Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
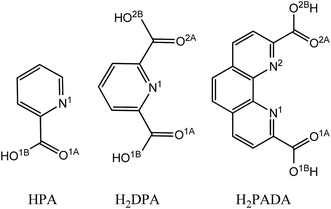
Influence of denticity and combined soft–hard strategy on the interaction of picolinic-type ligands with NpO2+†
Hongcai Lingab,
Miaoren Xiab,
Wenkai Chen *ac,
Zhifang Chaibd and
Dongqi Wang*b
*ac,
Zhifang Chaibd and
Dongqi Wang*b
aCollege of Chemistry, Fuzhou University, Fuzhou 350116, P. R. China. E-mail: qc2008@fzu.edu.cn
bMultidisciplinary Initiative Center, Institute of High Energy Physics, Chinese Academy of Sciences, Beijing 100049, P. R. China. E-mail: dwang@ihep.ac.cn
cKey Laboratory of Applied Nuclear Techniques in Geosciences Sichuan, Chengdu University of Technology, Chengdu 610059, P. R. China
dSchool of Radiation Medicine and Interdisciplinary Sciences (RAD-X), Soochow University, Suzhou 215123, P. R. China
First published on 21st February 2017
Abstract
The interaction of neptunyl ions (NpO2+) with three picolinic type ligands (L), including the deprotonated picolinic acid anion (PA−), the deprotonated dipicolinic acid anion (DPA2−) and the 1,10-phenanthroline-2,9-dicarboxylic acid anion (PADA2−), was investigated by using a density functional theory method with various stoichiometric ratios of Np![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) L = 1
L = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, and 1
2, and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3. The coordination modes, the influence of the denticity of the ligands, and the stoichiometry of the complexes were evaluated in terms of geometry, electronic structure, and thermodynamics. The calculations show that the coordination of NpO2+ to tetradentate ligands is more stable than that to tridentate and bidentate ones, and the coordination ability of the three deprotonated ligands follows the order: PADA2− > DPA2− > PA−. Quantum theory of atoms-in-molecules (QTAIM) analysis, charge decomposition analysis (CDA) and natural atomic orbital (NAO) analysis were used to understand the bonding nature and electronic properties of the complexes, and the metal–ligand dative bond was identified to be mainly ionic. In view of the favorable coordination modes and the distinct ability of the ligands in binding to neptunyl, we conclude that the denticity of the ligands and the combined hard–soft donor strategy work cooperatively in the coordination of NpO2+ with ligands. This work is expected to contribute to the rational design of new types of ligand with enhanced ability to extract neptunyl.
3. The coordination modes, the influence of the denticity of the ligands, and the stoichiometry of the complexes were evaluated in terms of geometry, electronic structure, and thermodynamics. The calculations show that the coordination of NpO2+ to tetradentate ligands is more stable than that to tridentate and bidentate ones, and the coordination ability of the three deprotonated ligands follows the order: PADA2− > DPA2− > PA−. Quantum theory of atoms-in-molecules (QTAIM) analysis, charge decomposition analysis (CDA) and natural atomic orbital (NAO) analysis were used to understand the bonding nature and electronic properties of the complexes, and the metal–ligand dative bond was identified to be mainly ionic. In view of the favorable coordination modes and the distinct ability of the ligands in binding to neptunyl, we conclude that the denticity of the ligands and the combined hard–soft donor strategy work cooperatively in the coordination of NpO2+ with ligands. This work is expected to contribute to the rational design of new types of ligand with enhanced ability to extract neptunyl.
Introduction
As one of the minor actinides (neptunium, americium, and curium) in spent nuclear fuel (SNF),1–3 neptunium (Np) is considered to be one of the major issues in nuclear waste management owing to its high radioactivity and long half-lifetime. Np has an electronic configuration of [Rn]5f57s2 (or [Rn]5f46d17s2) with multiple known oxidation states, i.e. III, IV, V, VI and VII, among which the most stable one in aqueous solutions is the penta-valent state,4,5 and predominantly exists as neptunyl cations (NpO2+). The NpO2+ ion does not form strong complexes with the commonly used ligands and is hard to extract during spent fuel reprocessing. In the development of advanced SNF reprocessing protocols, the efficient separation of Np remains a challenge, and calls for extensive study, from both the experimental and theoretical sides, to shed light on the chemical behavior of Np in the condensed phase.6–11In spent nuclear fuel reprocessing using extraction techniques, extractants containing heterocyclic N donors are attractive owing to their compositions of only C, H, O and N, thus being completely incinerable to avoid secondary waste in nuclear waste treatment. The N donor ligands developed in recent years, such as bis(triazinyl)pyridines (BTPs),12,13 bis(triazinyl) bipyridines (BTBPs),14–16 bis(triazinyl)-1,10-phenanthrolines (BTPhens),9,17–19 have been considered as promising extractants for minor actinides. These earlier studies mainly focused on the efficient separation of trivalent lanthanides and actinides, and neptunyl was rarely considered due to its weak extractability by organic ligands.
In recent years, Rao et al.20–26 and other groups27,28 conducted a series of experimental studies to evaluate the performance of ligands containing O and N in their binding with neptunyl. These cover the determination of the thermodynamic parameters (stability constants, enthalpy, and entropy) by spectrophotometry and microcalorimetry, and X-ray crystallographic studies of neptunyl complexes with dicarboxylic acids as well as the diamide derivatives, such as oxydiacetic acid (ODA), N,N-dimethyl-3-oxa-glutaramic acid (DMOGA) and, N,N,N′,N′-tetramethyl-3-oxa-glutaramide (TMOGA), and 1,10-phenanthrolin-2,9-dicarboxylic acid (H2PADA). In their recent work of H2PADA, Rao et al. compared25 it with picolinic acid (HPA)29 and dipicolinic acid (H2DPA),23 and found that the complexation of neptunyl ions with the tetradentate H2PADA ligand is much stronger than with other ligands (HPA and H2DPA), and proposed that it could be an excellent extractant in the separation of neptunyl ions. Note that, both H2DPA and H2PADA, comparing to some other κ3 and κ4 chelating ligands, have preorganized planar structures with their donor atoms, i.e. O and N, aligned on the same side to prepare for the coordination with neptunyl in the equatorial plane, thus save the energy cost that may be needed for the ligands with backbone dihedral freedoms.30
In earlier work, we have reported B3LYP studies on the coordination chemistry and thermodynamics of neptunyl with the ligands of N,N,N′,N′-tetramethyl-3-oxa-glutaramide (TMOGA), N,N-dimethyl-3-oxa-glutaramic acid (DMOGA), deprotonated oxydiacetic analog (ODA), and BTPs, BTBPs, BTPhens.9,31 which show that the denticity of the chelating ligands is key to their interaction with neptunyl. In this work, we extend our study and aim to understand the influence of denticity of ligands and the importance to consider the combined soft–hard donor strategy in developing new types of ligands to extract actinides. For this purpose, we investigated the complexation behavior of NpO2+ with H2PADA and its two picolinic derivatives by using density functional theory method. The geometries of the complexes have been optimized, and the free energy change of ligand exchange processes have been calculated and analyzed to find the most probable coordination modes of each ligands. To understand the coordination modes and bonding nature of NpO2+ with these ligands, the quantum theory of atoms-in-molecules (QTAIM) topological analysis, charge decomposition analysis (CDA), and natural atomic orbital (NAO) analysis were carried to reveal the feature of the metal–ligand dative bonds. The results were compared to the experimental data to show that the computational work may complement experimental studies by providing molecular level of details.
Methods
The ligands in experimental studies,25 HPA, H2DPA, and H2PADA, as shown in Scheme 1, were used as the prototype models to investigate the complexation of neptunyl ion (NpO2+) with the deprotonated picolinic acid anion (PA−), the deprotonated dipicolinic acid anion (DPA2−), and the 1,10-phenanthrolin-2,9-dicarboxylic acid anion (PADA2−).All geometry optimization and frequency calculations were carried out by using the B3LYP functional32–34 as implemented in the Gaussian 09 program.35 Frequency analysis was done for all of the optimized stationary points to identify their nature as minima, and to obtain the thermodynamic parameters (enthalpy (ΔH), Gibbs free energy (ΔG), and entropy (ΔS)), which were used to evaluate the thermodynamic feasibility of the binding process36,37 of hydrated neptunyl with the picolinic-type ligands. Two combined basis sets have been used differing in the treatment of C, H, O and N, one with the 6-31+G* (ref. 38) basis set (BS1), which was used for geometry optimization and frequency calculations, and another one with the larger basis set 6-311++G(d,p) (BS2) to refine energies.39–41 In both basis sets, the Np atom was treated by a small-core quasi-relativistic effective core potentials (5f-in-valence RECPs) for the 60 core electrons, and the corresponding valence basis set adopted a contraction scheme of (14s13p10d8f6g)/[10s9p5d4f3g] (ECP60MWB basis)42–44 to describe the valence shells.
The solvent effect of water was taken into account with the polarizable continuum model (PCM).45,46 GaussView 5.0 program47 was used for visualization of structures and molecular orbitals. The Multiwfn 3.2 program48 was used to carry out the quantum theory of atoms-in-molecules (QTAIM)49–52 topological analysis to understand the coordination modes and bonding nature of complexes53 described by five parameters at the (3, −1) bond critical point (BCP), i.e. the electron density at BCP (ρb), the Laplacian of electron density at BCP (∇2ρb), the total energy density at BCP (Hb), the delocalization index (δ(A,B)), and the bond ellipticity (ε). Charge decomposition analysis (CDA) and natural atomic orbital (NAO) analysis were also done to evaluate the ionic interaction in complex formation and the covalency of the metal–ligand dative bond.
Results and discussion
A. Geometries and relative energies
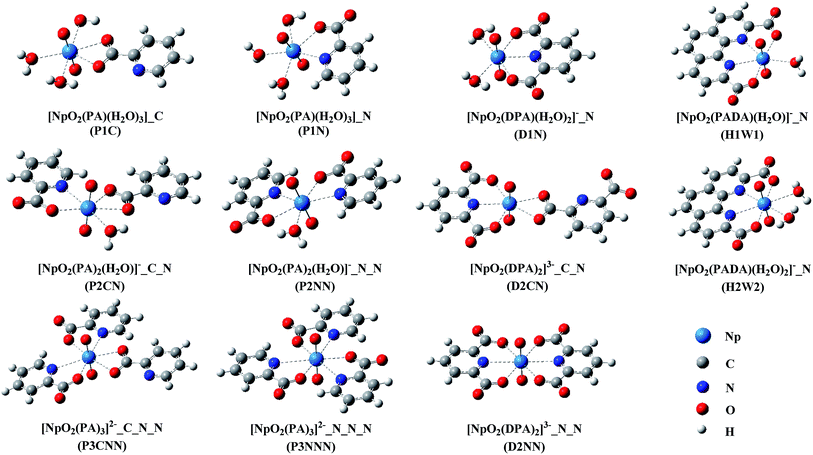
As shown in Scheme 1, the three ligands differ in their denticities when coordinating to metal ions, which are 2, 3, and 4 for PA−, DPA2− and PADA2−, respectively. As all of the three ligands may interact with neptunyl with their carboxyl groups, it is also possible for them to coordinate with neptunyl in a η1 (with one of the two Ocarb atoms) or κ2 (with both Ocarb atoms) manner. Thus, in this work, we have done an exhaustive search of coordination modes that the neptunyl–ligand complexes may take. In these complexes, a coordination number (C.N.) of neptunyl in its equatorial plane as 5 or 6 is retained, and the excess coordination sites may be occupied by additional same ligand, either in the identical or distinct coordination modes, or water molecules. This results in 17, 10, and 4 possible complexes for PA−, DPA2− and PADA2−, respectively. The representative stationary points with the lowest energy in each set of stoichiometric ratio from the calculations in the aqueous phase are shown in Fig. 1, and the rest complexes are collected in the ESI.†As a bidentate ligand, PA− may coordinate with neptunyl either in the manner of end-on with its carboxyl group (denoted as _C) or side-on with its N and one O1A of the carboxyl group (denoted as _N). In Fig. 1, six complexes of neptunyl–PA− are shown, differing in the coordination mode and the stoichiometric ratio, which is up to Np![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PA− = 1
PA− = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 with Np atom remaining penta- or hex-coordinated in its equatorial plane. In addition, in Fig. 1, three complexes of DPA2−, with one, two and two DPA2− in the complex, respectively, and two of PADA2− are also shown. In the complexes with Np
3 with Np atom remaining penta- or hex-coordinated in its equatorial plane. In addition, in Fig. 1, three complexes of DPA2−, with one, two and two DPA2− in the complex, respectively, and two of PADA2− are also shown. In the complexes with Np![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DPA2− = 1
DPA2− = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, one DPA2− appears as a tridentate ligand, and another one may coordinate with Np either in κ2 (D2CN) or κ3 (D2NN) manner. In the case of PADA2−, as the ligand binds with Np in κ4 manner, and constitutes substantial steric hindrance to prevent the co-appearance of additional PADA2−, here we only considered the stoichiometric ratio of Np
2, one DPA2− appears as a tridentate ligand, and another one may coordinate with Np either in κ2 (D2CN) or κ3 (D2NN) manner. In the case of PADA2−, as the ligand binds with Np in κ4 manner, and constitutes substantial steric hindrance to prevent the co-appearance of additional PADA2−, here we only considered the stoichiometric ratio of Np![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DAPA2− = 1
DAPA2− = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (H1W1 and H2W2, differing in the presence of one or two water molecules respectively).
1 (H1W1 and H2W2, differing in the presence of one or two water molecules respectively).
The averaged bond lengths d (Å) of Np–Oyl, Np–Npy, Np–Ocarb, and Np–Owat bonds were summarized in Table 1 and ESI.† In bare neptunyl, the Np–Oyl bond distance is calculated to be 1.737 and 1.781 Å in gas phase and in aqueous phase, respectively. This value increase to 1.780 and 1.794 Å in its penta-hydrated form (N1 in Table 1), which suggests that the coordination of water ligands to neptunyl in its equatorial plane weakens the Np–Oyl bonds. Upon its coordination to the three negatively charged organic ligands, the Np–Oyl bond is perturbed and elongated to 1.81–1.83 Å, suggesting a stronger perturbation brought by these ligands than by water.
| Np–Oyl | Np–Npy | Np–Ocarb | Np–Owat | Np–Oyl | Np–Npy | Np–Ocarb | Np–Owat | ||
|---|---|---|---|---|---|---|---|---|---|
| a N1: [NpO2(H2O)5]+. | |||||||||
| N1a | 1.794 | — | — | 2.555 | P1C | 1.802 | — | 2.555 | 2.597 |
| D1N | 1.812 | 2.593 | 2.487 | 2.658 | P1N | 1.804 | 2.644 | 2.444 | 2.574 |
| D2CN | 1.823 | 2.594 | 2.524 | — | P2CN | 1.821 | 2.662 | 2.542 | 2.686 |
| D2NN | 1.824 | 2.786 | 2.581 | — | P2NN | 1.814 | 2.667 | 2.458 | 2.686 |
| H1W1 | 1.823 | 2.621 | 2.480 | 2.603 | P3CNN | 1.827 | 2.917 | 2.578 | — |
| H1W2 | 1.812 | 2.690 | 2.524 | 2.714 | P3NNN | 1.821 | 3.006 | 2.454 | — |
We also note that for the dative bonds in the complexes, in general the bond length of Np–Ocarb is about 0.1–0.2 Å shorter than that of Np–Npy and Np–Owat bonds. This may be determined by the stronger electrostatic interaction between Np and Ocarb than between Np and the other two types of coordinating atoms.
In aqueous phase, neptunyl exists in its hydrated form with five water molecules bound in the first coordination shell. Starting from [NpO2(H2O)5]+, the thermodynamics of the formation of the above-mentioned complexes was evaluated via ligand exchange process to replace the water ligands by corresponding organic ligands. The data are collected in Table 2.23,25,29
| Complexation reactions | Aqueous | Exp. | ||||||
|---|---|---|---|---|---|---|---|---|
| ΔG | ΔH | TΔS | ΔG | ΔH | TΔS | |||
| a Data from ref. 29.b Data from ref. 23.c Data from ref. 25. | ||||||||
| (1) [NpO2(H2O)5]+ + PA− → P1C + 2H2O | −16.09 | −7.54 | 8.55 | — | — | — | ||
| (2) [NpO2(H2O)5]+ + PA− → P1N + 2H2O | −20.75 | −11.64 | 9.12 | −4.90a | −0.72a | 4.18a | ||
| (3) [NpO2(H2O)5]+ + 2PA− → P2CN + 4H2O | −41.24 | −25.24 | 16.00 | −8.91a | — | — | ||
| (4) [NpO2(H2O)5]+ + 2PA− → P2NN + 4H2O | −36.78 | −21.58 | 15.20 | — | — | — | ||
| (5) [NpO2(H2O)5]+ + 3PA− → P3CNN + 5H2O | −41.03 | −23.97 | 17.07 | — | — | — | ||
| (6) [NpO2(H2O)5]+ + 3PA− → P3NNN + 5H2O | −30.91 | −17.12 | 13.79 | — | — | — | ||
| (7) [NpO2(H2O)5]+ + DPA2− → D1N + 3H2O | −40.71 | −24.37 | 16.33 | −11.85b | −6.02b | 5.83b | ||
| (8) [NpO2(H2O)5]+ + 2DPA2− → D2NN + 5H2O | −53.32 | −27.55 | 25.78 | −16.75b | −10.99b | 5.76b | ||
| (9) [NpO2(H2O)5]+ + 2DPA2− → D2CN + 5H2O | −51.41 | −24.11 | 27.30 | — | — | — | ||
| (10) [NpO2(H2O)5]+ + PADA2− → H1W1 + 4H2O | −55.10 | −32.11 | 23.00 | −15.99c | −6.86c | 9.13c | ||
| (11) [NpO2(H2O)5]+ + PADA2− → H1W2 + 3H2O | −39.27 | −27.46 | 11.80 | — | — | — | ||
| (12) [NpO2(H2O)5]+ + H2O → [NpO2(H2O)6]+ | 4.47 | −4.48 | −8.95 | — | — | — | ||
In Table 2, the eqn (1) to (6) were used to calculate the thermodynamics for the formation of the neptunyl–PA− complexes with the stoichiometric ratio of Np![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) L varies from 1
L varies from 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 1
1 to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 to 1
2 to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3. It can be seen that the NpL2 (P2CN and P2NN) complexes is thermodynamically more stable than NpL (P1C and P1N) and comparable with NpL3 (P3CNN and P3NNN) both in the gas phase (data in ESI†) and in water, suggesting that neptunyl has stronger affinity to PA− than to water ligand, but with an optimal stoichiometric ratio of 1
3. It can be seen that the NpL2 (P2CN and P2NN) complexes is thermodynamically more stable than NpL (P1C and P1N) and comparable with NpL3 (P3CNN and P3NNN) both in the gas phase (data in ESI†) and in water, suggesting that neptunyl has stronger affinity to PA− than to water ligand, but with an optimal stoichiometric ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2. Regarding to the coordination mode, with one PA− bound, neptunyl favors the _N mode to benefit from both the excess negative charge of the carboxyl group (P1C: C–O1A = 1.269 Å, C–O1B = 1.273 Å vs. P1N: C–O1A = 1.286 Å, C–O1B = 1.242 Å) and pyridine N atom which is relatively “softer” than the carbonyl O1B atom according to Pierson's Hard–Soft–Acid–Base (HSAB) principle.54,55 This rule holds with one or two more PA− bound, and neptunyl does not favor to bind with all of the PA− ligands in the _N mode, but rather with one PA− in the _C mode and the rest in the _N mode.
2. Regarding to the coordination mode, with one PA− bound, neptunyl favors the _N mode to benefit from both the excess negative charge of the carboxyl group (P1C: C–O1A = 1.269 Å, C–O1B = 1.273 Å vs. P1N: C–O1A = 1.286 Å, C–O1B = 1.242 Å) and pyridine N atom which is relatively “softer” than the carbonyl O1B atom according to Pierson's Hard–Soft–Acid–Base (HSAB) principle.54,55 This rule holds with one or two more PA− bound, and neptunyl does not favor to bind with all of the PA− ligands in the _N mode, but rather with one PA− in the _C mode and the rest in the _N mode.
The DPA2− ligand behaves similarly to PA−, and in the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complex, it binds with neptunyl in the κ3 (_N) mode rather than the κ2 (_C) mode. The presence of one more DPA2− ligand brings additional stabilization energy to the complexes, with the κ3 mode (−53.32 kcal mol−1) moderately more favorable than the κ2 mode (−51.41 kcal mol−1).
1 complex, it binds with neptunyl in the κ3 (_N) mode rather than the κ2 (_C) mode. The presence of one more DPA2− ligand brings additional stabilization energy to the complexes, with the κ3 mode (−53.32 kcal mol−1) moderately more favorable than the κ2 mode (−51.41 kcal mol−1).
The PADA2− ligand displays exceptional binding affinity to neptunyl and the substitution of four water ligands by one PADA2− is calculated to be exothermic by 55.10 kcal mol−1. This suggests that at the same stoichiometric ratio, PADA2− is superior over the other two ligands toward to the coordination with neptunyl. This trend is consistent with the experimental observations23,25,29 as shown in Table 2.
In summary, the thermodynamic stability of the complexes of NpO2+ with the three pyridine-based carboxylate ligands follow the order: PADA2− (−55.1 kcal mol−1) > DPA2− (−53.32 kcal mol−1) > PA− (−41.24 kcal mol−1) in the aqueous phase, suggesting that the phenanthroline-based tetradentate ligand (PADA2−) has the best binding affinity with neptunyl than the other two ligands. The calculations also show that the binding mode with combined “hard–soft” donors brings more stabilization energy to the complexes than that with only “hard” donors. The former binding mode gains enthalpy without the loss of entropic contribution. This is reasonable concerning that the carboxylate group builds stronger hydrogen bonding with water solvent than the Npy does, thus the _C binding mode requires more energy to reorganize solvent environment than the _N mode does.
B. QTAIM topological analysis
The quantum theory of atoms-in-molecules (QTAIM) topological analysis of Bader49,52 and Matta and Boyd56 et al. were used to understand the bonding nature and electronic properties of the complexes. Five descriptors in the framework of AIM, i.e. the density ρb at the (3, −1) bond critical point (BCP), the Laplacian of electron density at BCP (∇2ρb), the total energy density at BCP (Hb), the delocalization index (δ(A,B)), and the bond ellipticity (ε), were used to characterize the interactions between Np and ligands. The values of these descriptors are tabulated in Table 3 and ESI.†![[small delta, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0c5.gif) (a.u.) and averaged bond ellipticity
(a.u.) and averaged bond ellipticity ![[small epsilon, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0c6.gif) (a.u.) of the Np–Oyl, Np–Npy, Np–Ocarb, and Np–Owater bonds of the complexes obtained from QTAIM analysis (N0: NpO2+, N1: [NpO2(H2O)5]+)
(a.u.) of the Np–Oyl, Np–Npy, Np–Ocarb, and Np–Owater bonds of the complexes obtained from QTAIM analysis (N0: NpO2+, N1: [NpO2(H2O)5]+)
| ρb | ∇2ρb | Hb | ![[small delta, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0c5.gif) |
![[small epsilon, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0c6.gif) |
ρb | ∇2ρb | Hb | ![[small delta, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0c5.gif) |
![[small epsilon, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0c6.gif) |
|
|---|---|---|---|---|---|---|---|---|---|---|
| Np–Oyl | Np–Npy | |||||||||
| N0 | 0.294 | 0.179 | −0.269 | 2.860 | 0.000 | — | — | — | — | — |
| N1 | 0.283 | 0.215 | −0.249 | 2.823 | 0.000 | — | — | — | — | — |
| P1C | 0.278 | 0.223 | −0.239 | 2.810 | 0.001 | — | — | — | — | — |
| P1N | 0.276 | 0.229 | −0.236 | 2.808 | 0.002 | 0.042 | 0.131 | −0.001 | 0.869 | 0.392 |
| P2CN | 0.266 | 0.247 | −0.218 | 2.792 | 0.004 | 0.040 | 0.126 | −0.001 | 0.851 | 0.390 |
| P2NN | 0.270 | 0.242 | −0.225 | 2.794 | 0.004 | 0.039 | 0.126 | −0.001 | 0.846 | 0.392 |
| P3CNN | 0.261 | 0.256 | −0.209 | 2.782 | 0.001 | 0.023 | 0.070 | 0.000 | 0.683 | 0.265 |
| P3NNN | 0.264 | 0.249 | −0.215 | 2.784 | 0.000 | 0.019 | 0.058 | 0.001 | 0.624 | 0.192 |
| D1N | 0.271 | 0.237 | −0.227 | 2.795 | 0.002 | 0.046 | 0.149 | −0.001 | 0.891 | 0.375 |
| D2CN | 0.265 | 0.273 | −0.215 | 2.777 | 0.005 | 0.045 | 0.158 | −0.001 | 0.875 | 0.132 |
| D2NN | 0.262 | 0.253 | −0.212 | 2.779 | 0.001 | 0.030 | 0.096 | 0.001 | 0.747 | 0.299 |
| H1W1 | 0.266 | 0.264 | −0.216 | 2.789 | 0.004 | 0.043 | 0.145 | −0.001 | 0.867 | 0.226 |
| H1W2 | 0.270 | 0.239 | −0.226 | 2.796 | 0.002 | 0.037 | 0.120 | 0.000 | 0.822 | 0.361 |
| Np–Ocarb | Np–Owater | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N0 | — | — | — | — | — | — | — | — | — | — |
| N1 | — | — | — | — | — | 0.041 | 0.164 | 0.001 | 0.864 | 0.220 |
| P1C | 0.044 | 0.158 | 0.001 | 0.918 | 0.357 | 0.037 | 0.146 | 0.001 | 0.827 | 0.305 |
| P1N | 0.054 | 0.214 | −0.001 | 1.073 | 0.363 | 0.039 | 0.157 | 0.001 | 0.848 | 0.249 |
| P2CN | 0.046 | 0.168 | 0.001 | 0.973 | 0.332 | 0.031 | 0.115 | 0.001 | 0.741 | 0.327 |
| P2NN | 0.053 | 0.208 | −0.001 | 1.070 | 0.345 | 0.032 | 0.115 | 0.000 | 0.732 | 0.337 |
| P3CNN | 0.041 | 0.155 | 0.001, −0.001 | 0.952 | 0.304 | — | — | — | — | — |
| P3NNN | 0.051 | 0.209 | 0.001 | 1.084 | 0.292 | — | — | — | — | — |
| D1N | 0.049 | 0.192 | −0.000 | 1.019 | 0.343 | 0.034 | 0.123 | 0.001 | 0.751 | 0.345 |
| D2CN | 0.047 | 0.180 | −0.001 | 1.003 | 0.069 | — | — | — | — | — |
| D2NN | 0.039 | 0.148 | 0.001 | 0.952 | 0.331 | — | — | — | — | — |
| H1W1 | 0.050 | 0.199 | −0.000 | 1.078 | 0.289 | 0.037 | 0.147 | 0.001 | 0.839 | 0.151 |
| H1W2 | 0.045 | 0.175 | 0.000 | 0.977 | 0.334 | 0.030 | 0.106 | −0.000 | 0.690 | 0.355 |
In a bare neptunyl ion (N0), for the Np–Oyl bond, the electron density ρb, the Laplacian of electron density (∇2ρb), and the total energy density (Hb) at the (3, −1) bond critical point (BCP) were [0.335, 0.120, −0.347] and [0.294, 0.179, −0.269] respectively in the gas phase and in the aqueous phase, ρb > 0.20, ∇2ρb > 0, Hb < 0. According to Matta and Boyd,56 a ρb greater than 0.20 e bohr−3, a positive ∇2ρb, and a negative Hb are the feature of a shared bond. This means that the interactions between Np and Oyl has strong covalent feature.
In contrast, the interaction between Np and the coordinating atoms, both for the Ocarb and Npy, displays predominant ionic feature. For these dative bonds, the electronic density ρb is close to 0, ∇2ρb is positive, and Hb ≈ 0, indicating a depleted nature.
The δ(A,B) and ε provides consistent results for the nature of the Np–Oyl and the coordination bonds. In the bare neptunyl ion, the delocalization index (δ(A,B)) and the bond ellipticity (ε) were calculated to be 2.970 and 0.002 in the gas phase and 2.860 and 0.000 in the aqueous phase, respectively, which suggests a triple bond feature for the Np–Oyl bond and is consistent with previous work.31 The explicit consideration of water ligands in the first coordination shell of neptunyl ([NpO2(H2O)5]+, N1) caused a marginal decrease of δ(A,B) of Np–Oyl bonds of about 0.04, suggesting a weakening of the Np–Oyl, while this does not change its triple bond feature.
Upon its coordination with the organic ligands studied here, the δ(Np, Oyl) decreases further by 0.01–0.04 with a slight increase of the bond ellipticity (ε), indicating a stronger perturbation of the organic ligands to the bond nature of Np–Oyl than the water ligands.
For the dative bonds of the complexes, i.e. Np–Npy, Np–Ocarb, and Np–Owater, the delocalization indices (δ(A,B)) are in the range of 0.62–1.08, and the bond ellipticity (ε) values are in the range of 0.06–0.39. Among the dative bonds, the Np–Ocarb bonds appear with the largest δ(A,B), and for the same coordination mode, the more ligands coordinated to Np, the lower the δ(A,B) value (P1C and P1N vs. P2CN and P2NN vs. P3CNN and P3NNN, D1N vs. D2CN and D2NN, H1W1 vs. H1W2), indicating the saturation in the coordination of neptunyl and the competition of the ligands in interacting with neptunyl.
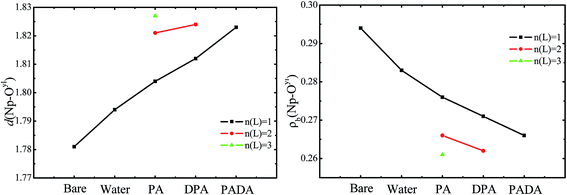
In Fig. 2, the distance and the ρb at BCP of Np–Oyl bond as a function of the type of ligand are plotted. As seen in Fig. 2, the bond length d increase from 1.781 Å to 1.823 Å, and the electron density ρb decrease from 0.294 e bohr−3 to 0.266 e bohr−3 at BCP of Np–Oyl bond in NpO2Ln (L = H2O, PA−, DPA2−, PADA2−, n(L) = 0, 1) complexes in the aqueous phase, which indicates enhanced perturbation on the strength of Np–Oyl bond by the coordination of the organic ligands in the complexes with the same stoichiometric ratio M![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) L = 1
L = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, suggesting the binding affinity of neptunyl to the ligands decreases in the order: PADA2− > DPA2− > PA− > H2O. The higher presence of the ligands brings more perturbation to the Np–Oyl bond.
1, suggesting the binding affinity of neptunyl to the ligands decreases in the order: PADA2− > DPA2− > PA− > H2O. The higher presence of the ligands brings more perturbation to the Np–Oyl bond.
In Fig. 3, the electron density in the equatorial plane transverse to the axis of neptunyl through Np is shown for the representative complexes complexes (NpO2Ln(H2O)m)j (L = PA−, DPA2−, and PADA2−, n = 0–3, m = 0−3, 5, j = 1+, 0, 1−, 2−, 3−), and the bond lengths of the dative bonds in the plane are also given. The data of Np–Oyl for the bare neptunyl are also shown. In these complexes, the ρb of Np–N bond is always smaller than that of Np–Ocarb, suggesting a larger accumulation of electron density of the latter than the former. This is consistent with their delocalization indices collected in Table 3.
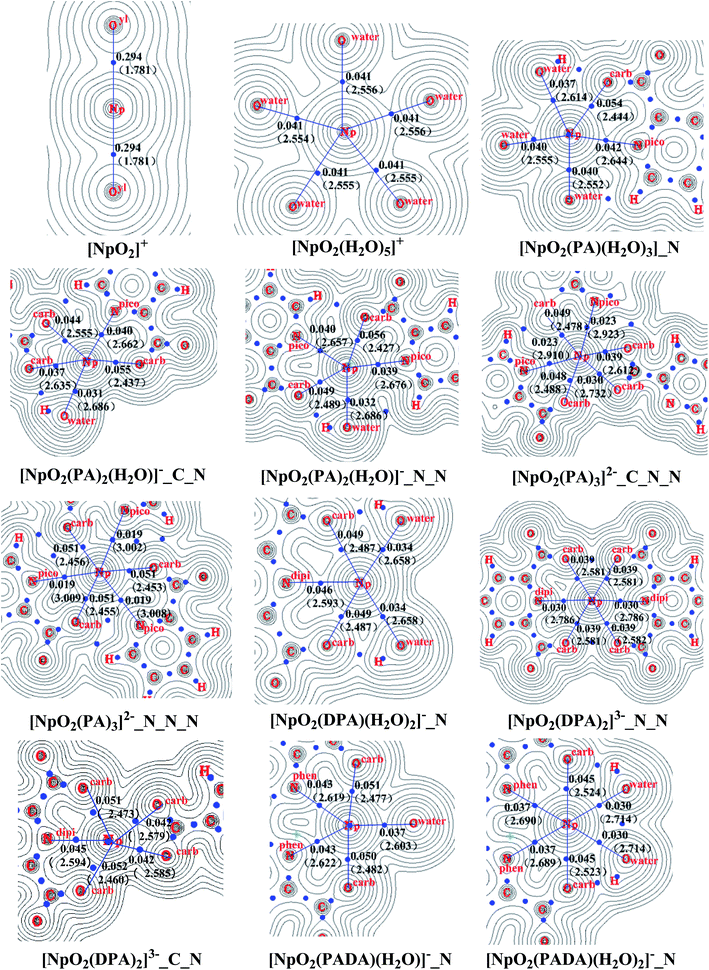 | ||
| Fig. 3 Electron density ρb (e− bohr−3) and bond length d (Å, in parenthesis) of NpO2+ complexes from calculations in aqueous phase. | ||
C. Charge transfer in the complexes
The charge decomposition analysis (CDA) proposed by Dapprich and Frenking,57,58 and the extended charge decomposition analysis (ECDA) by Gorelsky59,60 are used to calculate charge transfer between neptunyl and the ligands upon the ligand exchange. In CDA, the overall reorganization of electronic density is calculated which includes both the contributions of charge transfer (CT) and electronic polarization (PL). These are separated in ECDA, and the transferred charge can be directly obtained as CT(A → B) − CT(B → A) = [PL(A) + CT(A → B)] − [PL(A) + CT(B → A)].Here we consider the net charge transfer from the ligands to neptunyl in selected complexes by the CDA and ECDA methods at the B3LYP level, and the data are tabulated in Table 4. For Np![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) L = 1
L = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 type complexes, the net charge transfer from the ligands to neptunyl decreases in the order of PADA2− > DPA2− > PA− > H2O (in the range of 0.610–0.648, 0.594–0.615, 0.585–0.591, 0.573, and 0.900–0.999, 0.880–0.941, 0.871–0.878, 0.816, obtained by CDA and ECDA, respectively). This coincides the sequence of the relative stabilities, which suggests that the ligand-to-metal-charge-transfer (LMCT) plays an important role in the higher binding affinity of PADA2− and DPA2− than that of PA− and H2O.
1 type complexes, the net charge transfer from the ligands to neptunyl decreases in the order of PADA2− > DPA2− > PA− > H2O (in the range of 0.610–0.648, 0.594–0.615, 0.585–0.591, 0.573, and 0.900–0.999, 0.880–0.941, 0.871–0.878, 0.816, obtained by CDA and ECDA, respectively). This coincides the sequence of the relative stabilities, which suggests that the ligand-to-metal-charge-transfer (LMCT) plays an important role in the higher binding affinity of PADA2− and DPA2− than that of PA− and H2O.
| Complexes | Charge transfer | CDA | ECDA |
|---|---|---|---|
| (N1) [NpO2(H2O)5]+ | 5H2O → NpO2+ | 0.5734 | 0.8157 |
| (P1C) [NpO2 (PA)(H2O)3]_C | L + 3H2O → NpO2+ | 0.5908 | 0.8713 |
| (P1N) [NpO2 (PA)(H2O)3]_N | L + 3H2O → NpO2+ | 0.5852 | 0.8780 |
| (P2CN) [NpO2(PA)2(H2O)]−_C_N | 2L + H2O → NpO2+ | 0.6542 | 0.9885 |
| (P2NN) [NpO2(PA)2(H2O)]−_N_N | 2L + H2O → NpO2+ | 0.6315 | 0.9681 |
| (P3CNN) [NpO2(PA)3]2−_C_N_N | 3L → NpO2+ | 0.7408 | 1.1201 |
| (P3NNN) [NpO2(PA)3]2−_N_N_N | 3L → NpO2+ | 0.6970 | 1.0751 |
| (D1C) [NpO2(DPA)(H2O)3]−_C | L + 3H2O → NpO2+ | 0.5942 | 0.8799 |
| (D1N) [NpO2(DPA)(H2O)2]−_N | L + 2H2O → NpO2+ | 0.6152 | 0.9411 |
| (D2CN) [NpO2(DPA)2]3−_C_N | 2L → NpO2+ | 0.7429 | 1.1310 |
| (D2NN) [NpO2(DPA)2]3−_N_N | 2L → NpO2+ | 0.7897 | 1.2131 |
| (H1C) [NpO2(PADA)(H2O)3]−_C | L + 3H2O → NpO2+ | 0.6097 | 0.8996 |
| (H1W1) [NpO2(PADA)(H2O)]−_N | L + H2O → NpO2+ | 0.6211 | 0.9770 |
| (H1W2) [NpO2(PADA)(H2O)2]−_N | L + 2H2O → NpO2+ | 0.6483 | 0.9987 |
We note that the charge transfer is also correlated to the coordination mode. According to ECDA results, for each pair of isomers, e.g. P2CN vs. P2NN, P3CNN vs. P3NNN, and D2CN vs. D2CN, the co-presence of the _C and the _N coordination modes causes more charge transfer from ligands to neptunyl. In the complexes with a single organic ligands, i.e. P1C vs. P1N, D1C vs. D1N, and H1C vs. H1N, the latter appears with more charge transfer than the former. These results suggest that the combined hard–soft strategy, i.e. the harder Ocarb and the softer Npy, favors to stabilize the complexes with stronger electrostatic interaction between neptunyl and the ligands compared to the other coordination modes. This offers theoretical supports on the combined hard–soft strategy to develop extractants with higher selectivity towards the actinides.19,61,62
D. Molecular orbital (MO) and NAO analysis
The natural atomic orbital (NAO)63 was analyzed to understand the bonding of NpO2+ with ligands, and the representative α-spin frontier orbitals of P1C, P1N, D1N and H1W1 are shown in Fig. 4 and their compositions are tabulated in Table 5. These MOs are mainly contributed by the 2p atomic orbital of the O/N atoms and 5f or 6d atomic orbital of neptunium.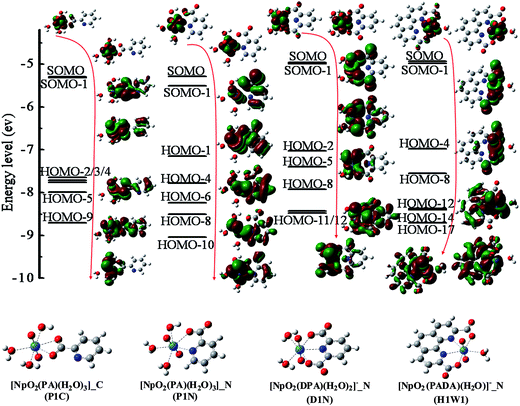 | ||
| Fig. 4 Representative α-spin frontier orbitals of NpO2+ complexes. The isosurface value of MO is 0.02 a.u. | ||
| P1C | HOMO−2 | HOMO−3 | HOMO−4 | HOMO−5 | HOMO−9 |
|---|---|---|---|---|---|
| Np | 4.1 | 30.2 | 23.0 | 9.2 | 9.3 |
| 5f/6d | 5fy(3x2−y2): 1.6 | 5fz3: 24.4 | 5fz3: 18.1, 5fxyz: 1.3 | 5fxz2: 3.9 | 6dyz: 8.8 |
| O1A/O1B | O1B: 7.0 (2px), 17.7 (2py) | O1B: 13.9 (2pz) | O1B: 22.3 (2pz) | O1B: 29.3 (2px), 2.7 (2py) | O1B: 1.8 (2pz) |
| O1A: 30.1 (2px), 25.8 (2py) | O1A: 19.7 (2pz) | O1A: 25.7 (2pz) | O1A: 11.9 (2px) | O1A: 1.9 (2pz) |
| P1N | HOMO−1 | HOMO−4 | HOMO−6 | HOMO−8 | HOMO−10 |
|---|---|---|---|---|---|
| Np | 6.5 | 39.9 | 11.0 | 21.4 | 13.9 |
| 5f/6d | 5fz3: 4.5 | 5fz3: 32.8 | 5fz3: 1.0, 5fxz2: 2.9, 5fyz2: 1.4 | 5fxz2: 15.6, 5fyz2: 3.3 | 6dxz: 11.3, 6dyz: 2.2 |
| N1/O1A | O1A: 32.2 (2pz) | N1: 2.4 (2pz), O1A: 9.6 (2pz) | N1: 26.4 (2px), 4.6 (2pz), O1A: 1.4 (2py) | N1: 1.6 (2s), 16.2 (2px) | N1: 1.2 (2pz) |
| D1N | HOMO−2 | HOMO−5 | HOMO−8 | HOMO−11 | HOMO−12 |
|---|---|---|---|---|---|
| Np | 18.8 | 5.5 | 23.9 | 5.8 | 10.8 |
| 5f/6d | 5fz3: 14.6 | 5fyz2: 1.3, 6dxy: 1.4 | 5fxz2: 21.2 | 5fxz2: 1.4, 5fx(x2−3y2): 1.3, 6dx2y2: 1.6 | 6dyz: 9.8 |
| N1/O2A/O1A | O2A: 16.2 (2pz), O1A: 16.2 (2pz) | O2A: 2.1 (2px), 29.1 (2py), O1A: 2.1 (2px), 29.1 (2py) | N1: 1.2 (2px), O2A: 2.5 (2py), O1A: 2.5 (2py) | N1: 3.5 (2s), 34.9 (2px), O2A: 2.2 (2py), O1A: 2.2 (2py) | N1: 0.9 (2px), O2A: 1.8 (2pz), O1A: 1.8 (2pz) |
| H1W1 | HOMO−4 | HOMO−8 | HOMO−12 | HOMO−14 | HOMO−17 |
|---|---|---|---|---|---|
| Np | 19.4 | 36.2 | 6.9 | 6.55 | 6.3 |
| 5f/6d | 5fz3: 13.5, 5fz(x2−y2): 2.5 | 5fz3: 29.8 | 5fxz2: 1.6, 6dxz: 2.7 | 5fy(3x2−y2): 2.0, 6dxy: 2.3 | 6dz2: 1.0 |
| N1/N2/O2A/O1A | O2A: 15.9 (2pz), O1A: 17.3 (2pz) | O2A: 6.3 (2pz), O1A: 6.5 (2pz) | N2: 5.7 (2px), 4.8 (2py), N1: 4.4 (2px), 2.5 (2py) | N2: 13.3 (2px), 6.1 (2py), 2.2 (2pz), N1: 16.4 (2px), 4.1 (2py), 2.7 (2pz) | N2: 4.8 (2px), 4.4 (2py), N1: 6.0 (2px), 3.2 (2py) |
As shown in Fig. 4, for all of the four complexes, the two singly occupied molecular orbitals (SOMO) are contributed by the Np and the Oyl atoms, which is consistent with the observations that the unpaired electrons are localized within the neptunyl moiety indicated by the spin density distribution. For the orbitals that constituted from both the neptunyl and the ligands, it is shown that from P1C to P1N to D1N and H1W1, there is increasing orbital overlap from these fragments, suggesting stronger ionic feature in P1C while more covalency in the other complexes. This indicates that the excess stabilization brought by _N coordination mode (P1N, D1N, and H1W1), compared to the _C mode (P1C), may be contributed by the enhanced covalent interaction between the neptunyl and the ligands.
As listed in Table 5, the compositions of the representative orbitals display localized feature, i.e. the major contribution comes from the Np and the ligand atoms coordinated to Np. For the complex P1C, some frontier MOs contains significant contributions from the two Ocarb atoms of the same carboxylate group. It is conceivable that this perturbs the delocalization feature of electrons in the carboxylate group. In the other complexes, the N atoms and one Ocarb atom of each carboxylate group have noticeable contributions. This avoids significant perturbation to the ligands and maintains the aromaticity of the hetero-rings and the local electronic feature of the carboxylate groups of the ligands.
E. Comparison with the experimental work
In Rao et al.'s work,25 the trends in the protonation constants of H2PADA and related ligands and the thermodynamic data for NpV complexes with three ligands were discussed. The first protonation constants of the ligands were observed to decrease in the order: HPA > H2DPA > H2PADA. This trend was explained by the intramolecular hydrogen bonding between the carboxylate group and the nitrogen atom which became weaker along the series. Though the preparation of the crystals of the NpV/PADA2− complex was not successful, the spectrophotometric and calorimetric data undoubtedly indicated that the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complex formed in solution and appeared to be much stronger than complexes formed by the other two ligands. This is reasonable concerning the increase in denticity from HPA to H2DPA and H2PADA that stabilizes the NpV complexes: PA− < DPA2− < PADA2−. Our calculations are consistent with the experimental observations on the variation of the stability constants of the complexes in view of the relative free energies of complexation, which are tabulated in Table 2.
1 complex formed in solution and appeared to be much stronger than complexes formed by the other two ligands. This is reasonable concerning the increase in denticity from HPA to H2DPA and H2PADA that stabilizes the NpV complexes: PA− < DPA2− < PADA2−. Our calculations are consistent with the experimental observations on the variation of the stability constants of the complexes in view of the relative free energies of complexation, which are tabulated in Table 2.
The calculated entropic and enthalpic contributions are plotted in Fig. 5. For Np![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) L = 1
L = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 type complexes, based on our calculations, their formation of relative Gibbs free energies decreased by the order: PADA2− (−55.10 kcal mol−1) > DPA2− (−40.71 kcal mol−1) > PA− (−20.75 kcal mol−1) in the aqueous phase. This trend is consistent with the experimental data which report the free energy changes of −15.99 kcal mol−1 for PADA2−, −11.85 kcal mol−1 for DPA2−, and −4.90 kcal mol−1 for PA−. We note that there is substantial difference in the values between the calculated and the experimentally derived ones, which may be due to the treatment of the model systems in this work, e.g. the insufficient sampling of the model systems, the implicit treatment of solvent effect, and the omitting of the counterion effect. This makes it hard to make a direct comparison between them, and molecular dynamic simulations at first-principle level are needed which is beyond the scope of this work.
1 type complexes, based on our calculations, their formation of relative Gibbs free energies decreased by the order: PADA2− (−55.10 kcal mol−1) > DPA2− (−40.71 kcal mol−1) > PA− (−20.75 kcal mol−1) in the aqueous phase. This trend is consistent with the experimental data which report the free energy changes of −15.99 kcal mol−1 for PADA2−, −11.85 kcal mol−1 for DPA2−, and −4.90 kcal mol−1 for PA−. We note that there is substantial difference in the values between the calculated and the experimentally derived ones, which may be due to the treatment of the model systems in this work, e.g. the insufficient sampling of the model systems, the implicit treatment of solvent effect, and the omitting of the counterion effect. This makes it hard to make a direct comparison between them, and molecular dynamic simulations at first-principle level are needed which is beyond the scope of this work.
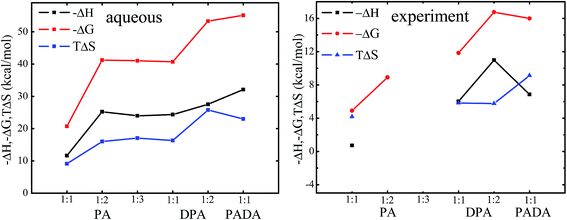 | ||
| Fig. 5 Thermodynamic trends of NpO2+ complexes from calculations in the aqueous phase (left) and from experiment (right). | ||
The energies were refined by using more sophisticated treatment (BS2) of the atoms where a larger basis set 6-311++G(d,p) was used for the atoms except for Np, and the results were plotted in Fig. 6. The data show that for the three ligands, when they form complexes with neptunyl with the same stoichiometric ratio, higher denticity brings more stabilization energy, i.e. the stability of the complexes decrease in the order of PADA2− > DPA2− > PA−. This trend is consistent with the data from the calculations at the B3LYP/BS1 level, and agrees with the reported experimental observations.
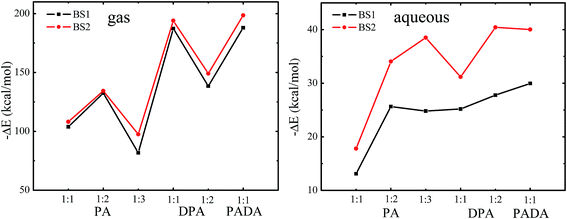 | ||
| Fig. 6 The relative electronic energies of NpO2+ complexes calculated using basis set BS1 and larger basis set BS2 in the gas phase and aqueous phase. | ||
Conclusions
In the present work, we report a DFT study of the interactions between neptunyl ion (NpO2+) and the deprotonated 1,10-phenanthrolin-2,9-dicarboxylic acid anion (PADA2−). Its analogs, the deprotonated picolinic acid anion (PA−) and dipicolinic acid anion (DPA2−), were also investigated and compared. The geometries, thermodynamics of the complexation reactions, and the electronic structures of the complexes were analyzed to evaluate the coordination modes and stoichiometry ratio of neptunyl ion with ligands. The calculations indicate that the coordination of NpO2+ to tetradentate chelators is more favorable than that to tridentate and bidentate ones, and the coordination ability of three deprotonated ligands follows the order: PADA2− > DPA2− > PA−.The QTAIM analysis showed that the metal–ligand interactions have strong ionic feature. In addition to the QTAIM analysis, the charge decomposition analysis (CDA) and extended charge decomposition analysis (ECDA) were performed to quantify the charge donation and back-donation between the metal and ligand fragments in complexes. For Np![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) L = 1
L = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 type complexes, the net charge transfer from the ligands to neptunyl decreases in the order of PADA2− > DPA2− > PA− > H2O, which is in good agreement with the relative thermodynamic stabilities of the corresponding complexes. The natural atomic orbital (NAO) analysis revealed that the 5f orbitals of Np participated in the metal–ligand dative bond and contribute to its covalency.
1 type complexes, the net charge transfer from the ligands to neptunyl decreases in the order of PADA2− > DPA2− > PA− > H2O, which is in good agreement with the relative thermodynamic stabilities of the corresponding complexes. The natural atomic orbital (NAO) analysis revealed that the 5f orbitals of Np participated in the metal–ligand dative bond and contribute to its covalency.
In summary, our calculations show that the denticity of ligand and the combined hard–soft donor strategy work cooperatively in the coordination of Np with ligands, which should be taken into account in the rational design of new type of extractants for the separation of Np.
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China to W. Chen (10676007), to Z. Chai (91026000) and to D. Wang (21473206, 91226105), which are gratefully acknowledged. The Special Program for Applied Research on Super Computation of the NSFC-Guangdong Joint Fund (the second phase), the National Supercomputing Center in Tianjin (NSCC-TJ), the Supercomputing Center of Chinese Academy of Sciences are acknowledged for providing computational grids.References
- J. Bruno and R. C. Ewing, Elements, 2006, 2, 343–349 CrossRef CAS.
- P. C. Burns, K. M. Deely and S. Skanthakumar, Radiochim. Acta, 2004, 92, 151–160 CrossRef CAS.
- P. A. Baisden and G. R. Choppin, Radiochemistry and Nuclear Chemistry, 2007, pp. 1–63 Search PubMed.
- E. Bondietti, Environmental migration of long-lived radionuclides, 1982, pp. 81–96 Search PubMed.
- W. Runde, S. D. Conradson, D. W. Efurd, N. Lu, C. E. VanPelt and C. D. Tait, Appl. Geochem., 2002, 17, 837–853 CrossRef CAS.
- K. Balasubramanian and Z. Cao, Inorg. Chem., 2007, 46, 10510–10519 CrossRef CAS PubMed.
- M. Garcia-Hernandez, C. Willnauer, S. Kruger, L. V. Moskaleva and N. Rosch, Inorg. Chem., 2006, 45, 1356–1366 CrossRef CAS PubMed.
- F. Gendron, D. Paez-Hernandez, F. P. Notter, B. Pritchard, H. Bolvin and J. Autschbach, Chem.–Eur. J., 2014, 20, 7994–8011 CrossRef CAS PubMed.
- X. Yang, Y. N. Liang, S. D. Ding, S. J. Li, Z. F. Chai and D. Q. Wang, Inorg. Chem., 2014, 53, 7848–7860 CrossRef CAS PubMed.
- D. Rios, P. X. Rutkowski, M. J. Van Stipdonk and J. K. Gibson, Inorg. Chem., 2011, 50, 4781–4790 CrossRef CAS PubMed.
- V. N. Serezhkin, M. A. Krivopalova and L. B. Serezhkina, Russ. J. Coord. Chem., 1998, 24, 59–66 CAS.
- Z. Kolarik, U. Müllich and F. Gassner, Solvent Extr. Ion Exch., 1999, 17, 23–32 CrossRef CAS.
- Z. Kolarik, U. Mullich and F. Gassner, Solvent Extr. Ion Exch., 1999, 17, 1155–1170 CrossRef CAS.
- M. G. Drew, M. R. Foreman, C. Hill, M. J. Hudson and C. Madic, Inorg. Chem. Commun., 2005, 8, 239–241 CrossRef CAS.
- M. R. S. J. Foreman, M. J. Hudson, A. Geist, C. Madic and M. Weigl, Solvent Extr. Ion Exch., 2005, 23, 645–662 CrossRef CAS.
- A. Geist, C. Hill, G. Modolo, M. R. S. J. Foreman, M. Weigl, K. Gompper and M. J. Hudson, Solvent Extr. Ion Exch., 2006, 24, 463–483 CrossRef CAS.
- F. W. Lewis, L. M. Harwood, M. J. Hudson, M. G. Drew, V. r. Hubscher-Bruder, V. Videva, F. O. Arnaud-Neu, K. Stamberg and S. Vyas, Inorg. Chem., 2013, 52, 4993–5005 CrossRef CAS PubMed.
- F. W. Lewis, L. M. Harwood, M. J. Hudson, M. G. Drew, J. F. Desreux, G. Vidick, N. Bouslimani, G. Modolo, A. Wilden and M. Sypula, J. Am. Chem. Soc., 2011, 133, 13093–13102 CrossRef CAS PubMed.
- C. L. Xiao, C. Z. Wang, L. Y. Yuan, B. Li, H. He, S. Wang, Y. L. Zhao, Z. F. Chai and W. Q. Shi, Inorg. Chem., 2014, 53, 1712–1720 CrossRef CAS PubMed.
- G. Tian, J. Xu and L. Rao, Angew. Chem., Int. Ed., 2005, 44, 6200–6203 CrossRef CAS PubMed.
- G. Tian, L. Rao and A. Oliver, Chem. Commun., 2007, 4119–4121, 10.1039/B706825E.
- L. Rao and G. Tian, Symmetry, 2009, 2, 1–14 CrossRef.
- G. Tian, L. Rao and S. J. Teat, Inorg. Chem., 2009, 48, 10158–10164 CrossRef CAS PubMed.
- Y. Gong, H.-S. Hu, L. Rao, J. Li and J. K. Gibson, J. Phys. Chem. A, 2013, 117, 10544–10550 CrossRef CAS PubMed.
- Y. Yang, Z. Zhang, S. Luo and L. Rao, Eur. J. Inorg. Chem., 2014, 2014, 5561–5566 CrossRef CAS.
- S. A. Ansari, A. Bhattacharyya, Z. Zhang and L. Rao, Inorg. Chem., 2015, 54, 8693–8698 CrossRef CAS PubMed.
- S. A. Ansari, P. Pathak, P. K. Mohapatra and V. K. Manchanda, Chem. Rev., 2011, 112, 1751–1772 CrossRef PubMed.
- J. M. Combes, C. J. Chisholm-Brause, G. E. Brown Jr, G. A. Parks, S. D. Conradson, P. G. Eller, I. R. Triay, D. E. Hobart and A. Miejer, Environ. Sci. Technol., 1992, 26, 376–382 CrossRef CAS.
- Y. Inoue and O. Tochiyama, Polyhedron, 1983, 2, 627–630 CrossRef CAS.
- Y.-N. Liang, X. Yang, S. Ding, S. Li, F. Wang, Z. Chai and D. Wang, New J. Chem., 2015, 39, 7716–7729 RSC.
- J. Zeng, X. Yang, J. Liao, N. Liu, Y. Yang, Z. Chai and D. Wang, Phys. Chem. Chem. Phys., 2014, 16, 16536–16546 RSC.
- A. D. Becke, Phys. Rev. A, 1988, 38, 3098 CrossRef CAS.
- C. Lee, W. Yang and R. G. Parr, Phys. Rev. B: Condens. Matter Mater. Phys., 1988, 37, 785 CrossRef CAS.
- R. Conte, A. Aspuru-Guzik and M. Ceotto, J. Phys. Chem. Lett., 2013, 4, 3407–3412 CrossRef CAS PubMed.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G. A. Petersson, H. Nakatsuji, M. Caricato, X. Li, H. P. Hratchian, A. F. Izmaylov, J. Bloino, G. Zheng, J. L. Sonnenberg, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, J. A. Montgomery Jr, J. E. Peralta, F. Ogliaro, M. Bearpark, J. J. Heyd, E. Brothers, K. N. Kudin, V. N. Staroverov, R. Kobayashi, J. Normand, K. Raghavachari, A. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, N. Rega, J. M. Millam, M. Klene, J. E. Knox, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, R. L. Martin, K. Morokuma, V. G. Zakrzewski, G. A. Voth, P. Salvador, J. J. Dannenberg, S. Dapprich, A. D. Daniels, O. Farkas, J. B. Foresman, J. V. Ortiz, J. Cioslowski and D. J. Fox, Gaussian 09, Revision C.1, Gaussian, Inc., Wallingford, CT, 2009 Search PubMed.
- P. L. Houston, R. Conte and J. M. Bowman, J. Phys. Chem. A, 2014, 118, 7758–7775 CrossRef CAS PubMed.
- P. L. Houston, R. Conte and J. M. Bowman, J. Phys. Chem. A, 2015, 119, 4695–4710 CrossRef CAS PubMed.
- S. Huzinaga, Comput. Phys. Rep., 1985, 2, 281–339 CrossRef.
- C. Xie, J. Li, D. Xie and H. Guo, J. Chem. Phys., 2012, 137, 024308 CrossRef PubMed.
- R. Conte, C. Qu and J. M. Bowman, J. Chem. Theory Comput., 2015, 11, 1631–1638 CrossRef CAS PubMed.
- Z. Homayoon, R. Conte, C. Qu and J. M. Bowman, J. Chem. Phys., 2015, 143, 084302 CrossRef PubMed.
- X. Cao, M. Dolg and H. Stoll, J. Chem. Phys., 2003, 118, 487–496 CrossRef CAS.
- X. Cao and M. Dolg, J. Mol. Struct.: THEOCHEM, 2004, 673, 203–209 CrossRef CAS.
- W. Küchle, M. Dolg, H. Stoll and H. Preuss, J. Chem. Phys., 1994, 100, 7535–7542 CrossRef.
- M. Cossi, N. Rega, G. Scalmani and V. Barone, J. Comput. Chem., 2003, 24, 669–681 CrossRef CAS PubMed.
- J. Tomasi, B. Mennucci and R. Cammi, Chem. Rev., 2005, 105, 2999–3094 CrossRef CAS PubMed.
- R. Dennington, T. Keith and J. Millam, GaussView, Version 5, Semichem Inc., Shawnee Mission, KS, 2009 Search PubMed.
- T. Lu and F. Chen, J. Comput. Chem., 2012, 33, 580–592 CrossRef CAS PubMed.
- R. F. Bader, J. Phys. Chem. A, 1998, 102, 7314–7323 CrossRef CAS.
- A. Becke, C. F. Matta and R. J. Boyd, The quantum theory of atoms in molecules: from solid state to DNA and drug design, John Wiley & Sons, 2007 Search PubMed.
- M. J. Tassell and N. Kaltsoyannis, Dalton Trans., 2010, 39, 6719–6725 RSC.
- M. W. Schmidt, K. K. Baldridge, J. A. Boatz, S. T. Elbert, M. S. Gordon, J. H. Jensen, S. Koseki, N. Matsunaga, K. A. Nguyen and S. Su, J. Comput. Chem., 1993, 14, 1347–1363 CrossRef CAS.
- R. Conte, B. Fu, E. Kamarchik and J. M. Bowman, J. Chem. Phys., 2013, 139, 044104 CrossRef PubMed.
- L.-H. Lee, in Fundamentals of Adhesion, Springer, 1991, pp. 349–362 Search PubMed.
- R. G. Pearson, J. Am. Chem. Soc., 1963, 85, 3533–3539 CrossRef CAS.
- C. F. Matta and R. J. Boyd, The Quantum Theory of Atoms in Molecules: From Solid State to DNA and Drug Design, 2007 Search PubMed.
- S. Dapprich and G. Frenking, J. Phys. Chem., 1995, 99, 9352–9362 CrossRef CAS.
- G. Frenking and N. Fröhlich, Chem. Rev., 2000, 100, 717–774 CrossRef CAS PubMed.
- S. I. Gorelsky, S. Ghosh and E. I. Solomon, J. Am. Chem. Soc., 2006, 128, 278–290 CrossRef CAS PubMed.
- S. I. Gorelsky and E. I. Solomon, Theor. Chem. Acc., 2008, 119, 57–65 CrossRef CAS.
- D. Manna and T. K. Ghanty, Phys. Chem. Chem. Phys., 2012, 14, 11060–11069 RSC.
- P. R. Zalupski, D. D. Ensor, C. L. Riddle and D. R. Peterman, Solvent Extr. Ion Exch., 2013, 31, 430–441 CrossRef CAS.
- J. Foster and F. Weinhold, J. Am. Chem. Soc., 1980, 102, 7211–7218 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra26114k |
| This journal is © The Royal Society of Chemistry 2017 |