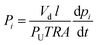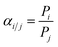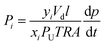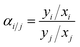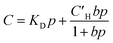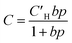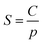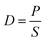Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Carbon molecular sieve membrane from a microporous spirobisindane-based polyimide precursor with enhanced ethylene/ethane mixed-gas selectivity†
Octavio Salinas,
Xiaohua Ma,
Yingge Wang,
Yu Han and
Ingo Pinnau*
King Abdullah University of Science and Technology (KAUST), Advanced Membranes and Porous Materials Center (AMPMC), Physical Sciences and Engineering Division, Chemical and Biological Engineering Program, Thuwal 23955-6900, Saudi Arabia. E-mail: ingo.pinnau@kaust.edu.sa
First published on 13th January 2017
Abstract
Ethylene is typically produced by steam cracking of various hydrocarbon feedstocks. The gaseous products are then separated in a demethanizer followed by a deethanizer unit and finally sent to a C2 splitter for the final purification step. Cryogenic distillation of ethylene from ethane is the most energy-intensive unit operation process in the chemical industry. Therefore, the development of more energy-efficient processes for ethylene purification is highly desirable. Membrane-based separation has been proposed as an alternative option for replacement or debottlenecking of C2 splitters but current polymer membrane materials exhibit insufficient mixed-gas C2H4/C2H6 selectivity (<7) to be technically and economically attractive. In this work, a highly selective carbon molecular sieve (CMS) membrane derived from a novel spirobisindane-based polyimide of intrinsic microporosity (PIM-6FDA) was developed and characterized. PIM-6FDA showed a single-stage degradation process under an inert nitrogen atmosphere which commenced at ∼480 °C. The CMS formed by pyrolysis at 800 °C had a diffusion/size-sieving-controlled morphology with a mixed-gas (50% C2H4/50% C2H6) ethylene/ethane selectivity of 15.6 at 20 bar feed pressure at 35 °C. The mixed-gas ethylene/ethane selectivity is the highest reported value for CMS-type membranes to date.
Introduction
Ethylene (C2H4) is produced in larger quantity than any other organic compound with a world-wide production of 141 million tons in 2011.1 Most commonly, C2H4 is produced by steam cracking of hydrocarbon-based feedstock, such as naphtha, ethane etc., which is the most energy-intensive process in the chemical process industry. After cracking, hydrogen and methane are first removed in a demethanizer unit and the dried hydrocarbon feed is then sent to a deethanizer to split the C2 from C3+ hydrocarbons. In the final process step, ethylene and ethane are separated in a highly energy-intensive cryogenic distillation C2 splitter. The required distillation columns operate typically at 20 bar and ∼−20 °C, are often more than 100 m tall and contain over 200 trays. The total world energy consumption for ethylene production was about 2–3 EJ (1 EJ = 1 × 1018 J) in 2004.1 Therefore, alternative unit operation processes, such as membrane technology, which may require less energy input for efficient ethylene/ethane separation, are highly desirable.2 Recent process modeling suggested that a membrane/distillation hybrid process to debottleneck ethylene/ethane distillation units could potentially save energy,3–5 provided that a membrane material has high ethylene/ethane selectivity of ≥10 and withstands realistic process conditions.4,6Currently available commercial membrane materials used for gas separation applications mainly comprise low-free-volume, solution-processable, glassy polymers, which exhibit an inverse relationship between permeability and selectivity.7 In 2002, Fuertes and Menendez reported a permeability/selectivity trade-off relationship for the ethylene/ethane system,8 which was updated in 2013 by Rungta et al.9 So far, the best performing polymers are polyimides that offer pure-gas ethylene/ethane selectivities of up to ∼7 when tested at low feed pressures.10–13 Under high pressure mixed-gas conditions (i.e. high hydrocarbon activity) penetrant-induced plasticization and competitive sorption typically cause even lower selectivity.11 Recently, mixed-matrix membranes containing polyimides with various metal–organic frameworks were reported to increase the ethylene permeability and/or ethylene/ethane selectivity. Ploegmakers et al. demonstrated that addition of 20 wt% Cu3BTC2 to P84® polyimide increased the pure-gas selectivity from 4.1 for the pristine polymer to 7.1 for the mixed-matrix membrane. However, the ethylene permeability of 0.051 Barrer (1 Barrer = 1 × 10−10 cm3 (STP) cm cm−2 s−1 cmHg−1) was extremely low.14 Bachman et al. reported highly permeable mixed-matrix membranes for ethylene/ethane separation based on 6FDA (4,4′-(hexafluoroisopropylidene)diphthalic anhydride)–DAM (2,4,6-trimethyl-1,3-phenylenediamine)polyimide blended with M2(dobdc) metal–organic framework nanocrystals (dobdc4− = 2,5-dioxido-1,4-benzenedicarboxylate).15 A 6FDA–DAM membrane containing 25% Ni2(dobdc) demonstrated excellent ethylene permeability of 268 Barrer when tested under mixed-gas conditions with an equimolar ethylene/ethane mixture at 20 bar feed pressure. However, the high permeability was coupled with an ethylene/ethane selectivity of only 4.1.
Ultramicroporous carbon molecular sieve (CMS) membranes derived from polyimide precursors have so far demonstrated the most promising gas separation performance for ethylene/ethane separation.9 For example, a CMS membrane made from Matrimid® vacuum-pyrolized at 675 °C showed excellent mixed-gas permeation properties (feed conditions: 63.2% C2H4/36.8% C2H6; feed pressure: 3.4 bar; permeate pressure: vacuum; T: 35 °C) with a C2H4 permeability of 10.9 Barrer and C2H4/C2H6 selectivity of 12.3. Pyrolysis at 800 °C resulted in a large drop in C2H4 permeability to only 0.2 Barrer without much effect on selectivity.16 CMS membranes comprise a disordered array of stacked graphene sheets generated from thermally degraded polymeric matrices.17,18 Fundamentally, the formation of size-sieving ultramicropores in CMS membranes is determined by a series of complex reactions (e.g. cyclization, dehydrogenation, crosslinking, and oxidation) during the pyrolysis of the polymer precursor structure.19 The evolution of the pore size distribution (PSD) during the pyrolysis protocol is a strong function of the polymer precursor structure itself.20–22 Functional groups, such as –OH, –COOH, –CN etc. can lead to potential reactions during the various stages of the pyrolysis process. In general, heat-reactive segments of a polymer chain may create voids left by gas phase evolved molecules23 or may form pre-crosslinks that open the CMS structure leading to higher permeability.24
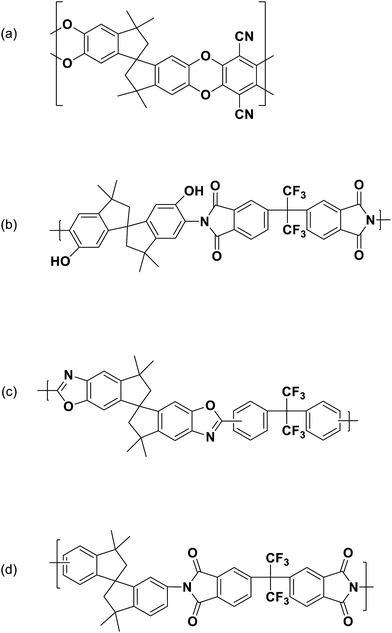
Recently, solution-processable polymers of intrinsic microporosity (PIMs) were introduced as potential membrane materials for various gas separation applications.25–31 PIMs are characterized by high BET (Brunauer–Teller–Emmett) surface area of up to ∼1000 m2 g−1 with micropores of less than 2 nm. Because of their large free volume and microporous structure, most PIMs are significantly more permeable than low-free-volume glassy polymers, such as polysulfone or cellulose acetate, but have lower selectivity. However, optimized PIMs containing a large fraction of ultramicropores of less than 7 Å, can discriminate between gases with small differences in molecular dimensions and some types based on triptycene- and Tröger's base molecular building blocks have defined the 2015 polymeric upper bound performance for air and hydrogen separations.32 Pure-gas ethylene/ethane separation properties of PIM-materials have only been reported for a very limited number of polymers.33–35 PIM-1 (Fig. 1a) was reported to have high ethylene permeability of 1640 Barrer but with very low C2H4/C2H6 selectivity of 1.8.34 A polyimide-based PIM, PIM-6FDA-OH (Fig. 1b), exhibited much lower ethylene permeability of 5.5 Barrer with an ethylene/ethane selectivity of 4.35
Interestingly, only a few reports are available on the formation and gas separation properties of CMS membranes made from intrinsically microporous polymer precursors.34–38 The CMS membrane formed at 800 °C from the prototype ladder polymer of intrinsic microporosity, PIM-1, demonstrated significant densification upon formation of its CMS structure with a dominant crosslinking/sintering effect, resulting in a ∼1000-fold reduction in the ethylene permeability compared to the pristine PIM-1 from 1640 to 1.3 Barrer. The PIM-1-derived CMS membrane had a pure-gas C2H4/C2H4 selectivity of 13, similar to that reported for a CMS made from low-free-volume Matrimid® polyimide at 675 °C.16 In another study, a CMS membrane derived from a hydroxyl-functionalized PIM polyimide at 800 °C, PIM-6FDA-OH, showed an ideal pure-gas C2H4/C2H6 selectivity of ∼18 measured at 2 bar.35 When tested under mixed-gas conditions with an equimolar feed at a pressure of 20 bar and 35 °C, the C2H4/C2H6 selectivity dropped to 8.3 with a C2H4 permeability of 9.6 Barrer.35 The CMS precursor polyimide, PIM-6FDA-OH, contained hydroxyl groups in ortho position to the imide linkages. Consequently, its pyrolysis process proceeded in three major stages: (i) transformation of the o-OH-functionalized polyimide to the corresponding polybenzoxazole (PBO) by thermal rearrangement at ∼400 °C (Fig. 1c) – in this step, an opening of the micropore structure is typically observed with a significant increase in gas permeability and decrease in selectivity;23,39–41 (ii) partial carbonization of the PBO from ∼500–550 °C, characterized by creation of larger pores and increase in permeability; (iii) development of an amorphous, ultramicroporous carbon structure from 600–800 °C with decrease in pore size and permeability but significant increase in selectivity.36 The last two steps are equivalent to the CMS formation of polyimides that do not undergo PBO formation during the pyrolysis process.16,42
In this study, a new hydroxyl-free spirobisindane-based polyimide precursor, PIM-6FDA (Fig. 1d), was used to fine-tune the morphology of high-performance CMS membranes for C2H4/C2H6 separation. Pure- and mixed-gas permeation tests were conducted with an equimolar feed up to 20 bar and 35 °C for the 800 °C CMS membrane derived from PIM-6FDA to determine its potential for industrial ethylene/ethane separation.
Experimental
Materials and characterization methods
Pristine PIM-6FDA (Fig. 1d) was synthesized following the same general procedure previously reported for PIM-6FDA-OH.43 4,4′-(Hexafluoroisopropylidene)diphthalic anhydride (6FDA, 99%) was purchased from Sigma-Aldrich and used after vacuum sublimation. 3,3,3′,3′-Tetramethyl-1,1′-spirobisindane-6,6′ (5′)-diamine was synthesized according to a previously reported method.44 PIM-6FDA was obtained as light yellow filaments with a yield of 95%. 1H NMR (500 MHz, CDCl3): δ 8.06 (d, 2H, J = 8.05 Hz), 7.93 (s, 2H), 7.89 (d, 2H, J = 7.65 Hz), 7.19–7.20 (m, 4H), 7.02 (d, 2H, J = 8.65 Hz), 2.44 (d, 2H, J = 13.2 Hz), 2.36 (d, 2H, J = 13.2 Hz), 1.43 (s, 6H), 1.39 (s, 6H); FT-IR (powder, ν, cm−1): 2959, 2928, 2854 (m, C–H vibration), 1786, 1717, 1620 (vs., imide), 1481, 1358, 1261, 1186 (s, aromatic C˙C), 720 (s, C–F); Mn = 2.1 × 104 g mol−1; Mw = 6.1 × 104 g mol−1; PDI = 2.9. Td = 480 °C; SBET = 260 m2 g−1.1H NMR spectra (500 MHz) were recorded on a Bruker DRX spectrometer using tetramethylsilane as the internal standard. Chemical shifts (δ) are reported in ppm. The molecular weight of PIM-6FDA was determined by gel permeation chromatography (Malvern HT-350) using chloroform as an eluent. A thermogravimetric analyzer (TGA, TA Instruments) was used to measure polymer weight loss as a function of heat treatment temperature. Evolved gases were qualitatively analyzed with a quadrupole mass spectrometer (Hiden Analytical) coupled to the TGA with N2 as the carrier gas. Fourier transform infrared (FTIR) spectra were acquired using a Thermo Nicolet iS10 infrared spectrometer. X-ray diffraction (XRD) scattering was conducted on a Bruker D8 Advance diffractometer. Raman spectra of the carbonized films were obtained with a Horiba LabRam HR visible microscope at 473 nm excitation wavelength. The BET surface area was determined by N2 sorption at −196 °C using a Micromeritics ASAP-2020. Powder polymer samples were degassed under high vacuum at 150 °C for 24 hours prior to analysis. Ethylene and ethane sorption isotherms were determined with a Hiden IGA apparatus in the pressure range from 1 to 15 bar at 35 °C.
Membrane fabrication
Dense PIM-6FDA polyimide films were made by slowly evaporating 3% w/v polymer/THF solution from a leveled and covered Petri dish for three days. The films were then air dried for 12 h and further heat conditioned at 250 °C for 24 h under vacuum to remove traces of solvent. Thermal gravimetric analysis confirmed that the films were solvent-free. The final film thickness was 90 μm (±1 μm) as measured with a digital micrometer. The effective areas of the films prior to permeation measurements were determined with a scanner and image processing software.CMS membranes were made by placing 25 mm diameter PIM-6FDA films inside a Carbolite three-zone tube furnace in a quartz tube supplied with 1000 cm3 (STP) min−1 of N2 from a mass flow controller. The temperature was measured with a thermocouple placed directly adjacent to the film sample. The concentration of oxygen exiting the furnace was less than 2 ppm as continuously measured with an oxygen analyzer (Cambridge Sensotec Rapidox 3100). The furnace temperature was ramped at 3 °C min−1 and then held isothermally for 30 minutes at each desired pyrolysis temperature. After this isothermal stage, the furnace was allowed to cool down to room temperature. The resulting CMS films were immediately stored in a desiccator under vacuum to prevent any significant oxygen chemisorption or water vapor sorption prior to gas permeation testing. These measures were used to ensure data reproducibility.
Permeation measurements
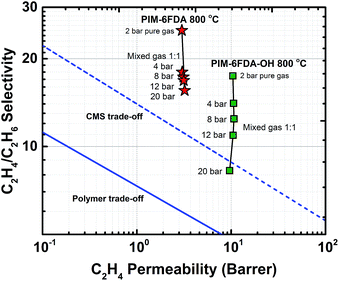
Pure- and mixed-gas permeation experiments were performed at 35 °C with feed pressures up to 20 bar in a constant-volume/variable-pressure apparatus as described in detail elsewhere.45 The films were masked with special care to prevent damage under vacuum as previously described.46 Samples were degassed under high vacuum for at least 24 h to remove adsorbed gases. Pure-gas permeability was calculated according to the following equation:where Pi is the pure-gas permeability in Barrer (1 Barrer = 1 × 10−10 cm3 (STP) cm cm−2 s−1 cmHg−1), Vd is the downstream volume, l is the membrane thickness, PU is the upstream pressure, T is the temperature in absolute units, R is the gas constant, A is the active permeation area and dpi/dt is the steady-state rise in pressure with respect to time. Pure-gas selectivity was calculated as the ratio of the pure-gas permeabilities:
The mixed-gas permeability was measured with a 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 v/v ethylene/ethane mixture by the general technique described by O'Brien et al.47 The stage-cut, that is, the permeate to feed flow rate, was less than 1%. Hence, the feed and residue concentrations were essentially equivalent and potential concentration polarization effects were minimized. The feed and permeate compositions were determined with an Agilent 3000A Micro GC. Mixed-gas permeabilities were calculated as follows:
50 v/v ethylene/ethane mixture by the general technique described by O'Brien et al.47 The stage-cut, that is, the permeate to feed flow rate, was less than 1%. Hence, the feed and residue concentrations were essentially equivalent and potential concentration polarization effects were minimized. The feed and permeate compositions were determined with an Agilent 3000A Micro GC. Mixed-gas permeabilities were calculated as follows:
Gravimetric pure-gas sorption
Gravimetric sorption isotherms for pure C2H4 and C2H6 in the pressure range 1 to 15 bar were determined at 35 °C. To determine the sorption capacity of the 6FDA-PIM and the CMS samples on a volumetric basis, their density was determined gravimetrically by measuring the weight, area, and film thickness (Table S1†).Gas sorption in glassy polymers can be described by the dual-mode sorption model:48
The gas solubility can be calculated for a fixed thermodynamic state as:
Then, the diffusion coefficient or diffusivity, D, can be estimated from the basic permeability definition as:
Results and discussion
Physical properties of PIM-6FDA
The molecular weight (Mn) of PIM-6FDA was ∼6.1 × 104 g mol−1 with a polydispersity index (PDI) of 2.9. The PIM-6FDA film annealed at 250 °C had a density of 1.24 g cm−3, which was slightly lower than that of its analogue PIM-6FDA-OH (ρ = 1.28 g cm−3) annealed under the same conditions (Table S1†). The degradation profiles of PIM-6FDA and PIM-6FDA-OH as a function of temperature are distinctively different, as shown in Fig. 2. PIM-6FDA remained in its polymeric glassy state prior to the onset of carbonization above 500 °C according to the gain in Raman sensitivity of the membranes (Fig. S1†). The onset of degradation in PIM-6FDA commenced at ∼480 °C and its backbone decomposed in a single stage that reached a maximum rate at ∼550 °C with H2O and CO2 as the main decomposition products. The PIM-6FDA membranes heated at 500 °C suffered a significant 23% weight loss after a 30 min isothermal soak (Fig. S2†). This degradation stage can be attributed to the cleavage of the polyimide rings and the creation of polar segments in the vestigial chains via free radicals mechanism. The decrease in the infrared spectra intensity confirmed the elimination of most methyl, carbonyl, and hexafluoroisopropylidene moieties as the samples were treated at higher temperatures (Fig. S3†). PIM-6FDA-OH showed a lower degradation temperature starting around 350 °C, and more importantly, its degradation profile proceeded in two stages. In the first stage, between 400–450 °C the polyimide was thermally converted to a polybenzoxazole, as previously reported for a wide variety of polyimides bearing an ortho-hydroxyl group adjacent to the imide linkage.23,39–41 This stage is characterized by the evolution of CO2 as a reaction by-product, as clearly shown in Fig. 2b. In the second stage, at temperatures higher than 500 °C, amorphous carbon was formed by main chain degradation as evidenced by the evolution of water. In the first stage of PIM-6FDA pyrolysis at 500 °C, the partially degraded polymer shows a widening in average d-spacing ranging from 4.11–6.26 Å, as indicated by the broadening of the amorphous peak of the pristine polyimide originally centered at 5.51 Å. This result is consistent with a significant increase in C2H4 permeability and drop in C2H4/C2H6 selectivity, as discussed below.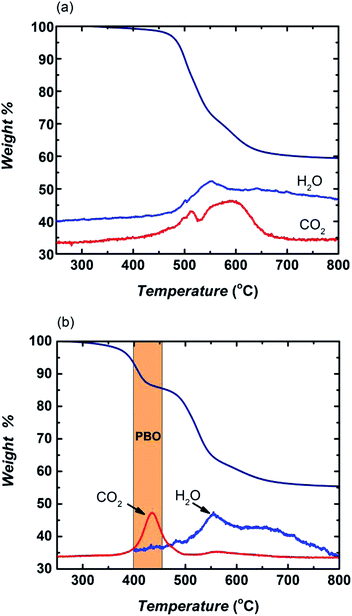 | ||
| Fig. 2 Weight loss and mass spectra of evolved gases during the degradation of (a) PIM-6FDA and (b) PIM-6FDA-OH. Ramping rate was 3 °C min−1 under N2 atmosphere. | ||
The carbonization process most likely induced a more ordered graphitic structure and ultramicroporosity into the CMS membranes at higher pyrolysis temperature as indicated by the narrowing in average d-spacing of the main amorphous peak in the XRD spectra in Fig. 3. The broad amorphous peak with the average d-spacing ranging from 4.11–6.26 Å at lower scattering angle (2-theta = 10–30°) showing in CMS 500 °C shrank to a more defined peak with a much narrower range of average d-spacing of the bimodal distribution (i.e. 3.75–4.34 Å) for CMS 600 °C. In addition, a new peak appeared at a d-spacing of 2.05 Å corresponding to the (1 0 0) plane of graphite layers. Such dramatic changes in XRD spectra indicate formation of a more ordered carbonaceous structure at higher pyrolysis temperature. Samples treated at 800 °C did not suffer significant additional weight loss, indicating that the membranes were mainly comprised of a honeycomb-arranged carbon skeleton with no thermally liable groups. Most importantly, the PIM-6FDA-based CMS generated at 800 °C had only one main amorphous peak with even smaller d-spacing centered at 3.69 Å, and the peak at d-spacing 2.05 Å became more pronounced, indicating generation of a more ordered graphitic structure in the resulting CMS membrane. This qualitative methodology using XRD tracked a definite improvement in tightening the membrane structure and increasing the ultramicroporous characteristics of the PIM-6FDA films after carbonization, and consequently, their molecular sieving capabilities. The subtle difference in the membrane structure of the 600 and 800 °C treated CMS membranes had significant effects on their gas separation performance. The 800 °C-treated CMS membrane consisted of a more tightly sintered structure with greatly enhanced C2H4/C2H6 selectivity but at the cost of lower ethylene permeability, as discussed below.
 | ||
| Fig. 3 XRD spectra of PIM-6FDA (pristine PI) and CMS membranes for each isothermal stage of carbonization. | ||
Pure-gas ethylene/ethane permeation, sorption and diffusion
The pure-gas C2H4 and C2H6 permeabilities of the thermally annealed PIM-6FDA film and its pyrolyzed CMS derivatives were determined at 2 bar and 35 °C (Table 1). The pristine PIM-6FDA film heat-treated at 250 °C showed an ethylene permeability of 7.9 Barrer with an ethylene/ethane permselectivity of 3.6. After partial degradation of the polymer backbone at 500 °C, the ethylene permeability of the resulting CMS membrane increased by a factor of ∼40 to 328 Barrer mainly due to the generation of new pores and pore opening (indicated by widening of average d-spacing in the range of ∼4.1–6.3 Å as shown in XRD spectra (Fig. 3)); however, the selectivity decreased from 3.6 to 2.1. Heat treatment at higher temperatures reduced permeability as a result of the chain compaction confirmed by XRD. For membranes treated at 600 to 800 °C, the ethylene permeability decreased sharply from 77 to 3.0 Barrer, whereas ethylene/ethane selectivity increased significantly from 4.1 to 25.To gain further insights to the contributions of gas solubility and diffusivity on the permeability and permselectivity of PIM-6FDA-based CMS membranes, gravimetric ethylene and ethane sorption measurements were carried out at 35 °C up to 15 bar. The films were dried in a vacuum oven at 120 °C for at least 12 h to remove any moisture. The sample cell was then evacuated for 1 day prior to testing and evacuated for 24 h after completion of each gas isotherm. The pure-gas sorption isotherms for C2H4 and C2H6 are shown in Fig. S4.† The CMS sorption isotherms followed Langmuir behavior with significantly higher C2H4 and C2H6 sorption capacity than their PIM-6FDA polyimide precursor. The dual-mode and Langmuir fitting parameters are listed in Table S2.† The ethylene solubility at 2 bar was 0.19, 0.34, 0.52, and 0.66 cm3 cm−3 cmHg−1 for the pristine PIM-6FDA, 500, 600, and 800 °C-treated CMS membranes, respectively. As expected, the sorption uptake for ethane was very similar to that of ethylene at a given pressure; hence, the C2H6/C2H6 solubility selectivity was essentially 1, as shown in Table 2. Consequently, the C2H6/C2H6 permselectivity of the CMS membranes was completely controlled by their diffusivity selectivity (Table 3).
| Membrane type | Solubility (cm3 cm−3 cmHg−1) | αS C2H4/C2H6 | |
|---|---|---|---|
| C2H4 | C2H6 | ||
| PIM-6FDA 250 °C | 0.19 | 0.19 | 1.0 |
| CMS 500 °C | 0.34 | 0.32 | 1.05 |
| CMS 600 °C | 0.52 | 0.50 | 1.04 |
| CMS 800 °C | 0.66 | 0.62 | 1.06 |
| Membrane type | Diffusivity (10−10 cm2 s−1) | αD C2H4/C2H6 | |
|---|---|---|---|
| C2H4 | C2H6 | ||
| PIM-6FDA 250 °C | 42 | 11.5 | 3.7 |
| CMS 500 °C | 970 | 490.5 | 2.0 |
| CMS 600 °C | 150 | 38.0 | 3.9 |
| CMS 800 °C | 4.6 | 0.195 | 23.5 |
The diffusivities of ethylene and ethane were calculated from the measured permeability and gravimetrically determined solubility at 2 bar and 35 °C from the relationship D = P/S. Because of the formation of larger micropores in the transition range from the pristine polymer to the CMS membrane pyrolized at 500 °C, the diffusivity increased 23-fold from 42 to 970 × 10−10 cm2 s−1; simultaneously, the diffusivity selectivity decreased from 3.7 to 2. Further heat treatment to 600 °C and 800 °C resulted in smaller average pore size, drop in diffusivity and increase in selectivity. Specifically, CMS membranes made at 800 °C exhibited outstanding molecular sieving properties with a pure-gas C2H6/C2H6 diffusivity selectivity of 23.5. The 6-fold increase in diffusivity selectivity from 3.9 for 600 °C to 23.5 for 800 °C-treated CMS membranes can be explained by the shift in their average d-spacing values from the range of 3.75–4.34 Å (600 °C) to 3.69 Å (800 °C), as shown in Fig. 3. Apparently, the small reduction in the average d-spacing of CMS membranes resulted in a very strong molecular sieving effect between ethylene and ethane in the 800 °C-treated CMS membrane. The size of ethylene and ethane are estimated to be 3.75 Å and 3.85 Å, respectively, using space-filling CPK models and calibrated “slits”.50 The highest C2H6/C2H6 selectivity exhibited in CMS membrane at 800 °C is not a coincidence because its unique CMS structure with average d-spacing of 3.69 Å has a close match with the ideal sieving effects for ethylene and ethane. Because its ethylene permeability of 3 Barrer was relatively low, higher temperature pyrolysis conditions to potentially further enhance the selectivity were not pursued in this study.
Mixed-gas permeation
Because the PIM-6FDA sample carbonized at 800 °C showed the highest pure-gas selectivity, additional experiments were performed with a 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 v/v ethylene/ethane mixture at 35 °C as a function of feed pressure. The results are summarized in Table 4. The overall response to increasing the upstream pressure indicated a relatively small permeability enhancement for both hydrocarbons. Ethylene permeability increased from 3.0 to 3.2 Barrer, whereas ethane permeability increased from 0.167 to 0.205 Barrer over the feed pressure range of 4 to 20 bar. The mixed-gas C2H4/C2H6 selectivity of the CMS membrane dropped only slightly from 18 to 15.6 by increasing the feed pressure from 4 to 20 bar.
50 v/v ethylene/ethane mixture at 35 °C as a function of feed pressure. The results are summarized in Table 4. The overall response to increasing the upstream pressure indicated a relatively small permeability enhancement for both hydrocarbons. Ethylene permeability increased from 3.0 to 3.2 Barrer, whereas ethane permeability increased from 0.167 to 0.205 Barrer over the feed pressure range of 4 to 20 bar. The mixed-gas C2H4/C2H6 selectivity of the CMS membrane dropped only slightly from 18 to 15.6 by increasing the feed pressure from 4 to 20 bar.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 vol% C2H6; T = 35 °C)
50 vol% C2H6; T = 35 °C)
| Feed pressure (Bar) | Permeability (Barrer) | αP C2H4/C2H6 | |
|---|---|---|---|
| C2H4 | C2H6 | ||
| 4 | 3.02 | 0.168 | 17.9 |
| 8 | 3.11 | 0.181 | 17.2 |
| 12 | 3.13 | 0.187 | 16.7 |
| 20 | 3.21 | 0.205 | 15.6 |
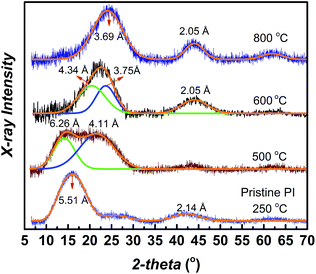
The time dependence of the mixed-gas permeation experiments is shown in Fig. S5.† In order to reach pseudo steady-state permeation conditions for each feed pressure over the range of 4 to 20 bar it took a total time of about 80 days. This significant time-dependent effect of the physical state of the CMS membrane tested at each pressure point on the mixed-gas permeation properties can possibly be attributed to a small matrix dilation of its ultramicropores. This subtle dilation resulted in the observed small increase in mixed-gas permeability of the two hydrocarbons and a concurrent small selectivity drop. This behavior agreed with previous mixed-gas permeation results for ethylene/ethane and CO2/CH4 obtained for the 800 °C heat-treated CMS membrane derived from PIM-6FDA-OH.35,37 However, the drop in selectivity is less pronounced compared to the CMS membrane derived from its OH-functionalized counterpart, as shown in the C2H4/C2H6 upper bound Robeson plot (Fig. 4), possibly due to its more tightly packed CMS structure as discussed in the following section.
 | ||
| Fig. 4 C2H4/C2H6 mix-gas permeation results for PIM-6FDA and PIM-6FDA-OH CMS membranes made at 800 °C and location on pure-gas polymer and CMS upper bound plots.35 | ||
The effect of PIM-6FDA polyimide precursor on CMS performance
Similar to PIM-6FDA-OH,35 increasing the pyrolysis temperature resulted in less permeable but highly C2H4/C2H6 selective CMS membranes made from PIM-6FDA, as shown in Fig. 4. However, the 800 °C CMS derived from OH-free PIM-6FDA polyimide has higher C2H4/C2H6 selectivity compared to its OH-functionalized counterpart PIM-6FDA-OH. The XRD spectra show a broader amorphous peak with average d-spacing at 3.75 Å for PIM-6FDA-OH as compared to its non-functionalized counterpart PIM-6FDA (3.69 Å), indicating formation of a more ordered CMS membrane structure with the OH-free PIM-6FDA precursor (see Fig. S6†). Raman spectra of both CMS membranes show the presence of both D and G bands (see Fig. S7†), suggesting that CMS membranes consist of highly disordered graphene-like sheets. However, a slightly lower D to G ratio (2.08) was found in PIM-6FDA compared to PIM-6FDA-OH (2.14) (see Fig. S7 and Table S3†), which suggests an increase in order and graphitization for CMS at 800 °C derived from PIM-6FDA as compared to PIM-6FDA-OH. This confirms the observed difference in XRD spectra between the two CMS membranes although using Raman spectra should be taken with caution (see Table S3 in ESI†). Therefore, the enhanced C2H4/C2H6 selectivity can be attributed to a more ordered graphitic structure obtained for OH-free PIM-6FDA CMS membranes. The presence of functional OH groups in the polymer precursor has shown detrimental effects for 800 °C CMS membrane performance for C2H4/C2H6 separation; however, the reason is not completely clear at this stage. It is likely that the additional larger pores created during PBO transformation for PIM-6FDA-OH precursor CMS membranes during the pyrolysis process undermine or slow down the formation of a more tightly sintered CMS structure with more optimum C2H4/C2H6 molecular sieving capabilities. It is possible that higher pyrolysis temperature is needed for PIM-6FDA-OH precursor to create a more ordered CMS structure with higher C2H4/C2H6 selectivity. However, the weak mechanical strength of the resulting CMS membranes prohibited any further exploration.Conclusions
In this work, a novel polyimide of intrinsic microporosity, PIM-6FDA, was employed to generate isotropic CMS membranes with excellent performance for ethylene/ethane separation. Development of a fine-tuned ultramicroporous structure from the PIM-6FDA precursor at 800 °C produced a superior ethylene/ethane diffusion-selective membrane. The CMS membrane derived from PIM-6FDA at 800 °C exhibited a pure-gas selectivity of 25 with an ethylene permeability of 3 Barrer (measured at 2 bar and 35 °C). Mixed-gas permeation tests using a binary 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v ethylene/ethane feed under more realistic operating pressures revealed a small performance loss due to a subtle matrix dilation of the selective CMS cavities. Notwithstanding, even the reduced mixed-gas C2H4/C2H6 selectivity of ∼16 measured at 20 bar and 35 °C is the highest value reported to date for any CMS membrane.
1 v/v ethylene/ethane feed under more realistic operating pressures revealed a small performance loss due to a subtle matrix dilation of the selective CMS cavities. Notwithstanding, even the reduced mixed-gas C2H4/C2H6 selectivity of ∼16 measured at 20 bar and 35 °C is the highest value reported to date for any CMS membrane.
Acknowledgements
The work reported in this publication was supported by funding from King Abdullah University of Science and Technology (KAUST).Notes and references
- T. Ren, M. Patel and K. Blok, Energy, 2006, 31, 425–451 CrossRef CAS.
- W. J. Koros, AIChE J., 2004, 50, 2326–2334 CrossRef CAS.
- J. A. Caballero, I. E. Grossmann, M. Keyvani and E. S. Lenz, Ind. Eng. Chem. Res., 2009, 48, 9151–9162 CrossRef CAS.
- A. Motelica, O. S. L. Bruinsma, R. Kreiter, M. den Exter and J. F. Vente, Ind. Eng. Chem. Res., 2012, 51, 6977–6986 CrossRef CAS.
- J. Ploegmakers, A. R. T. Jelsma, A. G. J. van der Ham and K. Nijmeijer, Ind. Eng. Chem. Res., 2013, 52, 6524–6539 CrossRef CAS.
- R. W. Baker and B. T. Low, Macromolecules, 2014, 47, 6999–7013 CrossRef CAS.
- L. M. Robeson, J. Membr. Sci., 2008, 320, 390–400 CrossRef CAS.
- A. B. Fuertes and I. Menendez, Sep. Purif. Technol., 2002, 28, 29–41 CrossRef CAS.
- M. Rungta, C. Zhang, W. J. Koros and L. Xu, AIChE J., 2013, 59, 3475–3489 CrossRef CAS.
- K. Tanaka, A. Taguchi, J. Hao, H. Kita and K. Okamoto, J. Membr. Sci., 1996, 121, 197–207 CrossRef CAS.
- C. Staudt-Bickel and W. J. Koros, J. Membr. Sci., 2000, 170, 205–214 CrossRef CAS.
- S. S. Chan, R. Wang, T. S. Chung and Y. Lin, J. Membr. Sci., 2002, 210, 55–64 CrossRef CAS.
- S. S. Chan, T. S. Chung, Y. Lin and R. Wang, J. Membr. Sci., 2003, 218, 235–245 CrossRef CAS.
- J. Ploegmakers, S. Japip and K. Nijmeijer, J. Membr. Sci., 2013, 428, 445–453 CrossRef CAS.
- J. E. Bachman, Z. P. Smith, T. Li, T. Xu and J. R. Long, Nat. Mater., 2016, 15, 845–849 CrossRef CAS PubMed.
- M. Rungta, L. Xu and W. J. Koros, Carbon, 2012, 50, 1488–1502 CrossRef CAS.
- K. M. Steel and W. J. Koros, Carbon, 2003, 41, 253–266 CrossRef CAS.
- A. J. Bird and D. L. Trimm, Carbon, 1983, 21, 177–180 CrossRef CAS.
- S. M. Saufi and A. F. Ismail, Carbon, 2004, 42, 241–259 CrossRef CAS.
- L. Shao, T.-S. Chung and K. P. Pramoda, Microporous Mesoporous Mater., 2005, 84, 59–68 CrossRef CAS.
- S. Fu, E. S. Sanders, S. S. Kulkarni and W. J. Koros, J. Membr. Sci., 2015, 487, 60–73 CrossRef CAS.
- M. Inagaki, N. Ohta and Y. Hishiyama, Carbon, 2013, 61, 1–21 CrossRef CAS.
- H. B. Park, C. H. Jung, Y. M. Lee, A. J. Hill, S. J. Pas, S. T. Mudie, E. Van Wagner, B. D. Freeman and D. J. Cookson, Science, 2007, 318, 254–258 CrossRef CAS PubMed.
- S. Fu, G. B. Wenz, E. S. Sanders, S. S. Kulkarni, W. Qiu, C. Ma and W. J. Koros, J. Membr. Sci., 2016, 520, 699–711 CrossRef CAS.
- P. M. Budd, K. J. Msayib, C. E. Tattershall, B. S. Ghanem, K. J. Reynolds, N. B. McKeown and D. Fritsch, J. Membr. Sci., 2005, 251, 263–269 CrossRef CAS.
- N. Du, H. B. Park, G. P. Robertson, M. M. Dal-Cin, T. Visser, L. Scoles and M. D. Guiver, Nat. Mater., 2011, 10, 372–375 CrossRef CAS PubMed.
- Y. Zhuang, J. G. Seong, Y. S. Do, H. J. Jo, Z. Cui, J. Lee, Y. M. Lee and M. D. Guiver, Macromolecules, 2014, 47, 3254–3262 CrossRef CAS.
- M. Carta, M. Croad, R. Malpass-Evans, J. C. Jansen, P. Bernardo, G. Clarizia, K. Friess, M. Lanč and N. B. McKeown, Adv. Mater., 2014, 26, 3526–3531 CrossRef CAS PubMed.
- B. S. Ghanem, R. Swaidan, E. Litwiller and I. Pinnau, Adv. Mater., 2014, 26, 3688–3692 CrossRef CAS PubMed.
- B. S. Ghanem, R. Swaidan, X. Ma, E. Litwiller and I. Pinnau, Adv. Mater., 2014, 26, 6696–6700 CrossRef CAS PubMed.
- I. Rose, M. Carta, R. Malpass-Evans, M.-C. Ferrari, P. Bernardo, G. Clarizia, J. C. Jansen and N. B. McKeown, ACS Macro Lett., 2015, 4, 912–915 CrossRef CAS.
- R. Swaidan, B. Ghanem and I. Pinnau, ACS Macro Lett., 2015, 4, 947–951 CrossRef CAS.
- P. Li, T. S. Chung and D. R. Paul, J. Membr. Sci., 2013, 432, 50–57 CrossRef CAS.
- O. Salinas, X. Ma, E. Litwiller and I. Pinnau, J. Membr. Sci., 2016, 504, 133–140 CrossRef CAS.
- O. Salinas, X. Ma, E. Litwiller and I. Pinnau, J. Membr. Sci., 2016, 500, 115–123 CrossRef CAS.
- X. Ma, R. Swaidan, B. Teng, H. Tan, O. Salinas, E. Litwiller, Y. Han and I. Pinnau, Carbon, 2013, 62, 88–96 CrossRef CAS.
- R. Swaidan, X. Ma, E. Litwiller and I. Pinnau, J. Membr. Sci., 2013, 447, 387–394 CrossRef CAS.
- R. J. Swaidan, X. Ma and I. Pinnau, J. Membr. Sci., 2016, 520, 983–989 CrossRef CAS.
- S. Li, H. J. Jo, S. H. Han, C. H. Park, S. Kim, P. M. Budd and Y. M. Lee, J. Membr. Sci., 2013, 434, 137–147 CrossRef CAS.
- X. Ma, O. Salinas, E. Litwiller and I. Pinnau, Polym. Chem., 2014, 5, 6914–6922 RSC.
- H. Shamsipur, B. A. Dawood, P. M. Budd, P. Bernardo, G. Clarizia and J. C. Jansen, Macromolecules, 2014, 47, 5595–5606 CrossRef CAS.
- J. N. Barsema, S. D. Klijnstra, J. H. Balster, N. F. A. van der Vegt, G. H. Koops and M. Wessling, J. Membr. Sci., 2004, 238, 93–102 CrossRef CAS.
- X. Ma, R. Swaidan, Y. Belmabkhout, Y. Zhu, E. Litwiller, M. Jouiad, I. Pinnau and Y. Han, Macromolecules, 2012, 45, 3841–3849 CrossRef CAS.
- Y. Rogan, L. Starannikova, V. Ryzhikh, Y. Yampolskii, P. Bernardo, F. Bazzarelli, J. C. Jansen and N. B. McKeown, Polym. Chem., 2013, 4, 3813–3820 RSC.
- D. G. Pye, H. H. Hoehn and M. Panar, J. Appl. Polym. Sci., 1976, 20, 1921–1931 CrossRef CAS.
- T. T. Moore, S. Damle, P. J. Williams and W. J. Koros, J. Membr. Sci., 2004, 245, 227–231 CrossRef CAS.
- K. C. O'Brien, W. J. Koros, T. A. Barbari and E. S. Sanders, J. Membr. Sci., 1986, 29, 229–238 CrossRef.
- W. R. Vieth, P. M. Tam and A. S. Michaels, J. Colloid Interface Sci., 1966, 22, 360–370 CrossRef CAS.
- L. Xu, M. Rungta, J. Hessler, W. Qiu, M. Brayden, M. Martinez, G. Barbay and W. J. Koros, Carbon, 2014, 80, 155–166 CrossRef CAS.
- M. Rungta, Ph.D. thesis, Georgia Institute of Technology, 2012, p. 155.
Footnote |
| † Electronic supplementary information (ESI) available: Raman and FTIR spectra; densities; ethylene and ethane sorption isotherms; dual-mode and Langmuir parameters. See DOI: 10.1039/c6ra24699k |
| This journal is © The Royal Society of Chemistry 2017 |