Metal-catalyzed enyne cycloisomerization in natural product total synthesis
Abstract
Since the seminal work of B. M. Trost et al. in 1985, metal catalyzed enyne cycloisomerization has become a fast growing and fascinating field. Due to its diversified chemistry and ability to increase molecular complexity in an efficient, atom economical, step economic and redox economic way, enyne cycloisomerization has become a powerful and attractive strategy for the construction of cyclic compounds, and thus has great potential for applications in total synthesis of natural products and pharmaceuticals. In this review, a brief summary of the state-of-the-art mechanism studies, the classification of structures accessed through enyne cycloisomerizations, and recent completed total synthesis of representative natural products showcasing creative and ingenious incorporation of enyne cycloisomerization as a strategic manoeuver are included, with the aim of providing a complement to the existing reviews to inspire future developments.
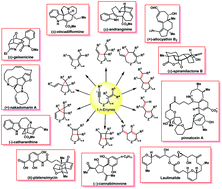
- This article is part of the themed collections: Synthetic Approaches to Natural Products via Catalytic Processes and 2017 Organic Chemistry Frontiers Review-type Articles


 Please wait while we load your content...
Please wait while we load your content...