Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Nine new compounds from the root bark of Lycium chinense and their α-glucosidase inhibitory activity†
Ya-Nan Yang‡
a,
Ya-Wen An‡a,
Zhi-Lai Zhanb,
Jing Xiea,
Jian-Shuang Jianga,
Zi-Ming Fenga,
Fei Yea and
Pei-Cheng Zhang*a
aState Key Laboratory of Bioactive Substance and Function of Natural Medicines, Institute of Materia Medica, Chinese Academy of Medical Sciences, Peking Union Medical College, Beijing 100050, People's Republic of China. E-mail: pczhang@imm.ac.cn
bState Key Laboratory Breeding Base of Dao-di Herbs, National Resource Center for Chinese Materia Medica, China Academy of Chinese Medical Sciences, Beijing, 100700, People's Republic of China
First published on 3rd January 2017
Abstract
Lycium chinense Mill. is a deciduous shrub in the Solanaceae family that is known for its fruits (Lycii fructus) and root bark (Lycii cortex). In our ongoing search for α-glucosidase inhibitors from the root bark of L. chinense, lyciumflavane A, one new flavane with an unusual benzofuran unit, one new amide possessing a naphthalene skeleton, one new sesquiterpene, three new lignan glucosides, and three new phenolic glucosides were isolated along with eight known compounds. Their structures were elucidated using NMR, HRESIMS, UV, ECD, and IR spectroscopic data. Their α-glucosidase inhibitory activity was screened using acarbose as a positive control (IC50 = 385 μM). Compound 1 showed strong inhibitory activity against α-glucosidase (IC50 = 20.89 μM).
Introduction
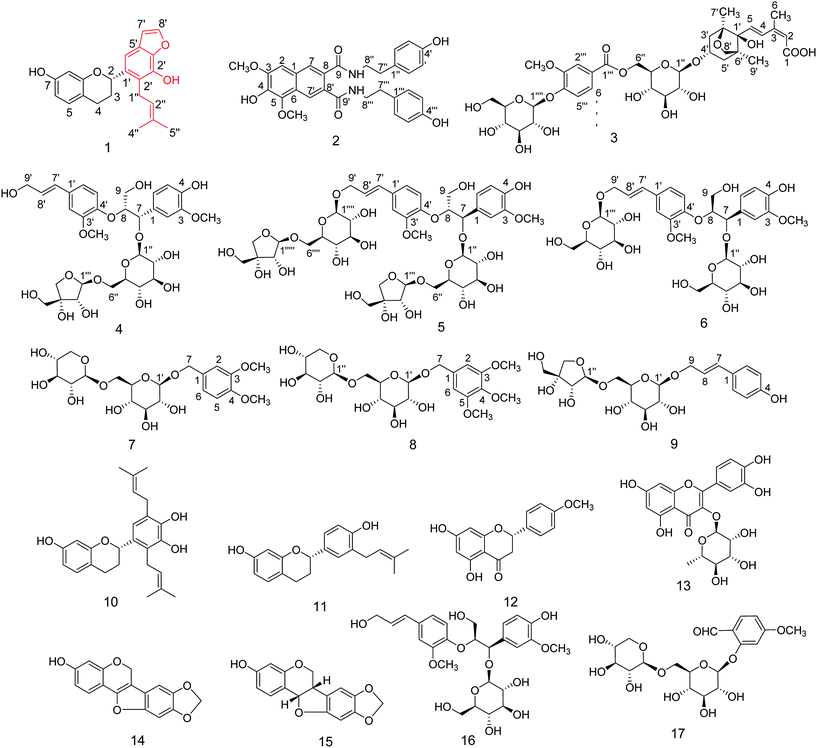
Lycium chinense Mill. is cultivated in the mainland of China and is an important source for health foods and traditional Chinese medicine.1–3 The root bark of L. chinense, named Digupi in China, is conventionally used in traditional Chinese medicine prescriptions to treat diabetes, coughs, hypertension, and fever. Modern pharmacological studies revealed that extracts from the root bark of L. chinense can lower serum glucose levels and improve insulin resistance.4,5 Previously, phytochemical investigations of this plant have shown the presence of alkaloids, lignanamides, cyclopeptides, lignans, and sterols.6–12With the aim of discovering new bioactive natural products with hypoglycemic effects from the root bark of L. chinense, an oral sucrose tolerance test (OSTT) was performed. The results demonstrated that the water-soluble portion of the root bark of L. chinense obtained from an 80% EtOH extract could significantly decrease the postprandial blood glucose levels in normal ICR mice at a dose of 200 mg kg−1 (Fig. S1, ESI†), which is similar to the hypoglycemic effect of acarbose (20 mg kg−1). Through bioactivity-guided isolation, one new flavane with an unusual benzofuran unit, one new amide possessing a naphthalene skeleton, one new sesquiterpene, three new lignan glucosides, and three new phenolic glucosides were obtained from the root bark of L. chinense along with eight known compounds (Fig. 1). In this paper, we reported the isolation and structure elucidation of compounds 1–9 and evaluated these compounds as α-glucosidase inhibitors.
Results and discussion
The molecular formula of 1 was determined to be C22H22O4 by the positive ion peak at m/z 351.1586 [M + H]+ in the HRESIMS, which indicated 12 degrees of unsaturation. The 1H NMR spectrum of 1 (Table 1) revealed an ABX system aromatic ring at δH 6.88 (1H, d, J = 8.0 Hz), 6.29 (1H, dd, J = 2.0, 8.0 Hz), and 6.18 (1H, d, J = 2.0 Hz), a pentasubstituted aromatic ring at δH 7.17 (1H, s), three olefinic protons at δH 6.88 (1H, d, J = 2.0 Hz), 7.91 (1H, d, J = 2.0 Hz), and 5.07 (1H, m), an oxymethylene proton at δH 5.08 (1H, br d, J = 11.0 Hz), three methylene protons at δH 3.49 (1H, m), 3.42 (1H, m), 2.82 (1H, m), 2.66 (1H, m), 2.09 (1H, m), and 1.88 (1H, m), and two methyl groups at δH 1.67 (3H, s) and 1.61 (3H, s). The 13C NMR and HSQC spectra of 1 displayed 22 carbon resonances including an isopentene group, two olefinic carbons and a C6–C3–C6 unit, which could be attributed to a flavone skeleton.| No. | 1 | 2 | 3 | 4 | 5 | 6 |
|---|---|---|---|---|---|---|
| 2 | 5.08, br d (11.0) | 7.16, s | 5.65, s | 7.07, d (2.0) | 7.07, d (2.0) | 6.97, d (2.0) |
| 3 | 2.09, m | |||||
| 1.88, m | ||||||
| 4 | 2.82, m | 8.05, d (16.5) | ||||
| 2.66, m | ||||||
| 5 | 6.88, d (8.0) | 6.02, d (16.5) | 6.67, d (8.0) | 6.67, d (8.0) | 6.77, d (8.0) | |
| 6 | 6.29, dd (2.0, 8.0) | 1.81, s | 6.80, dd (2.0, 8.0) | 6.80, dd (2.0, 8.0) | 7.00, d (8.0) | |
| 7 | 7.75, s | 4.96, d (4.5) | 4.95, d (4.5) | 4.94, d (4.0) | ||
| 8 | 6.18, d (2.0) | 4.38, m | 4.39, m | 4.50, m | ||
| 9 | 3.65, m | 3.62, m | 3.66, m | |||
| 3.21, m | 3.23, m | 3.21, m | ||||
| 2′ | 7.01, d (2.0) | 7.03, d (2.0) | 7.05, d (1.5) | |||
| 3′ | 1.96, m | |||||
| 1.73, m | ||||||
| 4′ | 3.95, m | |||||
| 5′ | 1.63, m | 6.96, d (8.0) | 6.97, d (8.0) | 6.67, d (8.0) | ||
| 1.57, m | ||||||
| 6′ | 7.17, s | 6.87, dd (2.0, 8.0) | 6.89, dd (2.0, 8.0) | 6.87, dd (8.0, 2.0) | ||
| 7′ | 6.88, d (2.0) | 7.83, s | 0.99, s | 6.43, d (16.0) | 6.55, d (16.0) | 6.55, d (16.5) |
| 8′ | 7.91, d (2.0) | 3.48, m | 6.23, dt (6.0, 16.0) | 6.20, m | 6.23, dt (16.0) | |
| 9′ | 0.67, s | 4.08, m | 4.15, m | 4.39, d (6.0) | ||
| 4.37, m | 4.16, m | |||||
| 1′′ | 3.42, m | 4.25, d (8.0) | 3.98, d (7.5) | 3.97, d (7.0) | 4.41, d (7.5) | |
| 3.49, m | ||||||
| 2′′ | 5.07, m | 7.06, d (8.5) | 2.94, m | 3.61, m | 3.00, m | 2.99, m |
| 3′′ | 6.69, d (8.5) | 3.28, m | 3.12, m | 3.01, m | 3.13, m | |
| 4′′ | 1.67, s | 3.11, m | 3.11, m | 2.99, m | 3.03, m | |
| 5′′ | 1.61, s | 6.69, d (8.5) | 3.17, m | 3.13, m | 3.27, m | 3.06, m |
| 6′′ | 7.06, d (8.5) | 4.49, d (10.5) | 3.83, d (10.0) | 3.48, m | 3.68, m, | |
| 4.29, m | 3.49, m | 3.85, m | 3.57, d (1.0) | |||
| 7′′ | 2.72, m | |||||
| 8′′ | 3.36, m | |||||
| 1′′′ | 4.89, d (3.0) | 4.88, d (3.0) | 4.20, d (8.0) | |||
| 2′′′ | 7.08, d (8.5) | 7.47, d (1.5) | 3.83, m | 3.78, m | 3.00, m | |
| 3′′′ | 6.71, d (8.5) | 3.14, m | ||||
| 4′′′ | 3.87, d (9.5) | 3.85, m | 3.04, m | |||
| 3.60, d (9.5) | 3.59, m | |||||
| 5′′′ | 6.71, d (8.5) | 7.15, d (8.5) | 3.36, m | 3.35, m | 3.08, m | |
| 6′′′ | 7.08, d (8.5) | 7.54, dd (8.5, 1.5) | 3.68, m | |||
| 3.45, m | ||||||
| 1′′′′ | 4.99, d (6.5) | 4.20, d (8.0) | ||||
| 2′′′′ | 3.18, m | 2.99, m | ||||
| 3′′′′ | 3.28, m | 3.13, m | ||||
| 4′′′′ | 3.17, m | 3.06, m | ||||
| 5′′′′ | 3.28, m | 3.13, m | ||||
| 6′′′′ | 3.64, d (10.5) | 3.42, m | ||||
| 3.47, m | 3.84, m | |||||
| 1′′′′ | 4.89, d (3.0) | |||||
| 2′′′′ | 3.71, m | |||||
| 4′′′′ | 3.59, m | |||||
| 3.87, m | ||||||
| 5′′′′ | 3.36, m | |||||
| 3-OCH3 | 3.93, s | 3.71, s | 3.71, s | 3.78, s | ||
| 5-OCH3 | 3.87, s | |||||
| 3′-OCH3 | 3.77, s | 3.77, s | 3.71, s | |||
| 3′′′-OCH3 | 3.80, s |
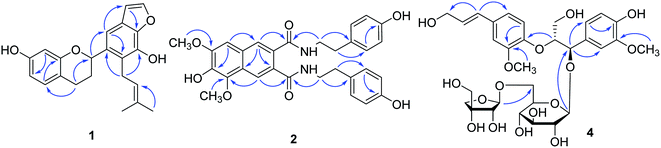
From the HMBC spectrum, the correlations of H-2 [δH 5.08 (1H, d, J = 10.0 Hz)] to C-1′ (δC 135.6) and C-6′ (δC 109.1) as well as the correlations of H-6′ [δH 7.17 (1H, s)] to C-2 (δC 74.6), C-1′ (δC 135.6), C-2′ (δC 122.3), C-4′ (δC 143.5), and C-5′ (δC 126.2) confirmed the basic skeleton of the flavane. The correlations of H-5 [δH 6.88 (1H, d, J = 8.0 Hz)] to C-9 (δC 156.4) and C-7 (δC 155.7) suggested the existence of 7-OH at ring A of the flavane. Moreover, the isopentene group was connected to the C-2′ (δC 122.3) of ring B because the H-1′′ [δH 3.42 (1H, m)] was correlated with C-1′ (δC 135.6), C-2′ (δC 122.3), and C-3′ (δC 139.3). By considering the remaining two degrees of unsaturation and the molecular formula, the benzofuran unit was established, which was supported by the HMBC correlations (Fig. 2) from H-7′ [δH 6.88 (1H, d, J = 2.0 Hz)] to C-4′ (δC 143.5), C-5′ (δC 126.2), and C-8′ (δC 145.3) and from H-8′ [δH 7.91 (1H, d, J = 2.0 Hz)] to C-4′ (δC 143.5) and C-5′ (δC 126.2). In addition, the 2S configuration was confirmed by the negative Cotton effect at 280 nm in the ECD spectrum.15 So, compound 1 was established as shown, and was accorded the trivial name lyciumflavane A.
Compound 2 was obtained as a yellow amorphous powder. The molecular formula, C30H30N2O7, was established by the protonated molecular ion peak at m/z 531.2131 [M + H]+ (calcd for C30H31N2O7, 531.2131) in the HRESIMS, which corresponded to 17 degrees of unsaturation. In the 1H NMR spectrum of 2 (Table 1), the presence of two p-tyramine moieties was deduced from two AA′BB′ spin system aromatic rings [δH 7.06 (2H, d, J = 8.5 Hz), 6.69 (2H, d, J = 8.5 Hz), 7.08 (2H, d, J = 8.5 Hz) and 6.71 (2H, d, J = 8.5 Hz)], two pairs of methylene proton signals [δH 2.72 (4H, m) and 3.36 (4H, m)], and two NH signals [δH 8.40 (1H, t, J = 5.5 Hz) and 8.34 (1H, t, J = 5.5 Hz)]. Simultaneously, another three aromatic protons at δH 7.16 (1H, s), 7.75 (1H, s), and 7.83 (1H, s), and two methoxy groups at δH 3.93 (3H, s) and 3.87 (3H, s) were also observed. The 13C NMR spectrum (Table 2) of 2 revealed 30 carbon signals, 20 of which were assigned to two p-tyramine moieties, two carbonyl groups and two methoxy groups, and the remaining 10 carbons were assigned to a naphthalene unit combined with the degrees of unsaturation. The HMBC correlations (Fig. 2) of H-8′′/C-9, H-8′′′/C-9′, H-7/C-9, and H-7′/C-9′ suggested that 2 was an amide possessing a naphthalene skeleton. The linkage points of the two methoxy groups were confirmed to be at C-3 and C-5 based on the correlations between the methoxy protons (δH 3.93) with C-3 and the methoxy protons (δH 3.87) with C-5 in the HMBC spectrum. Thus, compound 2 was elucidated as shown and was named lyciumamide A.
| No. | 1 | 2 | 3 | 4 | 5 | 6 |
|---|---|---|---|---|---|---|
| 1 | 123.2 | 177.5 | 128.6 | 128.4 | 129.7 | |
| 2 | 74.6 | 102.4 | 126.8 | 111.6 | 111.7 | 111.8 |
| 3 | 29.6 | 151.0 | 141.4 | 147.1 | 147.2 | 149.6 |
| 4 | 24.6 | 139.8 | 131.0 | 145.8 | 145.8 | 145.7 |
| 5 | 129.9 | 140.5 | 129.9 | 114.7 | 114.7 | 115.1 |
| 6 | 108.0 | 126.7 | 20.5 | 120.1 | 120.1 | 119.7 |
| 7 | 155.7 | 126.3 | 76.1 | 76.3 | 77.6 | |
| 8 | 102.8 | 126.8 | 82.9 | 83.0 | 81.8 | |
| 9 | 156.4 | 168.4 | 60.5 | 60.6 | 59.8 | |
| 10 | 112.1 | |||||
| 1′ | 135.6 | 81.0 | 130.2 | 129.7 | 129.7 | |
| 2′ | 122.3 | 85.3 | 110.0 | 110.1 | 109.7 | |
| 3′ | 139.3 | 42.0 | 149.6 | 149.6 | 147.6 | |
| 4′ | 143.5 | 72.8 | 147.7 | 148.1 | 147.6 | |
| 5′ | 126.2 | 41.8 | 115.7 | 115.7 | 114.6 | |
| 6′ | 109.1 | 47.5 | 119.1 | 119.4 | 119.4 | |
| 7′ | 107.7 | 120.0 | 19.5 | 128.5 | 131.7 | 131.4 |
| 8′ | 145.3 | 131.7 | 75.0 | 128.4 | 123.8 | 124.1 |
| 9′ | 168.6 | 16.0 | 61.6 | 68.6 | 68.7 | |
| 1′′ | 23.9 | 129.6 | 102.2 | 100.3 | 100.2 | 102.1 |
| 2′′ | 123.8 | 129.5 | 73.3 | 73.3 | 73.4 | 74.2 |
| 3′′ | 129.8 | 115.0 | 77.1 | 76.6 | 76.6 | 77.1 |
| 4′′ | 25.4 | 155.6 | 70.6 | 70.0 | 70.0 | 69.9 |
| 5′′ | 17.7 | 115.0 | 73.6 | 75.3 | 75.3 | 76.8 |
| 6′′ | 129.5 | 64.3 | 67.3 | 67.4 | 61.0 | |
| 7′′ | 34.1 | |||||
| 8′′ | 41.1 | |||||
| 1′′′ | 129.6 | 123.0 | 109.3 | 109.2 | 102.2 | |
| 2′′′ | 129.7 | 112.7 | 75.9 | 75.9 | 73.5 | |
| 3′′′ | 115.1 | 148.6 | 78.8 | 78.8 | 76.9 | |
| 4′′′ | 155.6 | 150.7 | 73.4 | 73.3 | 70.1 | |
| 5′′′ | 115.1 | 114.5 | 63.3 | 63.1 | 76.5 | |
| 6′′′ | 129.7 | 122.8 | 61.1 | |||
| 7′′′ | 34.3 | 165.1 | ||||
| 8′′′ | 41.1 | |||||
| 1′′′′ | 99.6 | 101.8 | ||||
| 2′′′′ | 73.1 | 73.4 | ||||
| 3′′′′ | 76.8 | 76.6 | ||||
| 4′′′′ | 69.4 | 70.3 | ||||
| 5′′′′ | 76.5 | 75.6 | ||||
| 6′′′′ | 60.4 | 67.7 | ||||
| 1′′′′ | 109.3 | |||||
| 2′′′′ | 75.9 | |||||
| 3′′′′ | 78.8 | |||||
| 4′′′′ | 73.2 | |||||
| 5′′′′ | 63.3 | |||||
| 3-OCH3 | 55.8 | 55.4 | 55.7 | 55.5 | ||
| 5-OCH3 | 60.4 | |||||
| 3′-OCH3 | 55.7 | 55.4 | 55.4 | |||
| 3′′′-OCH3 | 55.7 |
Compound 3 was isolated as a yellow powder. The molecular formula was established to be C35H48O18 by the HRESIMS ion peak at m/z [M + Na]+ 779.2723 (C35H48O18Na, calcd for 779.2733). The UV, IR, and NMR spectra of 3 were similar to dihydrophaseic acid 4′-O-(6′′-O-galloyl)-β-D-glucopyranoside;16 the differences between the compounds were that 3 contained an additional glucose unit and a vanilloyl group in place of the galloyl in dihydrophaseic acid 4′-O-(6′′-O-galloyl)-β-D-glucopyranoside. In the HMBC spectrum of 3, the correlations from H-1′′′′ (δH 4.99) to C-4′′′ (δC 150.7) established the linkage position of the additional glucose. Compound 3 was assigned as lyciumoside A.
Compound 4 was obtained as a white powder. The molecular formula, C31H42O16, was established by a sodiated molecular ion peak observed at m/z 693.2377 [M + Na]+ (calcd for C31H42O16Na, 693.2365) in the HRESIMS. The 1H NMR spectrum of 4 (Table 1) exhibited two ABX spin system aromatic protons at δH 7.07 (1H, d, J = 2.0 Hz), 6.67 (1H, d, J = 8.0 Hz), 6.80 (1H, dd, J = 2.0, 8.0 Hz), 7.01 (1H, d, J = 2.0 Hz), 6.96 (1H, d, J = 8.0 Hz), and 6.87 (1H, dd, J = 2.0, 8.0 Hz), and two olefinic protons at δH 6.43 (1H, d, J = 16.0 Hz) and 6.23 (1H, dt, J = 6.0, 16.0 Hz). Two oxygenated methine protons at δH 4.96 (1H, d, J = 4.5 Hz) and 4.38 (1H, m), four oxygenated methylene protons at δH 3.65 (1H, m), 3.21 (1H, m), and 4.08 (2H, m), two methoxy groups at δH 3.71 (3H, s) and 3.77 (3H, s), and two anomeric protons at δH 3.98 (1H, d, J = 7.5 Hz) and 4.89 (1H, d, J = 3.0 Hz) were observed in the upfield region. The 13C NMR and HSQC spectra displayed a total of 31 carbon signals. Apart from one glucopyranose moiety, one apiofuranose moiety, and two methoxy groups, the remaining 18 carbon signals were assigned to two C6–C3 units. The key HMBC correlations (Fig. 2) of H-7 at δH 4.96 with C-1, 2, 6, 8, and 9, of H-7′ at δH 6.43 with C-1′, 2′, 6′, 8′, and 9′, and of H-8 at δH 4.38 with C-4′ at δC 147.7 suggested that 4 was an 8-O-4′ system neolignan.17 The methoxy groups were confirmed to be at C-3 and C-3′ based on the HMBC correlations of the methoxy groups at δH 3.71 and 3.77 with C-3 and C-3′, respectively. The HMBC correlation of H-1′′ (δH 3.98) with C-7 (δC 76.1) revealed that the glucopyranose unit was attached to C-7, while the correlation of H-1′′′ (δH 4.89) with C-6′′ (δC 67.3) suggested that the apiofuranose unit was attached to C-6′′. On the basis of the above analysis, the planner structure of 4 was similar to that of ligusinenoside D,18 except for the position of sugar moiety. Acid hydrolysis of 4 yielded 4a, D-glucopyranose and D-apiofuranose, which were identified by GC analysis of their trimethylsilyl L-cysteine derivatives. Furthermore, the β-linkage of D-glucopyranose and D-apiofuranose was determined by their anomeric protons at δH 3.98 (1H, d, J = 7.5 Hz) and δH 4.89 (1H, d, J = 3.0 Hz). The 1H NMR experiment of 4a was performed in CDCl3. A small coupling constant (J7,8 = 4.5 Hz) between H-7 and H-8 indicated the erythro configuration of C-7 and C-8 unambiguously.19 This result combined with the negative Cotton effect at 231 nm in the CD spectrum of 4 established the 7S,8R configurations for 4.19 From the above data, the structure of 4 was assigned as shown (Fig. 1), and this compound was named lyciumlignan A.
The molecular formula of 5 was determined to be C42H60O25 on the basis of the sodiated molecular ion peak observed at m/z 987.3316 [M + Na]+ in the HRESIMS. A comparison of the UV and NMR data of 5 with those of 4 revealed that the aglycone of 5 was also an 8-O-4′ system neolignan. Its 1H NMR spectrum exhibited four anomeric protons at δH 3.97 (1H, d, J = 7.0 Hz), 4.88 (1H, d, J = 3.0 Hz), 4.20 (1H, d, J = 8.0 Hz), and 4.89 (1H, d, J = 3.0 Hz), which indicated the existence of two glucopyranose units and two apiofuranose units with the β-linkage. The key HMBC correlations of H-1′′ (δH 3.97) to C-7 (δC 76.3), H-1′′′′ (δH 4.20) to C-9′ (δC 68.6), H-1′′′ (δH 4.88) to C-6′′ (δC 67.4), and H-1′′′′ (δH 4.89) to C-6′′′′ (δC 67.7) clearly presented the planner structure for compound 5 as 4,9-dihydroxy-3,3′-dimethoxy-7-en-8,4′-oxyneolignan-7-O-β-D-apiofuranosyl-(1→6)-β-D-glucopyranosyl-9′-O-β-D-apiofuranosyl-(1→6)-β-D-glucopyranoside. Acid hydrolysis of 5 resulted in 5a, D-glucopyranose, and D-apiofuranose. The D-configurations of these two sugars were determined by GC analysis. In the 1H NMR spectrum of 5a (CDCl3), the characteristic coupling constant of H-7 (J7,8 = 7.5 Hz) allowed for the determination of the thero configuration of C-7 and C-8.18 By considering the negative Cotton effects at 231 nm (Δε −1.82) in 5 that were exhibited in the CD spectrum, the 7R,8R configurations were confirmed.18 Thus, 5 was characterized as shown and named lyciumlignan B.
Detailed analysis of the 1D NMR spectra (Tables 1 and 2) suggested that the structure of 6 was similar to that of 5. The difference between these two compounds was the absence of two apiofuranose units at C-6′′ and C-6′′′ in 6. Using the same method that was described for 5, the absolute configurations of C-7 and C-8 in 6 were established as 7R,8S. Therefore, the structure of 6 (lyciumlignan C) was determined to be (7R,8S)-4,9-dihydroxy-3,3′-dimethoxy-7-en-8,4′-oxyneolignan-7-O-β-D-glucopyranosyl-9′-O-β-D-glucopyranoside.
Compound 7 exhibited a molecular formula of C20H30O12 according to the positive HRESIMS ion observed at m/z 485.1633 [M + Na]+. The 1H NMR spectrum (Table 3) of 7 revealed a set of ABX system aromatic protons at δH 7.00 (1H, d, J = 2.0 Hz), 6.88 (1H, overlap), and 6.88 (1H, overlap), two oxymethylene protons at δH 4.70 (1H, d, J = 12.0 Hz) and 4.50 (1H, d, J = 12.0 Hz), and two methoxyl protons at δH 3.73 (3H, s) and 3.74 (3H, s). Moreover, two anomeric protons at δH 4.16 (1H, d, J = 8.0 Hz) and 4.24 (1H, d, J = 7.5 Hz) observed in the upfield region confirmed the presence of two sugar moieties with a β-linkage. The 13C NMR spectrum showed 20 carbon signals that could be assigned to a glucopyranose unit, a xylopyranose unit, two methoxy groups, and a benzylalcohol moiety. The HMBC correlation peaks of the two methoxy groups at δH 3.73 and 3.74 with C-3 and C-4 confirmed the linkage positions of these methoxy groups. The sequence of two sugar units was determined to be β-xylopyranosyl-(1→6)-O-β-glucopyranosyl based on correlations from H-1′′ to C-6′ in the HMBC spectrum. The unusual downfield shift of the H-7 (δH 4.70, 4.50) and C-7 (δC 69.3) was an important indicator that the sugar moiety occurred at C-7. This was supported by the HMBC correlation that was observed from H-1′ (δH 4.16) to C-7. Finally, the structure of 7 was elucidated as 3′,4′-dimethoxy-benzylalcohol-1-O-β-D-xylopyranosyl-(1→6)-O-β-D-glucopyranoside.
| No. | 7 | No. | 8 | No. | 9 | |||
|---|---|---|---|---|---|---|---|---|
| δH (J in Hz) | δC | δH (J in Hz) | δC | δH (J in Hz) | δC | |||
| 1 | 130.3 | 1 | 133.6 | 1 | 132.0 | |||
| 2 | 7.00, br s | 111.4 | 2 | 6.71, s | 104.8 | 2 | 7.25, d (9.0) | 127.6 |
| 3 | 148.1 | 3 | 152.7 | 3 | 6.71, d (9.0) | 115.4 | ||
| 4 | 148.5 | 4 | 136.5 | 4 | 157.2 | |||
| 5 | 6.88, overlap | 111.9 | 5 | 152.7 | 5 | 6.71, d (9.0) | 115.4 | |
| 6 | 6.88, overlap | 120.2 | 6 | 6.71, s | 104.8 | 6 | 7.25, d (9.0) | 127.6 |
| 7 | 4.70, d (12.0) | 69.3 | 7 | 4.70, d (12.5) | 69.3 | 7 | 6.53, d (16.0) | 132.0 |
| 4.50, d (12.0) | 4.54, d (12.5) | |||||||
| 8 | 8 | 8 | 6.10, dt (16.0) | 122.4 | ||||
| 9 | 9 | 9 | 4.44, m | 68.8 | ||||
| 4.13, m | ||||||||
| 1′ | 4.16, d (8.0) | 101.5 | 1′ | 4.17, d (7.5) | 101.7 | 1′ | 4.19, d (7.5) | 101.7 |
| 2′ | 3.01, m | 73.3 | 2′ | 3.00, m | 73.3 | 2′ | 2.98, m | 73.4 |
| 3′ | 3.08, m | 76.6 | 3′ | 3.08, m | 76.5 | 3′ | 3.13, m | 76.6 |
| 4′ | 3.55, m | 70.0 | 4′ | 3.55, m | 70.0 | 4′ | 2.99, m | 70.3 |
| 5′ | 3.27, m | 75.8 | 5′ | 3.24, m | 75.8 | 5′ | 3.25, m | 75.6 |
| 6′ | 3.95, m | 68.4 | 6′ | 3.95, d (10.5) | 68.4 | 6′ | 3.41, d (3.0) | 67.7 |
| 3.54, m | 3.55, m | 3.85, brd | ||||||
| 1′′ | 4.24, d (7.5) | 104.0 | 1′′ | 4.24, d (8.0) | 104.1 | 1′′ | 4.88, d (3.0) | 109.2 |
| 2′′ | 2.97, m | 73.3 | 2′′ | 2.98, m | 73.4 | 2′′ | 3.75, m | 75.9 |
| 3′′ | 3.05, m | 76.6 | 3′′ | 3.14, m | 76.6 | 3′′ | 78.8 | |
| 4′′ | 3.27, m | 69.6 | 4′′ | 3.27, m | 69.6 | 4′′ | 3.82, d (9.5) | 73.2 |
| 3.59, d (9.5) | ||||||||
| 5′′ | 3.02, m | 65.7 | 5′′ | 3.68, m | 65.7 | 5′′ | 3.34, m | 63.1 |
| 3.68, m | 3.02, m | |||||||
| 3-OCH3 | 3.73, s | 55.4 | 3,5-OCH3 | 3.73, s | 59.9 | |||
| 4-OCH3 | 3.74, s | 55.5 | 4-OCH3 | 3.63, s | 55.8 | |||
A comparison of the IR, UV, and NMR data (Table 3) of 8 with those of 7 revealed that the difference between these two compounds was the presence of an additional methoxy group at C-5 in 8. This result was supported by the HMBC correlation from OCH3-3, 5 to C-3, 5, and the singlet proton signal at δH 6.71 (2H, s) in the 1H NMR spectrum. So, compound 8 was elucidated as 3′,4′,5′-trimethoxy-benzylalcohol-1-O-β-D-xylopyranosyl-(1→6)-O-β-D-glucopyranoside.
Compound 9 was obtained as a white powder. The molecular formula, C20H28O11, was established by the positive molecular ion peak at m/z 467.1531 [M + Na]+ (calcd for C20H28O11Na, 467.1524) in the HRESIMS. The 1H NMR spectrum of 9 revealed an AA′BB′ spin system [δH 7.25 (2H, d, J = 9.0 Hz) and 6.71 (2H, d, J = 9.0 Hz)], two trans olefince proton signals [δH 6.53 (1H, d, J = 16.0 Hz) and 6.10 (1H, d, 16.0 Hz)], two oxymethylene proton signals [δH 4.44 (1H, m) and 4.13 (1H, m)], and two anomeric proton signals [δH 4.19 (1H, d, J = 7.5 Hz) and 4.88 (1H, d, J = 3.0 Hz)]. In the 13C NMR spectrum (Table 3), aside from the 11 carbon signals of a glucopyranose unit and an apiofuranose unit, the remaining nine carbon signals could be attributed to a p-coumaryl alcohol moiety. Subsequently, the two sugar units were determined to be β-apiofuranosyl-(1→6)-O-β-glucopyranosyl by the characteristic correlation of H-1′′ with C-6′ in the HMBC spectrum. In combination with the HMBC correlation of H-1′ with C-9, compound 9 was defined as 4′-hydroxystyrone-1-O-β-D-apiofuranosyl-(1→6)-O-β-D-glucopyranoside.
Based on the spectroscopic data and a comparison with those found in the literature, the eight known compounds (Fig. 1) were established as kazinol A (10),20,21 7,4′-dihydroxy-isopentene flavane (11),22 acacetin (12),23 quercitrin (13),24 maackianin (14),25 maackiain (15),26 (7R,8S)-4,9,9′-trihydroxy-3,3′-dimethoxy-7′-en-8,4′-oxyneolignan-7-O-β-D-glucopyranoside (16),27 and 2-hydroxy-4-methoxybenzaldehyde-2-O-β-D-glucopyraneosyl-1-6-O-β-D-xylopyranoside (17).28
Experimental
General experimental procedures
The optical rotations, UV spectra, and ECD spectra were measured on JASCO P-2000 polarimeter, JASCO V650 spectrometer, and JASCO J-815 spectrometer (JASCO, Easton, MD, USA), respectively. Infrared (IR) spectra were recorded on a Nicolet 5700 spectrometer (Thermo Scientific, Waltham, MA, USA). 1D and 2D NMR spectra were recorded with a Bruker 500 MHz spectrometer (Bruker-Biospin, Billerica, MA, USA). High-resolution electrospray ionization mass spectrometry (HRESIMS) was performed on an Agilent 6520 HPLC-Q-TOF (Agilent Technologies, Waldbronn, Germany). Flash chromatography was performed using a Combiflash RF200 apparatus (Teledyne Isco Corp., Lincoln, NE, USA). Preparative and semi-preparative HPLC (pHPLC and semi-pHPLC) were performed using a Shimadzu preparative chromatography system (Shimadzu Corp., Tokyo, Japan) with YMC-Pack ODS-A columns (250 mm × 20 mm, 5 μm; 250 mm × 10 mm, 5 μm; YMC Corp., Kyoto, Japan). GC analysis was performed using on an Agilent 7890A instrument. HPLC-DAD analysis was conducted on an Agilent 1200 instrument with a YMC C18 column (250 mm × 4.6 mm, 5 μm).Plant material
The root bark of the L. chinense was collected from Ningan Town, Zhongning County, Ningxia Hui autonomous region, People's Republic of China, in March 2012. A voucher specimen (ID-S-2592) was deposited in the Herbarium of the Institute of Materia Medica, Chinese Academy of Medical Science, Beijing.Extraction and isolation
The powdered plant material (100 kg) was extracted three times with 80% EtOH (600 L) under reflux. The solvent was evaporated and the crude residue (8.0 kg) was partitioned with EtOAc (3 × 45 L). The EtOAc and H2O solvent were removed under reduced pressure, which yielded Fr. 1 and Fr. 2, respectively. Fr. 2 (1.375 kg) was chromatographed with a macroporous resin column (HP-20, 200 × 15 cm) and eluted successively with H2O, 15%, 30%, 50%, 70%, and 95% EtOH. The 30% EtOH fraction was concentrated and was further chromatographed over a macroporous resin (SP-700, 200 × 15 cm) and eluted successively with 15%, 20%, 25%, 30%, 45%, 50%, and 95% EtOH, which yielded fractions A–G.Fr. C (72 g) was subjected to Combiflash RF200 apparatus with a C18 column (55 × 8 cm, 50 μm) and eluted with MeOH–H2O (from 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 95 to 100
95 to 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0) to provide Fr. C1–Fr. C25. Fr. C5 (3 g) was chromatographed over Sephadex LH-20 and eluted with gradient mixtures of MeOH–H2O (from 10
0) to provide Fr. C1–Fr. C25. Fr. C5 (3 g) was chromatographed over Sephadex LH-20 and eluted with gradient mixtures of MeOH–H2O (from 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 90 to 95
90 to 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5) to yield Fr. C5-1–C5-15. Fr. C5-4 was purified with pHPLC (MeOH–H2O, 45
5) to yield Fr. C5-1–C5-15. Fr. C5-4 was purified with pHPLC (MeOH–H2O, 45![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 55) to yield 5 (20 mg). Fraction C5-11 was chromatographed over Sephadex LH-20 using MeOH–H2O (from 0
55) to yield 5 (20 mg). Fraction C5-11 was chromatographed over Sephadex LH-20 using MeOH–H2O (from 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 to 60
100 to 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40) as the gradient mobile phase system and was further purified with pHPLC to yield 3 (26 mg), 6 (16 mg), and 17 (35 mg). Fraction C7 (5 g) was subjected to the Rp-C18 (50 μm) and eluted with MeOH–H2O (from 10
40) as the gradient mobile phase system and was further purified with pHPLC to yield 3 (26 mg), 6 (16 mg), and 17 (35 mg). Fraction C7 (5 g) was subjected to the Rp-C18 (50 μm) and eluted with MeOH–H2O (from 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 90 to 100
90 to 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0) to yield Fr. C7-1–C7-8. 4 (17 mg) was obtained from Fr. C7-3 by pHPLC (MeOH–H2O, 30
0) to yield Fr. C7-1–C7-8. 4 (17 mg) was obtained from Fr. C7-3 by pHPLC (MeOH–H2O, 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 70). Fr. C7-5 was purified using pHPLC with MeOH–H2O (40
70). Fr. C7-5 was purified using pHPLC with MeOH–H2O (40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 60) as the mobile phase to yield 16 (12 mg) and 9 (24 mg). Fr. C10 (1.8 g) was subjected to the Rp-C18 (50 μm) and eluted with MeOH–H2O (15
60) as the mobile phase to yield 16 (12 mg) and 9 (24 mg). Fr. C10 (1.8 g) was subjected to the Rp-C18 (50 μm) and eluted with MeOH–H2O (15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 85) to give six fractions (Fr. C10-1–C10-6). Purification of Fr. C10-2 (1.8 g) with MeOH–H2O (20
85) to give six fractions (Fr. C10-1–C10-6). Purification of Fr. C10-2 (1.8 g) with MeOH–H2O (20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 80) on pHPLC yielded 7 (32 mg) and 8 (19 mg).
80) on pHPLC yielded 7 (32 mg) and 8 (19 mg).
Fraction G (4 g) was chromatographed over Sephadex LH-20 with a gradient of H2O–MeOH (from 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 to 0
40 to 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100) to yield Fr. G1–G11. Fr. G2 was purified by pHPLC (MeOH–H2O, 50
100) to yield Fr. G1–G11. Fr. G2 was purified by pHPLC (MeOH–H2O, 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50) to yield 13 (25 mg) and 2 (20 mg). 11 (33 mg) and 12 (27 mg) were obtained from Fr. G5 by pHPLC (MeOH–H2O, 65
50) to yield 13 (25 mg) and 2 (20 mg). 11 (33 mg) and 12 (27 mg) were obtained from Fr. G5 by pHPLC (MeOH–H2O, 65![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 35) as the mobile phase. Fr. G7 was purified by pHPLC (MeOH–H2O, 70
35) as the mobile phase. Fr. G7 was purified by pHPLC (MeOH–H2O, 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30) to yield 14 (29 mg) and 15 (22 mg). Fr. G9 and Fr. G10 were purified by pHPLC with MeOH–H2O (70
30) to yield 14 (29 mg) and 15 (22 mg). Fr. G9 and Fr. G10 were purified by pHPLC with MeOH–H2O (70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30) to yield 1 (28 mg) and 10 (15 mg), respectively.
30) to yield 1 (28 mg) and 10 (15 mg), respectively.
Structure characterization
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ε): 207 (4.24), 255 (2.95), and 285 (2.53) nm; IR (KBr) νmax: 3364, 2926, 1598, 1509, 1460, 1378, 1261, 1155, 1114, 1041, 956, and 801 cm−1; and (+)-HRESIMS: m/z 351.1586 [M + H]+ (calcd for C22H23O4, 351.1596). For the NMR data, see Tables 1 and 2.
ε): 207 (4.24), 255 (2.95), and 285 (2.53) nm; IR (KBr) νmax: 3364, 2926, 1598, 1509, 1460, 1378, 1261, 1155, 1114, 1041, 956, and 801 cm−1; and (+)-HRESIMS: m/z 351.1586 [M + H]+ (calcd for C22H23O4, 351.1596). For the NMR data, see Tables 1 and 2.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ε): 202 (4.15), 237 (3.79), and 258 (4.05) nm; IR (KBr) νmax: 3325, 2940, 1635, 1515, 1452 1241, 1122, 1087, 910, 827, and 560 cm−1; and (+)-HRESIMS: m/z 531.2131 [M + H]+ (calcd for C30H31N2O7, 531.2131). For the NMR data, see Tables 1 and 2.
ε): 202 (4.15), 237 (3.79), and 258 (4.05) nm; IR (KBr) νmax: 3325, 2940, 1635, 1515, 1452 1241, 1122, 1087, 910, 827, and 560 cm−1; and (+)-HRESIMS: m/z 531.2131 [M + H]+ (calcd for C30H31N2O7, 531.2131). For the NMR data, see Tables 1 and 2.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ε): 207 (4.25), 215 (4.28), 256 (4.26), and 297 (3.49) nR (KBr) νmax: 3379, 2930, 1712, 1601, 1417, 1274, 1074, 1026, 883, and 764 cm−1; and (+)-HRESIMS: m/z 779.2723 [M + Na]+ (calcd for C35H48O18Na, 779.2738). For the NMR data, see Tables 1 and 2.
ε): 207 (4.25), 215 (4.28), 256 (4.26), and 297 (3.49) nR (KBr) νmax: 3379, 2930, 1712, 1601, 1417, 1274, 1074, 1026, 883, and 764 cm−1; and (+)-HRESIMS: m/z 779.2723 [M + Na]+ (calcd for C35H48O18Na, 779.2738). For the NMR data, see Tables 1 and 2.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ε): 206 (4.57), 268 (3.89), and 301 (3.49); IR (KBr) νmax: 3371, 2936, 2881, 1698, 1604, 1513, 1268, 1034, 823, and 621 cm−1; and (+)-HRESIMS: m/z 693.2377 [M + Na]+ (calcd for C31H42O16Na, 693.2371). For the NMR data, see Tables 1 and 2.
ε): 206 (4.57), 268 (3.89), and 301 (3.49); IR (KBr) νmax: 3371, 2936, 2881, 1698, 1604, 1513, 1268, 1034, 823, and 621 cm−1; and (+)-HRESIMS: m/z 693.2377 [M + Na]+ (calcd for C31H42O16Na, 693.2371). For the NMR data, see Tables 1 and 2.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ε): 205 (4.54), 268 (3.95), and 304 (3.32); IR (KBr) νmax: 3368, 2974, 2931, 2885, 1513, 1419, 1268, 1049, 825, and 617 cm−1; and (+)-HRESIMS: m/z 987.3316 [M + Na]+ (calcd for C42H60O25Na, 987.3321). For the NMR data, see Tables 1 and 2.
ε): 205 (4.54), 268 (3.95), and 304 (3.32); IR (KBr) νmax: 3368, 2974, 2931, 2885, 1513, 1419, 1268, 1049, 825, and 617 cm−1; and (+)-HRESIMS: m/z 987.3316 [M + Na]+ (calcd for C42H60O25Na, 987.3321). For the NMR data, see Tables 1 and 2.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ε): 205 (4.35), 271 (3.71), and 301 (3.61); IR (KBr) νmax: 3384, 2930, 1565, 1513, 1420, 1267, 1078, 1031, 670, and 618 cm−1; and (+)-HRESIMS: m/z 723.2485 [M + Na]+ (calcd for C32H44O17Na, 723.2476). For the NMR data, see Tables 1 and 2.
ε): 205 (4.35), 271 (3.71), and 301 (3.61); IR (KBr) νmax: 3384, 2930, 1565, 1513, 1420, 1267, 1078, 1031, 670, and 618 cm−1; and (+)-HRESIMS: m/z 723.2485 [M + Na]+ (calcd for C32H44O17Na, 723.2476). For the NMR data, see Tables 1 and 2.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ε): 203 (4.09), 221 (3.61), and 279 (2.93) nm; IR (KBr) νmax: 3612, 3429, 2932, 2835, 1514, 1449, 1368, 1258, 1050, and 668 cm−1; and (+)-HRESIMS: m/z 485.1633 [M + Na]+ (calcd for C20H30O12Na, 485.1635). For the NMR data, see Table 3.
ε): 203 (4.09), 221 (3.61), and 279 (2.93) nm; IR (KBr) νmax: 3612, 3429, 2932, 2835, 1514, 1449, 1368, 1258, 1050, and 668 cm−1; and (+)-HRESIMS: m/z 485.1633 [M + Na]+ (calcd for C20H30O12Na, 485.1635). For the NMR data, see Table 3.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ε): 207 (4.19), 233 (3.55), and 269 (3.17) nm; IR (KBr) νmax: 3368, 2935, 1594, 1461, 1423, 1334, 1239, 1126, 1044, and 832 cm−1; and (+)-HRESIMS: m/z 515.1741 [M + Na]+ (calcd for C21H32O13Na, 515.1741). For the NMR data, see Table 3.
ε): 207 (4.19), 233 (3.55), and 269 (3.17) nm; IR (KBr) νmax: 3368, 2935, 1594, 1461, 1423, 1334, 1239, 1126, 1044, and 832 cm−1; and (+)-HRESIMS: m/z 515.1741 [M + Na]+ (calcd for C21H32O13Na, 515.1741). For the NMR data, see Table 3.Determination of the absolute configuration of sugar
Compound 4 (8 mg) was dissolved in 0.5 M HCl (8 mL) and refluxed for 4 h. The reaction solution was then extracted with EtOAc (3 × 10 mL). The EtOAc layer was concentrated to create a residue that was further purified using pHPLC with MeOH–H2O (45![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 55) to yield 4a (3.5 mg). The aqueous layer was evaporated under a vacuum to create a monosaccharide residue. The residue was mixed with L-cysteine methyl ester hydrochloride (2 mg), and was dissolved in fresh anhydrous pyridine (2.0 mL). The reaction mixture was maintained at 60 °C for 2 h. Then, the dried N-trimethylsilylimidazole (0.2 mL) was added to the mixture. The solution was incubated at 60 °C for 2 h and partitioned between n-hexane and H2O. The n-hexane layer was subjected to GC analysis under the following conditions: capillary column, HP-5 (30 m × 0.32 mm, with a 0.25 μm film, Dikma); detector temperature, 300 °C; injection temperature, 300 °C; initial temperature, 200 °C that was raised to 280 °C at a rate of 10 °C min−1; the final temperature was maintained for 30 min; detection, FID; and carrier gas, N2 gas. D-glucose and D-apiose were confirmed by comparing the retention time of their derivatives to the standard sugar derivatized in a similar manner, which exhibited retention times of 20.5 and 14.5 min, respectively. The hydrolysis procedures for 5–9 were similar to that of 4. The D-xylose was confirmed by the retention time (14.9 min) of its derivative.
55) to yield 4a (3.5 mg). The aqueous layer was evaporated under a vacuum to create a monosaccharide residue. The residue was mixed with L-cysteine methyl ester hydrochloride (2 mg), and was dissolved in fresh anhydrous pyridine (2.0 mL). The reaction mixture was maintained at 60 °C for 2 h. Then, the dried N-trimethylsilylimidazole (0.2 mL) was added to the mixture. The solution was incubated at 60 °C for 2 h and partitioned between n-hexane and H2O. The n-hexane layer was subjected to GC analysis under the following conditions: capillary column, HP-5 (30 m × 0.32 mm, with a 0.25 μm film, Dikma); detector temperature, 300 °C; injection temperature, 300 °C; initial temperature, 200 °C that was raised to 280 °C at a rate of 10 °C min−1; the final temperature was maintained for 30 min; detection, FID; and carrier gas, N2 gas. D-glucose and D-apiose were confirmed by comparing the retention time of their derivatives to the standard sugar derivatized in a similar manner, which exhibited retention times of 20.5 and 14.5 min, respectively. The hydrolysis procedures for 5–9 were similar to that of 4. The D-xylose was confirmed by the retention time (14.9 min) of its derivative.
Inhibitory activity of α-glucosidase
The inhibitory activity of compounds 1–17 on α-glucosidase was determined by the same method as described in the literature.13,14Conclusions
In our ongoing effort to discover new bioactive natural products with hypoglycemic effects from the root bark of L. chinense, 17 compounds were isolated, including one new flavane with an unusual benzofuran unit, one new amide possessing a naphthalene skeleton, one new sesquiterpene, three new lignan glucosides, three new phenolic glucosides, and eight known compounds. A literature survey revealed that this is the first report of a flavane with an unusual benzofuran unit that showed strong inhibitory activity against α-glucosidase (IC50 = 20.89 μM).Acknowledgements
The research described in this publication was supported by the National Natural Science Foundation of China (No. 81303207) and the Beijing Natural Science Foundation (No. 7144227).References
- T. Wu, H. Y. Lv, F. Z. Wang and Y. Wang, J. Agric. Food Chem., 2016, 64, 2280–2288 CrossRef CAS PubMed.
- N. C. Lin, J. C. Lin, S. H. Chen, C. T. Ho and A. I. Yeh, J. Agric. Food Chem., 2011, 59, 10088–10096 CrossRef CAS PubMed.
- K. Gao, D. W. Ma, Y. Cheng, X. R. Tian, Y. Y. Lu, X. Y. Du, H. F. Tang and J. Z. Chen, J. Agric. Food Chem., 2015, 63, 1067–1075 CrossRef CAS PubMed.
- Z. Ye, Q. Huang, H. X. Ni and D. Wang, Phytother. Res., 2008, 22, 1665–1670 CrossRef PubMed.
- L. W. Xie, A. G. Atanasov, D. A. Guo, C. Malainer, J. X. Zhang, M. Zehl, S. H. Guan, E. H. Heiss, E. Urban, V. M. Dirsch and B. Kopp, J. Ethnopharmacol., 2014, 152, 470–477 CrossRef CAS PubMed.
- J. X. Zhang, S. H. Guan, R. H. Feng, Y. Wang, Z. Y. Wu, Y. B. Zhang, X. H. Chen, K. S. Bi and D. A. Guo, J. Nat. Prod., 2013, 76, 51–58 CrossRef CAS PubMed.
- S. Yahara, C. Shigeyama, T. Nohara, H. Okuda, K. Wakamatsu and T. Yasuhara, Tetrahedron Lett., 1989, 30, 6041–6042 CrossRef CAS.
- S. Yahara, C. Shigeyama, T. Ura, K. Wakamatsu, T. Yasuhara and T. Nohara, Chem. Pharm. Bull., 1993, 41, 703–709 CrossRef CAS PubMed.
- D. G. Lee, H. J. Jung and E. R. Woo, Arch. Pharmacal Res., 2005, 28, 1031–1036 CrossRef CAS.
- N. Mamoru and K. Mochida, J. Nat. Prod., 1985, 48, 342–343 CrossRef.
- N. Afza, I. H. Qureshi and Y. Ahmad, J. Chem. Soc. Pak., 1987, 9, 627–628 CAS.
- Y. W. An, Z. L. Zhan, J. Xie, Y. N. Yang, J. S. Jiang, Z. M. Feng, F. Ye and P. C. Zhang, J. Nat. Prod., 2016, 79, 1024–1034 CrossRef CAS PubMed.
- T. Li, X. D. Zhang, Y. W. Song and J. W. Liu, Chin. J. Clin. Pharmacol. Ther., 2005, 10, 1128–1134 Search PubMed.
- Y. N. Yang, F. S. Li, F. Liu, Z. M. Feng, J. S. Jiang and P. C. Zhang, RSC Adv., 2016, 6, 60741–60748 RSC.
- H. Achenbach, M. Stöcker and M. A. Constenla, Phytochemistry, 1988, 27, 1835–1841 CrossRef CAS.
- Y. Zhou, H. B. Chen, B. Wang, H. Liang, Y. Y. Zhao and Q. Y. Zhang, Planta Med., 2011, 77, 1944–1946 CrossRef CAS PubMed.
- X. Y. Huang, Z. M. Feng, Y. N. Yang, J. S. Jiang and P. C. Zhang, J. Asian Nat. Prod. Res., 2015, 17, 504–511 CrossRef CAS PubMed.
- J. P. Ma, C. H. Tan, D. Y. Zhu and L. Jin, Chin. Chem. Lett., 2011, 22, 1454–1456 CrossRef CAS.
- M. L. Gan, Y. L. Zhang, S. Lin, M. T. Liu, W. X. Song, J. C. Zi, Y. C. Yang, X. N. Fan, J. G. Shi, J. F. Hu, J. D. Sun and N. H. Chen, J. Nat. Prod., 2008, 71, 647–654 CrossRef CAS PubMed.
- J. Ikut, Y. Hano and T. Nomura, Heterocycles, 1985, 23, 2835–2842 CrossRef.
- H. W. Ryu, B. W. Lee, M. J. Curtis-Long, S. Jung, Y. B. Ryu, W. S. Lee and K. H. Park, J. Agric. Food Chem., 2010, 58, 202–208 CrossRef CAS PubMed.
- D. Lee, K. P. L. Bhat, H. H. S. Fong, N. R. Farnsworth, J. M. Pezzuto and A. D. Kinghorn, J. Nat. Prod., 2001, 64, 1286–1293 CrossRef CAS.
- J. M. J. Vasconcelos, A. M. S. Silva and J. A. S. Cavaleiro, Phytochemistry, 1998, 49, 1421–1424 CrossRef CAS.
- W. Peng, T. Han, Q. C. Liu and L. P. Qin, Chem. Nat. Compd., 2012, 48, 491–492 CrossRef CAS.
- R. B. Williams, M. O'Neil-Johnson, A. J. Williams, P. Wheeler, R. Pol and A. Moser, Org. Biomol. Chem., 2015, 13, 9957–9962 CAS.
- P. M. Dewick and D. Ward, Phytochemistry, 1978, 17, 1751–1754 CrossRef CAS.
- J. P. Ma, C. H. Tan and D. Y. Zhu, J. Asian Nat. Prod. Res., 2008, 10, 565–569 CrossRef CAS PubMed.
- J. D. Msonthi, Bull. Chem. Soc. Ethiop., 1991, 5, 107–110 CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: 1D NMR, 2D NMR HRMS, IR, and ECD spectra. See DOI: 10.1039/c6ra24751b |
| ‡ These authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2017 |