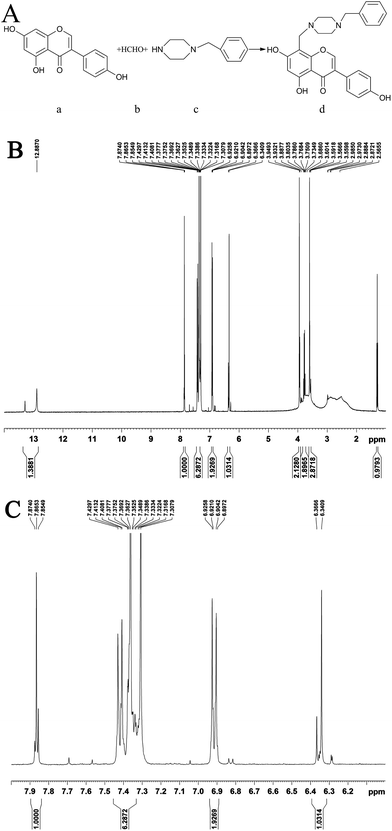 | ||
| Fig. 1 The synthetic route of GD ((Aa) genistein, (Ab) formaldehyde, (Ac) 1-benzylpiperazine and (Ad) GD). (B and C) 1H-NMR spectra of GD in DMSO-d6 (400 MHz). | ||
Experimental animals
White guinea pigs (350–380 g) were supplied by Shandong Lukang Animal Pharmaceutical Co. Ltd. The production license number of the company is Scxk Lu 2013001.UVB irradiation-induced pigmentation in white guinea pigs
All experimental procedures were approved by the Institutional Animal Care and Use Committee (IACUC) of China, and followed the institutional guidelines for animal welfare and experimental conduct (Permit Number: IACUC2015-011). White guinea pigs were housed in cages under standard experimental conditions (22 ± 1 °C; 55 ± 5% humidity; 12 h light and 12 h dark cycle) and were maintained on a standard diet with freely available water. After a week of adaptation, the dorsal hair of the white guinea pigs was shaved off. Then, six separate square (1 cm × 1 cm) dorsal areas on each guinea pig were exposed to 83 mJ cm−2 of UVB light with a distance of 10 cm between the light source (321 nm, Philips, China) and the target skin for 24 min per day for up to 5 days.12,13External inunctum
Guinea pigs (21) were used for all experiments. Each guinea pig had six separate squares (1 cm × 1 cm) on its back. These squares were the high dose group, middle dose group, low dose group, model group, DMSO group and arbutin group. GD and arbutin were dissolved in DMSO to prepare 1%, 2%, and 4% GD solutions and 2% arbutin solution, respectively. Starting from the day after the last dose of UVB irradiation, each sample solution was applied topically with a pipette to the separate shaved areas once a day for 30 days. The high dose group, medium dose group and low dose group were coated with 250 µL of 4%, 2% and 1% GD solution, respectively. The model group was only irradiated with UVB and not coated with anything. The DMSO group was coated with 250 µL of DMSO and the arbutin group was coated with 250 µL of 2% arbutin solution.Growth performance and skin color
The general state of the guinea pigs, including their diet and the color of the skin on the back of the guinea pigs, was observed every day.Histopathological analysis
The guinea pigs were suffocated to death by ethyl ether on the 31st day. The six separate squares of the dorsal skin tissues were surgically removed and incubated in 12% formalin to fix them. The skins were made into conventional paraffin-embedded sections. The slides were dried after an anti-shedding treatment. HE stain and Fontana-Masson stain were used to show the melanin granules in the epidermis, and the distribution of melanin in the skin was observed.14Determination of MDA, SOD, TYR, mTOR and AMPK
The dorsal glabrous skins of the guinea pigs were cleaned with saline (4 °C). The back skin tissue (0.5 g) of each guinea pig was placed in a 4 mL centrifuge tube to which was added 2.0 mL of pre-cooled saline in advance. Then, the skin tissue was homogenized 2 times (10 s/time) using a XHF-1 high speed disperser (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm). After mixing evenly, the supernatant was collected using a TDZ5-WS centrifuge (3500 rpm; 15 min; 4 °C). After centrifugation, the supernatant was preprocessed using the commercial kits’ protocols. Finally, the optical densities of the supernatant were measured with a RT-6100 microplate reader at 450 nm, and then compared to standard curves to get the concentrations of MDA, SOD, TYR, mTOR and AMPK in the sample.
000 rpm). After mixing evenly, the supernatant was collected using a TDZ5-WS centrifuge (3500 rpm; 15 min; 4 °C). After centrifugation, the supernatant was preprocessed using the commercial kits’ protocols. Finally, the optical densities of the supernatant were measured with a RT-6100 microplate reader at 450 nm, and then compared to standard curves to get the concentrations of MDA, SOD, TYR, mTOR and AMPK in the sample.
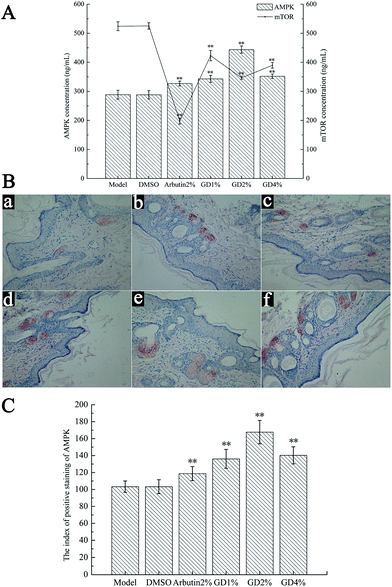
Immunohistochemistry for AMPK and COX-2
The expression of AMPK and COX-2 in the dorsal skin tissues was evaluated using an immunohistochemical technique (IHC). The slides were deparaffinized, hydrated, immersed in 0.3% H2O2 (30 min), covered with normal 6% goat serum (10 min), incubated with primary antibodies (Boster Biological Technology, Wuhan, China) (2 h; 4 °C), reacted with secondary antibodies (30 min; 37 °C), stained with 3,3-diaminobenzidine (DAB) (Sigma, St Louis, MO, USA), and counterstained. Finally, the slides were visualized under a light microscope at 200× magnification. The index of positive staining was expressed as the integral optical density (IOD) processed by an Image Pro-Plus 6.0.15Statistical analysis
Statistical analysis was performed using SPSS 19.0 statistical software. All data were expressed as mean ± standard error of mean (S.E.M.). The differences among the groups were analyzed by a one-way analysis of variance (ANOVA) followed by Dunnett’s test. A level of p < 0.05 was considered significant.Results and discussion
General situation
When the dose of UVB irradiation reached 6000 mJ cm−2, the guinea pigs were found to have a loss of appetite and a reduction in the amount of water they were drinking, and their dorsal skin turned red. The next day, the skin began to desquamate. The skin began to show pigmentation when the irradiation dose of UVB was 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 mJ cm−2.
000 mJ cm−2.
HE stain analysis
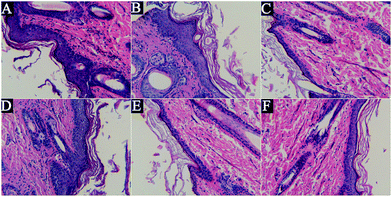
The HE stain can display the cellular and tissue morphology, but it cannot specifically identify melanin granules, because it is nonspecific.14 As shown in Fig. 2, after UVB irradiation, the spinous layer was obviously thickened, the epidermal structure was incomplete, the layer was obscure, the dermal layer was loose, the dermal fibers were degenerated and destructed, the dermal tissue was arranged in disorder and inflammatory cells were infiltrated in it uniformly. However, treatment with GD and arbutin could improve this situation, but the inhibitory effects of GD were not dose-dependent, and the 2% GD-treated specimens showed a significant result.Fontana-Masson stain analysis
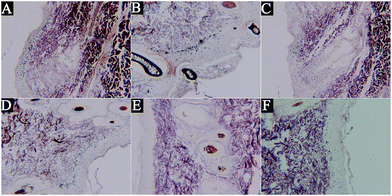
The biopsy specimens were stained with Fontana-Masson which can enhance visualisation of melanin granules to assess the content of epidermal melanin after treatment with the different concentrations of GD. From Fig. 3, we could see that the skin pigmentation model of guinea pigs induced by UVB irradiation was successful. The relative density of epidermal melanin was reduced in the specimens treated with arbutin and GD compared to that in the model group. In particular, the 2% GD-treated specimens showed a significant reduction in melanin content.Effect of GD on oxidative stress markers
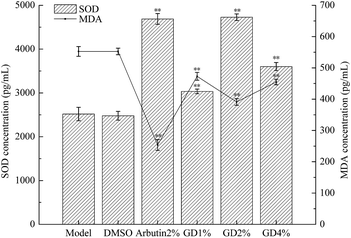
Superoxide dismutase (SOD) is a biological antioxidant enzyme. It has an antioxidant function and it is the only enzyme that can remove the superoxide anion free radical (O2−˙) produced by biological oxidation in nature.16 The antioxidant capacity and the ability in removing free radicals can increase as the level of SOD increases.17 Free radicals can cause lipid peroxidation. MDA is a degradation product of lipid peroxides, which can make the proteins crosslink to form pigments.3 Therefore, SOD scavenges free radicals to make the level of MDA decrease, which can show that the oxidation degree of the guinea pig skin is decreased.The results of Fig. 4 show that there was no significant difference between the DMSO group and the model group in the levels of SOD and MDA (P > 0.05). This indicated that DMSO had no effect on the levels of MDA and SOD in the guinea pig skin. In the GD group, the level of MDA was lower and the level of SOD was higher than in the model group, and there was a markedly significant difference between the two groups (P < 0.01). In particular, the effect of the middle dose group (2% GD) was best. It can be concluded that the effect of GD is similar to that of arbutin, which can inhibit the skin pigmentation of guinea pigs induced by UVB irradiation.
Effect of GD on the level of melanogenic enzyme
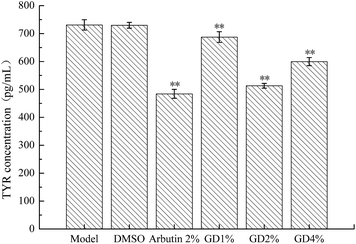
Tyrosinase (TYR), which is the main rate-limiting enzyme in the process of the production of melanin, is a kind of polyphenol oxidase containing copper.18 Its activity is positively related to the synthesis of melanin.19 TYR mainly controls the synthesis of tyrosine (Tyr) which is the main raw material for the formation of melanin. Inhibiting the activity of Tyr can suppress the formation of melanin.20As shown in Fig. 5, compared with the model group, DMSO administration did not achieve statistical significance (P > 0.05). GD could significantly reverse the overproduction of TYR (P < 0.01) and the effect of 2% GD was approximately the same as that of the arbutin which could decrease the level of TYR to inhibit the synthesis of tyrosine.
Effect of GD on the levels of AMPK and mTOR
AMP-activated protein kinase (AMPK) and the mammalian target of rapamycin (mTOR) are serine/threonine protein kinases. Moreover, the signal pathways of AMPK and mTOR are associated with each other, and the increase of AMPK concentration can inhibit mTOR and its effector.21 Through phosphorylation of S6K1 and 4E-BP1, on the one hand, mTOR can regulate the proliferation of melanocytes, while on the other hand, it can regulate and promote the synthesis of tyrosine.22 Therefore AMPK plays an important role in inhibiting the synthesis of tyrosine and the proliferation of melanocytes by regulating the mTOR pathway.23Fig. 6 indicates that DMSO had no effect on the levels of AMPK and mTOR in guinea pig skin. Pretreatment with GD resulted in a noticeable increase in the AMPK level (Fig. 6A) compared with that in the model group (P < 0.01). The expression of AMPK, tested by immunohistochemical staining (Fig. 6B and C), also increased. In addition, potent reduction of the mTOR (Fig. 6A) level was determined after GD administration. GD at the dose of 2% showed a potent effect that was similar to that of arbutin which could inhibit the synthesis of tyrosine and the growth and reproduction of melanocytes.
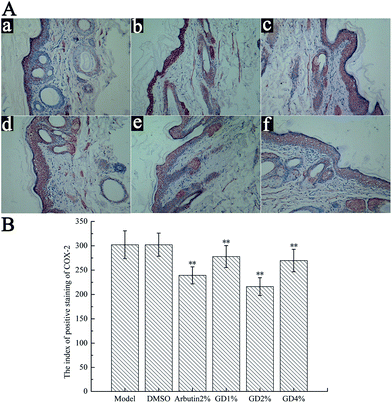
Effect of GD on the expression of COX-2
Cyclooxygenase-2 (COX-2) is the inducible form of the COX enzymes, and it can catalyze prostaglandin biosynthesis from arachidonic acid.24 UVB irradiation induces the production of COX-2 to increase the PGE-2 level which is the factor of melanin synthesis, and then promotes the synthesis of melanin.25 Some studies have found that AMPK is the “cellular energy regulator”, and COX-2 can accept the energy signal, so there is an AMPK–COX-2 signal pathway between AMPK and COX-2.26The expression of COX-2 in the skin tissues of white guinea pigs was tested using immunohistochemical staining. Fig. 7A and B indicate that UVB irradiation increased the expression of COX-2 in the skin tissues of white guinea pigs. Compared with the model group, GD (1%, 2% and 4%) and arbutin (2%) could significantly reverse the overproduction of COX-2. GD at doses of 1%, 2%, and 4% achieved statistical significance when compared with the model group (P > 0.05). In particular, an approximately 30% reduction of COX-2 expression in skin tissues was determined after 2% GD administration.
Conclusions
This study demonstrated the inhibitory effect of GD on pigmentation in a guinea pig model from pathology and physiological activity. From the pathological histology section, the skin pigmentation was obviously reduced after GD treatment. From the physiological activity, GD inhibited the formation of melanin by four pathways (Fig. 8). First, GD inhibited the formation of melanin by inhibiting the production of TYR. Second, GD increased the level of SOD to improve the antioxidant capacity of the skin, so that the ability of the skin to remove MDA was enhanced, and reduced protein cross-linking to form melanin. Third, GD inhibited the production of mTOR, which is a melanoma cell growth regulator, by promoting AMPK. The number of melanoma cells was reduced, which inhibited the synthesis of melanin. Last, GD decreased the expression of COX-2 by the AMPK–COX-2 signal pathway. The decrease of COX-2 expression caused the decrease of the PGE-2 level which was the factor of melanin synthesis. It also demonstrated the inhibitory effect of GD on pigmentation.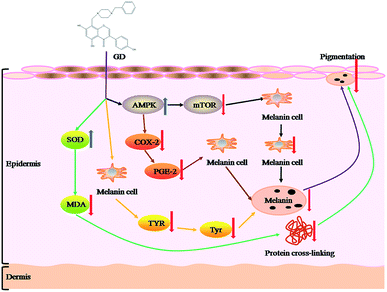 | ||
| Fig. 8 The mechanisms of the inhibitory effects of GD against UVB-induced pigmentation in guinea pig skin. | ||
Arbutin is the drug known to treat skin pigmentation. The results of this study found that the inhibitory effect of 2% GD on skin pigmentation is better than that of arbutin. In conclusion, this study provides a new method for the treatment of skin pigmentation.
Acknowledgements
This study was supported by the Open Project Program (SKLF-KF-201414) of State Key Laboratory of Food Science and Technology, Nanchang University.References
- J. Fang, W. Y. Zhu and M. H. Zhang, Am. J. Clin. Dermatol., 2007, 36, 141–144 CAS.
- A. K. Gupta, M. D. Gover, K. Nouri and S. Taylor, J. Am. Acad. Dermatol., 2007, 55, 1048–1065 CrossRef PubMed.
- L. Cao and Y. Dai, World Clin. Drugs, 2007, 28, 526–531 CAS.
- J. Fang, S. J. Du and Y. L. Jin, J. Hyg. Res., 2009, 38, 111–113 CAS.
- L. Liu, X. Li, F. Liu and X. W. Deng, Chin. J. Arterioscler., 2008, 16, 928–932 CAS.
- C. L. Liu, Z. Q. Li, H. X. Sun and C. S. Li, Soybean Sci., 2008, 27, 693–696 Search PubMed.
- C. M. Rassi, M. Lieberherr and G. Chaumaz, J. Bone Miner. Res., 2002, 17, 630–638 CrossRef CAS PubMed.
- J. L. Cai, Y. H. Lu, L. L. Gan, Y. Y. Zhang and C. H. Zhou, Chin. J. Antibiot., 2009, 34, 454–462 CAS.
- S. H. Lee, K. Baek, J. E. Lee and B. G. Kim, Biotechnol. Bioeng., 2016, 113, 735–743 CrossRef CAS PubMed.
- C. Danciu, F. Borcan, F. Bojin, I. Zupko and C. Dehelean, Nat. Prod. Commun., 2013, 8, 343–346 CAS.
- L. S. Feng and M. Frank, Mol. Nutr. Food Res., 2016, 60, 1264–1274 Search PubMed.
- G. Imokawa, M. Miyagishi and Y. Yada, J. Invest. Dermatol., 1995, 105, 32–37 CrossRef CAS PubMed.
- Y. Yoshida, A. Hachiya, P. Sriwiriyanont, A. Ohuchi, T. Kitahara, Y. Takema, M. O. Visscher and R. E. Boissy, FASEB J., 2007, 21, 2829–2839 CrossRef CAS PubMed.
- K. T. Park, J. K. Kim, D. Hwang, Y. Yoo and Y. H. Lim, Food Chem. Toxicol., 2011, 49, 3038–3045 CrossRef CAS PubMed.
- H. Zheng, Y. L. Chen, J. Z. Zhang, L. Wang, Z. X. Jin, H. H. Huang, S. L. Man and W. Y. Gao, Chem.–Biol. Interact., 2016, 250, 68–77 CrossRef CAS PubMed.
- Y. H. Hu and J. K. Niu, Biology Teaching, 2005, 30, 2–3 Search PubMed.
- A. D. Lin and A. Mannikarottu, Mol. Cell. Biochem., 2007, 296, 11–16 CrossRef CAS PubMed.
- A. Sánchez-ferrer, J. N. Rodríguez-Lopez, F. García-Cánovas and F. García-Carmona, Biochim. Biophys. Acta, Protein Struct. Mol. Enzymol., 1995, 1247, 1–11 CrossRef.
- H. M. Zhang, J. Gen and Q. C. Zhou, J. Chin. Cereals Oils Assoc., 2013, 28, 96–97 CAS.
- Q. X. Chen and K. K. Song, J. Xiamen Univ., Nat. Sci., 2009, 45, 733–734 Search PubMed.
- Q. Y. Fu and Y. Q. Gao, Chin. Sci. Bull., 2005, 17, 149 Search PubMed.
- L. J. Chen and D. H. Shi, J. Mod. Oncol., 2012, 20, 2435–2436 CAS.
- F. Cai, J. L. Su, P. Ge, Q. Ai and L. Zhang, Chemistry for Life, 2015, 35, 26–27 Search PubMed.
- Y. K. Lee, S. Y. Park, Y. M. Kim, W. S. Lee and O. J. Park, Exp. Mol. Med., 2009, 41, 201–207 CrossRef CAS PubMed.
- K. Kazue, M. H. Hiroko, T. Kiyoji, E. Naomi, I. Takashi, U. Yoshihiro and H. Takeshi, J. Invest. Dermatol., 2000, 114, 241–246 CrossRef PubMed.
- J. Zhang and B. G. Tim, Mol. Carcinog., 2008, 47, 974–983 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2017 |