Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Synthesis of aryl triflones by insertion of arynes into C–SO2CF3 bonds†
Xian Zhaoa,
Yangen Huanga,
Feng-Ling Qingab and
Xiu-Hua Xu*b
aCollege of Chemistry, Chemical Engineering and Biotechnology, Donghua University, 2999 North Renmin Lu, Shanghai 201620, China
bKey Laboratory of Organofluorine Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Science, 345 Lingling Lu, Shanghai 200032, China. E-mail: xuxiuhua@sioc.ac.cn
First published on 22nd December 2016
Abstract
A new approach toward the synthesis of aryl triflones was achieved by the formal insertion of arynes into C–SO2CF3 bonds. This reaction proceeds through addition of CF3SO2-containing nucleophiles to the in situ generated arynes and subsequent intramolecular rearrangement.
Largely as a result of their unique biological properties, fluorinated compounds have found wide applications in pharmaceuticals, agrochemicals, and materials.1 Compounds containing a trifluoromethanesulfonyl (triflyl, SO2CF3, Tf) group have received increasing interest due to the strong electron-withdrawing ability and high lipophilicity of SO2CF3.2 In particular, aryl triflones (ArSO2CF3) are frequently used as structural units in bioactive compounds,3 catalysts or ligands,4 and advanced functional materials.5 Consequently, a number of methods have been developed for the preparation of aryl triflones. The general methods include oxidation of aryl trifluoromethyl sulfides,6 trifluoromethylation of aryl sulfonyl fluorides or aryl sulfinates,7 and triflylation of aromatic compounds.8
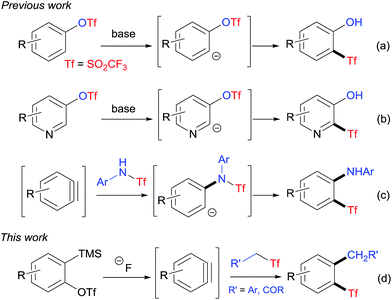
In 2003, Lloyd-Jones and co-workers reported an unprecedented method for the preparation of aryl triflones by anionic thia-Fries rearrangement of aryl triflates (Scheme 1a).9a Since then, Lloyd-Jones' group and Butenschön's group have applied this rearrangement reaction to the synthesis of various o-hydroxyaryl triflones.4d,9 Recently, one of the present authors together with collaborators synthesized a series of heteroaryl triflones with the same methodology (Scheme 1b).10 In these anionic thia-Fries rearrangement reactions, the carbanion intermediates are generated via directed ortho-metalation with organolithium reagents, which are not tolerant of a range of functional groups. We wondered if it was possible to develop new methods to generate carbanions under mild conditions. Considering that the addition of a nucleophile to the aryne is a general method to generate a transient aryl anion intermediate,11 we reasoned that the reaction of a Tf-containing nucleophile and the aryne would give the Tf-containing carbanion intermediate, which may subsequently undergo anionic thia-Fries rearrangement to afford the corresponding aryl triflone. Interestingly, during the investigation of our idea, Li and co-workers reported a novel synthesis of o-aminoaryl triflones from N-triflylated anilines and arynes by adopting a similar strategy (Scheme 1c).12 As a continuation of our research interest in triflyl chemistry,10,13 we herein disclose the first example of preparation of o-alkylaryl triflones by the insertion of arynes into C–SO2CF3 bond through the tandem nucleophilic attack/intramolecular rearrangement (Scheme 1d).
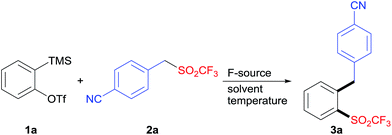
The insertion of arynes into element–element bonds, such as carbon–carbon,14 carbon–heteroatom,15 and heteroatom–heteroatom16 bonds, provides a convenient method for direct access to 1,2-disubstituted aromatics. However, this method has rarely been applied to synthesize SO2CF3-containing aromatics.12,17 Thus, we chose 2-(trimethylsilyl)phenyltriflate (1a) and 4-(((trifluoromethyl)sulfonyl)methyl)benzonitrile (2a) as the model substrates to explore the alkylation–triflylation of arynes for the preparation of o-alkylaryl triflones (Table 1). Initially, different fluoride sources, including CsF, KF, tetrabutylammonium fluoride (TBAF), and KF/18-crown-6, were explored employing THF as the solvent (entries 1–4). Among them, KF/18-crown-6 proved to be the optimal fluoride source, leading to desired product 3a in 60% yield (entry 4). In the subsequent solvent screen, we found that the use of other solvents such as MeCN, toluene, dioxane, and Et2O diminished the yield of 3a (entries 5–8). Furthermore, increasing the temperature to 50 °C could slightly improve the yield to 75% (entry 9). However, when the reaction was performed at 70 °C, lower yield was obtained (entry 10). Additional surveys of the reaction stoichiometry (entries 11–14) revealed that the instance with 1a as the limiting reagent, 1.0 equivalent of 2a, 2.0 equivalents of KF, and 2.0 equivalents of 18-crown-6 afforded the highest yield of 3a (entry 12). Finally, increasing or reducing the concentration of this reaction had no positive effects on the yield (entries 15 and 16).
| Entry | F-Source | Solvent | Temperature | Yieldb (%) |
|---|---|---|---|---|
| a Reaction conditions: 1a (0.10 mmol), 2a (0.15 mmol), fluoride source (0.20 mmol), solvent (3.0 mL), under N2, temperature, overnight.b Yields determined by 19F NMR spectroscopy using trifluoromethoxybenzene as an internal standard.c 2a (0.12 mmol).d 2a (0.10 mmol).e KF (0.10 mmol), 18-crown-6 (0.10 mmol).f KF (0.30 mmol), 18-crown-6 (0.30 mmol).g THF (1.5 mL).h THF (5.0 mL). | ||||
| 1 | CsF | THF | rt | Trace |
| 2 | KF | THF | rt | 10 |
| 3 | TBAF | THF | rt | 49 |
| 4 | KF/18-crown-6 | THF | rt | 60 |
| 5 | KF/18-crown-6 | MeCN | rt | 24 |
| 6 | KF/18-crown-6 | Toluene | rt | 5 |
| 7 | KF/18-crown-6 | Dioxane | rt | 45 |
| 8 | KF/18-crown-6 | Et2O | rt | 51 |
| 9 | KF/18-crown-6 | THF | 50 °C | 75 |
| 10 | KF/18-crown-6 | THF | 70 °C | 54 |
| 11c | KF/18-crown-6 | THF | 50 °C | 73 |
| 12d | KF/18-crown-6 | THF | 50 °C | 85 |
| 13e | KF/18-crown-6 | THF | 50 °C | 30 |
| 14f | KF/18-crown-6 | THF | 50 °C | 60 |
| 15g | KF/18-crown-6 | THF | 50 °C | 69 |
| 16h | KF/18-crown-6 | THF | 50 °C | 78 |
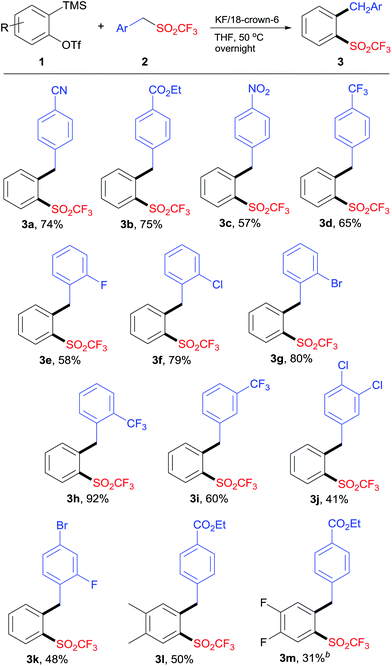
With the optimized reaction conditions (Table 1, entry 12) in hand, we then investigated the substrate scope of this reaction. The reaction of aryne precursor 1a with substituted benzyl triflones 2 carried out effectively to give the corresponding o-alkylaryl triflones 3a–k in moderate to excellent yields (Scheme 2). In general, the electron-withdrawing substituent on benzyl triflones was essential for this transformation. Benzyl triflones bearing fluoro (2e), chloro (2f), and bromo (2g) groups were suitable substrates, providing products 3e–g in good yields. The steric hindrance had no obvious effect on the yield, as benzyl triflone 2h proceeded well to afford 3h in high yield. Benzyl triflone 2i with a substituent into the meta position of the benzyl scaffold was also effective. In addition, disubstituted triflones 2j and 2k underwent this reaction smoothly. It was noteworthy that benzyl triflone 2b reacted with aryne precursors 1b and 1c to produce the corresponding insertion products 3l and 3m. The structure of product 3 was confirmed by X-ray crystallographic analysis of compound 3a (see the ESI†).
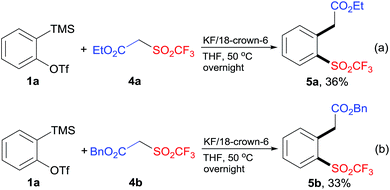
To extend the scope of this protocol, we further examined other CF3SO2-containing substrates. In a similar manner, the reaction of 1a with β-triflyl esters 4a or 4b gave the desired products 5a and 5b, albeit in low yields (Scheme 3). Some unknown byproducts (<10% yield) were also obtained. In the cases of β-triflyl amides, the yields of the desired products were even lower. Furthermore, when β-triflyl ketones were subjected to the standard reaction conditions, the insertion of arynes into C–SO2CF3 bond did not happen. Instead, the insertion of arynes into C(active methylene)–C(ketone) bond was detected.
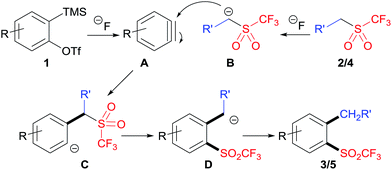
A plausible mechanism of this reaction is proposed in Scheme 4. The substrates 2-(trimethylsilyl)aryltriflate 1 and CF3SO2-containing nucleophile 2/4 were respectively converted to aryne A and carbanion B under the treatment of fluoride anion. Then, the addition of B to A afforded intermediate C, which underwent intramolecular migration of the triflyl group to give intermediate D. Finally, protonation of intermediate D gave the target product 3/5. It should be noted that the triflyl group plays an important role in this reaction. The analogous reaction of aryne precursors with substituted benzyl methanesulfones could not give the desired aryl methanesulfones.
In conclusion, we have developed a new access to aryl triflones starting from aryne precursors and CF3SO2-containing nucleophiles. This protocol proceeds through the tandem nucleophilic attack/intramolecular rearrangement to give the formal insertion products. Further exploration of this insertion reaction in the preparation of bioactive fluorinated compounds is in progress.
Acknowledgements
We are grateful for the financial support from National Natural Science Foundation of China (21502215, 21421002, 21332010, 21272036), Strategic Priority Research Program of the Chinese Academy of Sciences (XDB20020000), Youth Innovation Promotion Association CAS (2016234), and Shanghai Pujiang Program (15PJ1410300).Notes and references
- (a) K. Müller, C. Faeh and F. Diederich, Science, 2007, 317, 1881 CrossRef PubMed; (b) S. Purser, P. R. Moore, S. Swallow and V. Gouverneur, Chem. Soc. Rev., 2008, 37, 320 RSC; (c) N. A. Meanwell, J. Med. Chem., 2011, 54, 2529 CrossRef CAS PubMed; (d) M. Cametti, B. Crousse, P. Metrangolo, R. Milani and G. Resnati, Chem. Soc. Rev., 2012, 41, 31 RSC; (e) J. Wang, M. Sánchez-Roselló, J. L. Aceña, C. del Pozo, A. E. Sorochinsky, S. Fustero, V. A. Soloshonok and H. Liu, Chem. Rev., 2014, 114, 2432 CrossRef CAS PubMed; (f) E. P. Gillis, K. J. Eastman, M. D. Hill, D. J. Donnelly and N. A. Meanwell, J. Med. Chem., 2015, 58, 8315 CrossRef CAS PubMed.
- (a) W. Sheppard, J. Am. Chem. Soc., 1963, 85, 1314 CrossRef CAS; (b) C. Hansch, A. Leo and R. W. Taft, Chem. Rev., 1991, 91, 165 CrossRef CAS; (c) R. Goumont, E. Kizilian, E. Buncel and F. Terrier, Org. Biomol. Chem., 2003, 1, 1741 RSC; (d) F. Terrier, E. Magnier, E. Kizilian, C. Wakselman and E. Buncel, J. Am. Chem. Soc., 2005, 127, 5563 CrossRef CAS PubMed.
- (a) G. Wang, H. Zhang, J. Zhou, C. Ha, D. Pei and K. Ding, Synthesis, 2008, 2398 CAS; (b) B. E. Sleebs, P. E. Czabotar, W. J. Fairbrother, W. D. Fairlie, J. A. Flygare, D. C. S. Huang, W. J. A. Kersten, M. F. T. Koehler, G. Lessene, K. Lowes, J. P. Parisot, B. J. Smith, M. L. Smith, A. J. Souers, I. P. Street, H. Yang and J. B. Baell, J. Med. Chem., 2011, 54, 1914 CrossRef CAS PubMed; (c) P. Ondachi, A. Castro, C. W. Luetje, M. I. Damaj, S. W. Mascarella, H. A. Navarro and F. I. Carroll, J. Med. Chem., 2012, 55, 6512 CrossRef CAS PubMed; (d) J. Chen, H. Zhou, A. Aguilar, L. Liu, L. Bai, D. McEachern, C.-Y. Yang, J. L. Meagher, J. A. Stuckey and S. Wang, J. Med. Chem., 2012, 55, 8502 CrossRef CAS PubMed.
- (a) K. Ishihara, A. Hasegawa and H. Yamamoto, Angew. Chem., Int. Ed., 2001, 40, 4077 CrossRef CAS; (b) P. Wu, R. Hilgraf and V. V. Fokin, Adv. Synth. Catal., 2006, 348, 1079 CrossRef CAS; (c) A. Hasegawa, Y. Naganawa, M. Fushimi, K. Ishihara and H. Yamamoto, Org. Lett., 2006, 8, 3175 CrossRef CAS PubMed; (d) R. Kargbo, Y. Takahashi, S. Bhor, G. R. Cook, G. C. Lloyd-Jones and I. R. Shepperson, J. Am. Chem. Soc., 2007, 129, 3846 CrossRef CAS PubMed; (e) H. Yanai, H. Ogura, H. Fukaya, A. Kotani, F. Kusu and T. Taguchi, Chem.–Eur. J., 2011, 17, 11747 CrossRef CAS PubMed; (f) H. Yanai, T. Yoshino, M. Fujita, H. Fukaya, A. Kotani, F. Kusu and T. Taguchi, Angew. Chem., Int. Ed., 2013, 52, 1560 CrossRef CAS PubMed.
- (a) C. L. Droumaguet, O. Mongin, M. H. V. Werts and M. Blanchard-Desce, Chem. Commun., 2005, 2802 RSC; (b) O. Mongin, L. Porrès, M. Charlot, C. Katan and M. Blanchard-Desce, Chem.–Eur. J., 2007, 13, 1481 CrossRef CAS PubMed; (c) F. Terenziani, C. L. Droumaguet, C. Katan, O. Mongin and M. Blanchard-Desce, ChemPhysChem, 2007, 8, 723 CrossRef CAS PubMed; (d) C. Rouxel, C. L. Droumaguet, Y. Macé, S. Clift, O. Mongin, E. Magnier and M. Blanchard-Desce, Chem.–Eur. J., 2012, 18, 12487 CrossRef CAS PubMed.
- (a) Q.-Y. Chen and J.-X. Duan, J. Chem. Soc., Chem. Commun., 1993, 918 RSC; (b) M. E. González-Núñez, R. Mello, J. Royo, J. V. Ríos and G. Asensio, J. Am. Chem. Soc., 2002, 124, 9154 CrossRef; (c) L. Xu, J. Cheng and M. L. Trudell, J. Org. Chem., 2003, 68, 5388 CrossRef CAS PubMed; (d) P. Kirsch, M. Lenges, D. Kühne and K.-P. Wanczek, Eur. J. Org. Chem., 2005, 797 CrossRef CAS; (e) R. Pluta, P. Nikolaienko and M. Rueping, Angew. Chem., Int. Ed., 2014, 53, 1650 CrossRef CAS PubMed.
- (a) R. P. Singh, G. Cao, R. L. Kirchmeier and J. M. Shreeve, J. Org. Chem., 1999, 64, 2873 CrossRef CAS PubMed; (b) Y. Chang and C. Cai, J. Fluorine Chem., 2005, 126, 937 CrossRef CAS; (c) L. M. Yagupolskii, A. V. Matsnev, R. K. Orlova, B. G. Deryabkin and Y. L. Yagupolskii, J. Fluorine Chem., 2008, 129, 131 CrossRef CAS; (d) X. Lin, G. Wang, H. Li, Y. Huang, W. He, D. Ye, K.-W. Huang, Y. Yuan and Z. Weng, Tetrahedron, 2013, 69, 2628 CrossRef CAS; (e) G. K. S. Prakash, F. Wang, Z. Zhang, R. Haiges, M. Rahm, K. O. Christe, T. Mathew and G. A. Olah, Angew. Chem., Int. Ed., 2014, 53, 11575 CrossRef CAS PubMed.
- (a) J. B. Hendrickson and K. W. Bair, J. Org. Chem., 1977, 42, 3875 CrossRef CAS; (b) X. Creary, J. Org. Chem., 1980, 45, 2727 CrossRef CAS; (c) A. P. Avdeenko, S. A. Konovalova, O. N. Mikhailichenko, S. V. Shelyazhenko, V. V. Pirozhenko and L. M. Yagupol'skii, Russ. J. Org. Chem., 2012, 48, 221 CrossRef CAS; (d) S. C. Cullen, S. Shekhar and N. K. Nere, J. Org. Chem., 2013, 78, 12194 CrossRef CAS PubMed; (e) S. K. Aithagani, K. R. Yempalla, G. Munagala, R. A. Vishwakarma and P. P. Singh, RSC Adv., 2014, 4, 50208 RSC; (f) F. Xie, Z. Zhang, X. Yu, G. Tang and X. Li, Angew. Chem., Int. Ed., 2015, 54, 7405 CrossRef CAS PubMed; (g) K. Zhang, X.-H. Xu and F.-L. Qing, J. Org. Chem., 2015, 80, 7658 CrossRef CAS PubMed; (h) K. Matsuzaki, K. Okuyama, E. Tokunaga, N. Saito, M. Shiro and N. Shibata, Org. Lett., 2015, 17, 3038 CrossRef CAS PubMed; (i) F. Wang, X. Yu, Z. Qi and X. Li, Chem.–Eur. J., 2016, 22, 511 CrossRef CAS PubMed; (j) L. A. Smyth, E. M. Phillips, V. S. Chan, J. G. Napolitano, R. Henry and S. Shekhar, J. Org. Chem., 2016, 81, 1285 CrossRef CAS PubMed.
- (a) J. P. H. Charmant, A. M. Dyke and G. C. Lloyd-Jones, Chem. Commun., 2003, 381 Search PubMed; (b) Z. Zhao, J. Messinger, U. Schön, R. Wartchow and H. Butenschön, Chem. Commun., 2006, 3007 RSC; (c) K. Barta, G. Franciò, W. Leitner, G. C. Lloyd-Jones and I. R. Shepperson, Adv. Synth. Catal., 2008, 350, 2013 CrossRef CAS; (d) A. M. Dyke, D. M. Gill, J. N. Harvey, A. J. Hester, G. C. Lloyd-Jones, M. P. Muñoz and I. R. Shepperson, Angew. Chem., Int. Ed., 2008, 47, 5067 CrossRef CAS PubMed; (e) M. Kruck, M. P. Munoz, H. L. Bishop, C. G. Frost, C. J. Chapman, G. Kociok-Köhn, C. P. Butts and G. C. Lloyd-Jones, Chem.–Eur. J., 2008, 14, 7808 CrossRef CAS PubMed; (f) G. Werner, C. W. Lehmann and H. Butenschön, Adv. Synth. Catal., 2010, 352, 1345 CrossRef CAS; (g) G. Werner and H. Butenschön, Eur. J. Org. Chem., 2012, 3132 CrossRef CAS; (h) G. Werner and H. Butenschön, Organometallics, 2013, 32, 5798 CrossRef CAS.
- X.-H. Xu, X. Wang, G.-K. Liu, E. Tokunaga and N. Shibata, Org. Lett., 2012, 14, 2544 CrossRef CAS PubMed.
- For selected reviews, see: (a) C. M. Gampe and E. M. Carreira, Angew. Chem., Int. Ed., 2012, 51, 3766 CrossRef CAS PubMed; (b) P. M. Tadross and B. M. Stoltz, Chem. Rev., 2012, 112, 3550 CrossRef CAS PubMed; (c) A. Bhunia, S. R. Yetra and A. T. Biju, Chem. Soc. Rev., 2012, 41, 3140 RSC; (d) C.-L. Sun and Z.-J. Shi, Chem. Rev., 2014, 114, 9219 CrossRef CAS PubMed; (e) S. S. Bhojgude, A. Bhunia and A. T. Biju, Acc. Chem. Res., 2016, 49, 1658 CrossRef CAS PubMed; (f) R. Karmakar and D. Lee, Chem. Soc. Rev., 2016, 45, 4459 RSC.
- D. Qiu, J. He, X. Yue, J. Shi and Y. Li, Org. Lett., 2016, 18, 3130 CrossRef CAS PubMed.
- (a) X.-H. Xu, G.-K. Liu, A. Azuma, E. Tokunaga and N. Shibata, Org. Lett., 2011, 13, 4854 CrossRef CAS PubMed; (b) X.-H. Xu, M. Taniguchi, A. Azuma, G.-K. Liu, E. Tokunaga and N. Shibata, Org. Lett., 2013, 15, 686 CrossRef CAS PubMed; (c) X.-H. Xu, M. Taniguchi, X. Wang, E. Tokunaga, T. Ozawa, H. Masuda and N. Shibata, Angew. Chem., Int. Ed., 2013, 52, 12628 CrossRef CAS PubMed; (d) C. Chen, X.-H. Xu, B. Yang and F.-L. Qing, Org. Lett., 2014, 16, 3372 CrossRef CAS PubMed; (e) K. Zhang, X.-H. Xu and F.-L. Qing, J. Org. Chem., 2015, 80, 7658 CrossRef CAS PubMed; (f) L.-N. Hua, H. Li, F.-L. Qing, Y. Huang and X.-H. Xu, Org. Biomol. Chem., 2016, 14, 8443 RSC.
- For recent examples, see: (a) R. Li, H. Tang, H. Fu, H. Ren, X. Wang, C. Wu, C. Wu and F. Shi, J. Org. Chem., 2014, 79, 1344 CrossRef CAS PubMed; (b) B. Rao, J. Tang, Y. Wei and X. Zeng, Chem.–Asian J., 2016, 11, 991 CrossRef CAS PubMed; (c) R. Kranthikumar, R. Chegondi and S. Chandrasekhar, J. Org. Chem., 2016, 81, 2451 CrossRef CAS PubMed; (d) B. Rao, J. Tang and X. Zeng, Org. Lett., 2016, 18, 1678 CrossRef CAS PubMed; (e) P. Gouthami, R. Chegondi and S. Chandrasekhar, Org. Lett., 2016, 18, 2044 CrossRef CAS PubMed; (f) R. Samineni, P. Srihari and G. Mehta, Org. Lett., 2016, 18, 2832 CrossRef CAS PubMed.
- For recent examples, see: (a) E. Yoshioka, H. Tanaka, S. Kohtani and H. Miyabe, Org. Lett., 2013, 15, 3938 CrossRef CAS PubMed; (b) Y. Zhou, Y. Chi, F. Zhao, W.-X. Zhang and Z. Xi, Chem.–Eur. J., 2014, 20, 2463 CrossRef CAS PubMed; (c) Y. Zeng and J. Hu, Chem.–Eur. J., 2014, 20, 6866 CrossRef CAS PubMed; (d) B. Rao and X. Zeng, Org. Lett., 2014, 16, 314 CrossRef CAS PubMed; (e) M. Pawliczek, L. K. B. Garve and D. B. Werz, Org. Lett., 2015, 17, 1716 CrossRef CAS PubMed; (f) A. C. Wright, C. K. Haley, G. Lapointe and B. M. Stoltz, Org. Lett., 2016, 18, 2793 CrossRef CAS PubMed; (g) Y. Li, C. Mück-Lichtenfeld and A. Studer, Angew. Chem., Int. Ed., 2016, 55, 14435 CrossRef CAS PubMed.
- For recent examples, see: (a) T. R. Hoye, B. Baire, D. Niu, P. H. Willoughby and B. P. Woods, Nature, 2012, 490, 208 CrossRef CAS PubMed; (b) D. Rodríguez-Lojo, A. Cobas, D. Peña, D. Pérez and E. Guitián, Org. Lett., 2012, 14, 1363 CrossRef PubMed; (c) H. Yoshida, R. Yoshida and K. Takaki, Angew. Chem., Int. Ed., 2013, 52, 8629 CrossRef CAS PubMed; (d) C. E. Hendrick, S. L. McDonald and Q. Wang, Org. Lett., 2013, 15, 3444 CrossRef CAS PubMed; (e) C. Shen, G. Yang and W. Zhang, Org. Lett., 2013, 15, 5722 CrossRef CAS PubMed; (f) F.-L. Liu, J.-R. Chen, Y.-Q. Zou, Q. Wei and W.-J. Xiao, Org. Lett., 2014, 16, 3768 CrossRef CAS PubMed; (g) S. Yoshida, T. Yano, Y. Misawa, Y. Sugimura, K. Igawa, S. Shimizu, K. Tomooka and T. Hosoya, J. Am. Chem. Soc., 2015, 137, 14071 CrossRef CAS PubMed; (h) Z. Chen and Q. Wang, Org. Lett., 2015, 17, 6130 CrossRef CAS PubMed; (i) Y. Li, S. Chakrabarty, C. Mück-Lichtenfeld and A. Studer, Angew. Chem., Int. Ed., 2016, 55, 802 CrossRef CAS PubMed; (j) Y. Li, D. Qiu, R. Gu, J. Wang, J. Shi and Y. Li, J. Am. Chem. Soc., 2016, 138, 10814 CrossRef CAS PubMed.
- For a pioneering work of synthesis of SOCF3-containing aromatics by aryne insertion, see: Z. Liu and R. C. Larock, J. Am. Chem. Soc., 2005, 127, 13312 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 1509106. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c6ra26429h |
| This journal is © The Royal Society of Chemistry 2017 |