Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
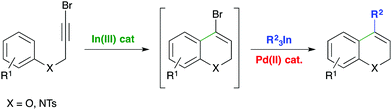
Sequential In-catalyzed intramolecular hydroarylation and Pd-catalyzed cross-coupling reactions using bromopropargyl aryl ethers and amines†
Lorena
Alonso-Marañón
 ,
Luis A.
Sarandeses
,
Luis A.
Sarandeses
 ,
M. Montserrat
Martínez
,
M. Montserrat
Martínez
 * and
José
Pérez Sestelo
* and
José
Pérez Sestelo
 *
*
Centro de Investigaciones Científicas Avanzadas (CICA) and Departamento de Química Fundamental, Universidade da Coruña, E-15071 A Coruña, Spain. E-mail: mmartinezc@udc.es; sestelo@udc.es
First published on 26th December 2016
Abstract
A sequential one-pot indium-catalyzed intramolecular hydroarylation (IMHA) of bromopropargyl aryl ethers and amines, and palladium-catalyzed cross-coupling reaction using triorganoindium reagents (R3In) has been developed. In this transformation, the IMHA of 3-bromo-2-propynyl aryl ethers under indium(III) catalysis, proceeds regioselectively through a 6-endo dig pathway to afford 4-bromo-2H-chromenes. Subsequent palladium-catalyzed cross-coupling with R3In gives 4-substituted-2H-chromenes in one-pot. This sequential transformation was extended to 3-bromo-2-propynyl-N-tosylanilines to afford 4-substituted-1,2-dihydroquinolines. The dual-catalyzed procedure takes place efficiently with a variety of propargyl aryl ethers and amines and R3In (R = aryl, heteroaryl, alkyl or alkynyl), showing the efficiency of these organometallics and proving the compatibility of indium and palladium in catalysis.
Introduction
The development of efficient and sustainable chemical methodologies has become one of the most important goals of modern organic chemistry.1 As an example, sequential one-pot reactions are attractive procedures because the possibility of forming several bonds in one vessel offers important synthetic advantages in terms of economy, sustainability and versatility. Although many sequential or tandem reactions involving classical organic transformations have been reported, orthogonal metal-catalyzed processes, in which more than one catalyst and reagents are combined in a single pot, are particularly challenging because the increased level of complexity enables redox processes or ligand-exchange reactions.2 Actually, several sequential one-pot systems, which range from isolated catalytic cycles to tandem or orthogonal catalysis, have been designed and this research has become an emerging area.3Recently, we reported the indium-catalyzed intramolecular hydroarylation (IMHA) of propargyl aryl ethers for the synthesis of 2H-chromenes.4 The reaction is highly versatile and takes place regioselectively with terminal and internal alkynes bearing electron-rich and electron-deficient substituents in the arene and alkyne affording the 6-endo dig cyclization product. Recent computational studies support a mechanism based on indium(III) activation of the alkyne.5 In addition, indium catalysis offers significant advantages in terms of cost and low toxicity.6 Interestingly, the reaction of halopropargyl aryl ethers gives 4-halo-2H-chromenes in high yields without 1,2-halogen migration, a side reaction usually observed under gold catalysis.7 Taking advantage of the halo-substituents, and in combination with our research on metal-catalyzed cross-coupling reactions with triorganoindium reagents (R3In),8 we envisioned a sequential one-pot indium-catalyzed IMHA of 3-halopropynyl aryl ethers with a palladium-catalyzed cross-coupling reaction using R3In (Scheme 1). Additionally, we also propose the extension of this procedure to halopropargyl anilines. The IMHA of N-propargyl-N-tosylanilines has been reported using precious metals (Au, Pt, Rh) with variable yields based on the alkyne and arene substitution and catalyst loading.9 The gold-catalyzed IMHA of N-(3-iodoprop-2-ynyl)-N-tosylanilines takes place with iodide migration affording 3-iodo-1,2-dihydroquinolines.9g This methodology should offer a straightforward route to 4-functionalized 2H-chromenes and 1,2-dihydroquinolines – structural motifs present in a large number of naturally occurring and biologically active compounds.10
During the last few years, the palladium-catalyzed cross-coupling reaction of triorganoindium reagents (R3In), discovered by this research group,8a has gained increasing utility in organic synthesis.11 R3In can be easily prepared from the corresponding organolithium using InCl3, or organic halides and the indium metal. Particular features of R3In are the high efficiency, versatility and selectivity in transferring the three organic groups attached to indium. In comparison with other organometallics, aryl–aryl and aryl–alkenyl couplings using R3In have become an efficient alternative.12
Results and discussion
Our investigation started studying the palladium-catalyzed coupling of R3In with the 4-bromo-2H-chromenes obtained from the indium-catalyzed IMHA of bromopropargyl aryl ethers. In this research, 4-bromo-6-methoxy-2H-chromene (2a, Table 1) was selected as the starting material since it can be obtained from indium-catalyzed IMHA in high yield.4 Given that the In-catalyzed IMHA requires a non-coordinating solvent, the coupling reaction was studied in toluene. Under these considerations, we found that the reaction of 0.5 equiv. of triphenylindium with 2a in toluene under palladium catalysis proceeded after 18 h at 80 °C to give the coupling product 3a in 87–88% yield (entries 1 and 2). The efficient coupling was also observed using either Pd(II) or Pd(0) complexes, and with a variety of R3In (heteroaryl-, alkynyl- or alkylindium), thus providing a variety of 4-substituted-2H-chromenes (3b–d, entries 3–5) in high yields. In order to compare the reactivity of R3In with other common organometallics in cross-coupling reactions, the coupling was tested using organotin, organozinc and organoboron compounds. Interestingly, the reaction using tributylphenyltin or phenylzinc chloride gave the coupling product 3a in lower yields, even when a large excess of the organometallic agent was employed (entries 6 and 7). Only the reaction with phenylboronic acid (2.2 equiv.) using Na2CO3 as a base afforded 3a in 87% yield (entry 8). These results illustrate the synthetic utility of R3In reagents in palladium-catalyzed coupling reactions.| Entry | R–M | (Equiv.) | [Pd] cat. | Product | Yielda (%) |
|---|---|---|---|---|---|
a Isolated yields.
b Toluene at 100 °C.
c Toluene/THF 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.
d Na2CO3 (6.0 equiv.) in toluene/EtOH 2 1.
d Na2CO3 (6.0 equiv.) in toluene/EtOH 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. 1.
|
|||||
| 1 | Ph3In | 0.5 | Pd(PPh3)2Cl2 | 3a | 88 |
| 2 | Ph3In | 0.5 | Pd(PPh3)4 | 3a | 87 |
| 3 | (2-Thienyl)3In | 0.5 | Pd(PPh3)2Cl2 | 3b | 89 |
| 4 | (PhC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C)3In C)3In |
0.5 | Pd(dppf)Cl2 | 3c | 67 |
| 5 | Bu3In | 0.5 | Pd(PPh3)2Cl2 | 3d | 60 |
| 6 | PhSnBu3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) b b |
4.0 | Pd(PPh3)4 | 3a | 30 |
| 7 | PhZnClc | 4.0 | Pd(PPh3)4 | 3a | 40 |
| 8 | PhB(OH)2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) d d |
2.2 | Pd(PPh3)4 | 3a | 87 |
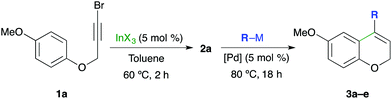
Once the reaction conditions for the Pd-catalyzed coupling of R3In with the 4-bromo-2H-chromene 2a had been established, the sequential one-pot In-catalyzed IMHA and Pd-catalyzed cross-coupling was explored (Table 2). In this scenario, we found that hydroarylation of the 3-bromo-2-propynyl aryl ether 1a using InCl3 (5 mol%) in toluene at 60 °C for 2 hours, followed by the addition of Ph3In (THF solution, 0.5 equiv.) and Pd(PPh3)2Cl2 (5 mol%) gave the 4-phenyl-2H-chromene 3a in 95% overall yield after 18 h at 80 °C (entry 1). Using InBr3 or InI3 as catalysts the sequential transformation also afforded the desired product 3a but in lower yields (57% and 60% respectively, entries 2 and 3). These results can be explained by the higher reactivity of these indium(III) halides, which led to the formation of by-products. Overall, these results demonstrate the compatibility of palladium cross-coupling with the indium IHMA reaction conditions.
| Entry | InX3 | R–M (0.5 equiv.) | [Pd] cat. | Product | Yielda (%) |
|---|---|---|---|---|---|
a Isolated yield.
b 4.0 equiv. of PhZnCl.
c 4.0 equiv. of PhSnBu3 at 100 °C.
d 2.2 equiv. of PhB(OH)2, Na2CO3 (6 equiv.) in toluene![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EtOH (2 EtOH (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). 1).
|
|||||
| 1 | InCl3 | Ph3In | Pd(PPh3)2Cl2 | 3a | 95 |
| 2 | InBr3 | Ph3In | Pd(PPh3)2Cl2 | 3a | 57 |
| 3 | InI3 | Ph3In | Pd(PPh3)2Cl2 | 3a | 60 |
| 4 | InCl3 | (2-Thienyl)3In | Pd(PPh3)2Cl2 | 3b | 85 |
| 5 | InCl3 | (PhC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C)3In C)3In |
Pd(dppf)Cl2 | 3c | 72 |
| 6 | InCl3 | Bu3In | Pd(PPh3)2Cl2 | 3d | 70 |
| 7 | InCl3 | Me3In | Pd(PPh3)2Cl2 | 3e | 89 |
| 8 | InCl3 | PhZnClb | Pd(PPh3)4 | 3a | 40 |
| 9 | InCl3 | PhSnBu3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) c c |
Pd(PPh3)4 | 3a | 40 |
| 10 | InCl3 | PhB(OH)2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) d d |
Pd(PPh3)4 | 3a | 87 |
Under the previously developed reaction conditions, the one-pot sequence was assessed with a variety of R3In reagents (Table 2). The procedure using tri(2-thienyl)indium (0.5 equiv.) gave the corresponding 2H-chromene 3b in 85% overall yield (entry 4). The reaction with tri(phenylethynyl)indium also gave the 4-phenylethynyl-2H-chromene 3c on using Pd(dppf)Cl2 as the catalyst (72%, entry 5). The reaction with trialkylindium reagents such as tributylindium and trimethylindium afforded the 4-substituted-2H-chromenes 3d and 3e in 70% and 89% yields, respectively (entries 6 and 7). In all of these examples the yields for the two-step one-pot sequence compare favourably with procedures where each step was performed separately and required isolation of the intermediate. The reactivity and compatibility of other organometallic reagents in this sequential process was also evaluated. The use of phenylzinc chloride (4 equiv.) and Pd(PPh3)4 (5 mol%) provided the coupling product 3a in a modest 40% isolated yield (entry 8). Analogously, the reaction with tributylphenyltin (4.0 equiv.) at 100 °C gave only 40% yield (entry 9). The use of phenylboronic acid (2.2 equiv.), Na2CO3 (6.0 equiv.) and Pd(PPh3)4 (5 mol%) in a mixture of toluene and ethanol gave the 4-phenyl-2H-chromene 3a in 87% yield (entry 10). These results demonstrate the compatibility of palladium-catalyzed coupling reactions with the indium-catalyzed IMHA in a one-pot protocol and triorganoindium compounds are shown as the reagents of choice.
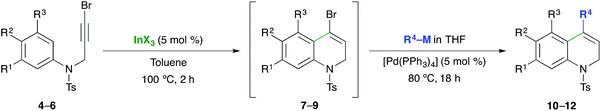
In an effort to increase the chemical diversity of this sequential dual-catalyzed In/Pd protocol, the methodology was then studied with 3-bromopropargyl anilines. This sequential transformation should allow the synthesis of 4-substituted-1,2-dihydroquinolines, a structural unit present in naturally occurring products, pharmaceuticals and also used as building blocks in organic synthesis.10 In our initial experiments we found that the IMHA of N-(3-bromoprop-2-ynyl)-N-tosylaniline 4 using InCl3 (5 mol%) takes place at 100 °C to give the 4-bromo-1,2-dihydroquinoline 7 in 95% yield (Table 3, entry 1). In comparison with the IMHA of propargyl aryl ethers, the amines are less reactive, probably due to electronic effects. The use of InBr3 or InI3 (5 mol%) as catalysts at 100 °C also gave 7 in shorter reaction times and high yields (92% and 80% respectively, entries 2 and 3). With these results in mind, the sequential IMHA-coupling using the different indium(III) halides in toluene at 100 °C and reaction with Ph3In (THF solution, 0.5 equiv.) and Pd(PPh3)4 (5 mol%) at 80 °C was tested. Under these conditions, the cross-coupling step was not complete after 18 h and the desired 4-phenyl-1,2-dihydroquinoline 10a was obtained in moderate yield. Optimal results were obtained using 0.7 equiv. of Ph3In and InBr3 as the catalyst (92%), although the use of InCl3 or InI3 also gave satisfactory yields (Table 3, entries 4–6).
| Entry | Substrate | InX3 | R1 | R2 | R3 | R4–M (0.7 equiv.) | Product | Yielda (%) |
|---|---|---|---|---|---|---|---|---|
a Isolated yields.
b Pd(dppf)Cl2 (5 mol%) as the catalyst.
c 2.2 equiv. of PhB(OH)2, Na2CO3 (6.0 equiv.) in toluene/EtOH 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 at 80 °C.
d 4.0 equiv. of PhSnBu3 at 100 °C for 24 h. 1 at 80 °C.
d 4.0 equiv. of PhSnBu3 at 100 °C for 24 h.
|
||||||||
| 1 | 4 | InCl3 | H | OMe | H | — | 7 | 95 |
| 2 | 4 | InBr3 | H | OMe | H | — | 7 | 92 |
| 3 | 4 | InI3 | H | OMe | H | — | 7 | 80 |
| 4 | 4 | InBr3 | H | OMe | H | Ph3In | 10a | 92 |
| 5 | 4 | InI3 | H | OMe | H | Ph3In | 10a | 80 |
| 6 | 4 | InCl3 | H | OMe | H | Ph3In | 10a | 70 |
| 7 | 4 | InBr3 | H | OMe | H | (2-Thienyl)3In | 10b | 86 |
| 8 | 4 | InBr3 | H | OMe | H | (PhC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C)3In C)3In |
10c | 65b |
| 9 | 4 | InBr3 | H | OMe | H | Bu3In | 10d | 60 |
| 10 | 4 | InBr3 | H | OMe | H | Me3In | 10e | 72 |
| 11 | 4 | InBr3 | H | OMe | H | PhSnBu3 | 10a | 38d |
| 12 | 4 | InBr3 | H | OMe | H | PhB(OH)2 | 10a | 80c |
| 13 | 5 | InBr3 | H | H | H | Ph3In | 11a | 92 |
| 14 | 5 | InBr3 | H | H | H | (2-Thienyl)3In | 11b | 98 |
| 15 | 5 | InBr3 | H | H | H | (PhC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C)3In C)3In |
11c | 78 |
| 16 | 5 | InBr3 | H | H | H | Bu3In | 11d | 74 |
| 17 | 5 | InBr3 | H | H | H | Me3In | 11e | 85 |
| 18 | 6 | InI3 | OMe | H | OMe | Ph3In | 12a | 75 |
| 19 | 6 | InI3 | OMe | H | OMe | (2-Thienyl)3In | 12b | 55 |
| 20 | 6 | InI3 | OMe | H | OMe | Me3In | 12e | 67 |
Under the previously developed conditions, the sequential procedure was tested using different triorganoindium reagents. The reaction using heteroarylindium reagents such as tri(2-thienyl)indium (0.7 equiv.) gave the 4-thienyldihydroquinoline 10b in 86% yield (entry 7). The use of trialkynylindium reagents such as tri(phenylethynyl)indium also afforded the dihydroquinoline 10c in 65% yield with Pd(dppf)2Cl2 as the catalyst (entry 8). The reaction with tributylindium and trimethylindium also gave the corresponding 4-substituted dihydroquinolines 10d and 10e in 60% and 72% yields respectively (entries 9 and 10), thus showing the utility of indium organometallics in the cross-coupling with alkyl nucleophiles. This sequential transformation was also assessed employing other organometallic reagents. Interestingly, the reaction using tributylphenyltin gave a low isolated yield (38%) (entry 11), and only the reaction of phenylboronic acid worked efficiently using Na2CO3 as the base (80%, entry 12).
With the purpose to expand the synthetic utility of the methodology, we tested the procedure with other bromopropargyl anilines. The indium-catalyzed IMHA of N-(3-bromoprop-2-ynyl)-N-tosylaniline 5 using InBr3 (5 mol%) in toluene at 100 °C for 2 h, followed by the addition of triphenylindium (THF solution, 0.7 equiv.) and Pd(PPh3)4 (5 mol%) overnight at 80 °C, afforded N-tosyl-4-phenyl-1,2-dihydroquinoline (11a) in an excellent 92% yield (entry 13).13 Analogously, the use of tri(2-thienyl)indium gave the corresponding dihydroquinoline 11b in 98% yield (entry 14). The dual-catalyzed process with the tri(phenylethynyl)indium reagent also gave 4-phenylethynyldihydroquinoline 11c in 78% yield (entry 15). Furthermore, butyl- and methyldihydroquinolines 11d and 11e were successfully prepared by using tributylindium and trimethylindium reagents in 74% and 85% yields (entries 16 and 17), respectively.
Additionally, the one-pot sequence with N-(3-bromo-2-propynyl)-3,5-dimethoxy-N-tosylaniline 6 also proceeded efficiently to give the desired dihydroquinolines. In this case, the best yields were obtained using InI3 as the catalyst and the 4-phenyl-1,2-dihydroquinoline 12a was obtained in 75% overall yield (entry 18).13 Under the established conditions, the reaction with tri(2-thienyl)indium and trimethylindium provided 12b and 12e in 55% and 67% overall yields (entries 19 and 20), respectively. These results show the versatility of this novel sequential one-pot In/Pd catalyzed IMHA-cross-coupling reaction using indium organometallics and demonstrate its application to the synthesis of 4-substituted-1,2-dihydroquinolines.
Conclusions
In summary, starting from easily available bromopropargyl aryl ethers and amines, we have developed a sequential one-pot indium-catalyzed IMHA and palladium-catalyzed cross-coupling process that allows the regioselective synthesis of 4-substituted 2H-chromenes and dihydroquinolines. This sequence demonstrates the possibility of combining dual-catalyzed In/Pd transformations and highlights the efficiency of triorganoindium reagents in this one-pot sequential procedure transferring a variety of organic groups (aryl, heteroaryl, alkyl and alkynyl) with high efficiency and good yields. Moreover, these results support the possibility to develop the orthogonal tandem catalytic processes combining indium and palladium.Experimental
General procedure for palladium-catalyzed cross-coupling reactions of 4-bromo-6-methoxy-2H-chromene (2a) with triorganoindium reagents (Table 1, entries 1–5)
In a Schlenk tube filled with argon, a THF solution of the corresponding triorganoindium reagent (0.5 equiv., 0.207 mmol) was added to a toluene solution of 2a4 (0.415 mmol) and the palladium catalyst (0.021 mmol). The reaction mixture was heated at 80 °C until the starting material was consumed. The mixture was allowed to cool down to room temperature and was quenched by the addition of a few drops of MeOH. The solvent was concentrated in vacuo and the residue was diluted with EtOAc (10 mL) and poured into a separating funnel with H2O (10 mL). The product was extracted with EtOAc (3 × 10 mL) and the combined organic layers were washed with brine (15 mL), dried, filtered and concentrated in vacuo. The residue was purified by flash chromatography on silica gel to afford, after concentration and high vacuum drying, the corresponding cross-coupling product (3a–d).General procedure for sequential IMHA-cross coupling reactions of 4-methoxyphenyl-(3-bromoprop-2-ynyl)ether (1a) with triorganoindium reagents (Table 2, entries 1–7)
In a Schlenk tube filled with argon, a solution of 1a (0.415 mmol) and InCl3 (0.021 mmol) in toluene (5 mL) was heated at 60 °C for 2 h. The reaction was cooled down to rt and the palladium catalyst (0.021 mmol) and a solution of R3In (50 mol%, 0.207 mmol, ∼0.1 M in THF) were added. The resulting mixture was heated at 80 °C for 16 h and then quenched by the addition of a few drops of MeOH. The solvent was removed in vacuo and the residue was diluted with EtOAc (10 mL) and poured into a separatory funnel with H2O (10 mL). The aqueous phase was extracted with EtOAc (3 × 10 mL) and the combined organic phases were washed with brine (15 mL), dried, filtered and concentrated in vacuo to afford the corresponding cross-coupling product (3a–e) after purification by column chromatography.General procedure for the preparation of N-(3-bromoprop-2-ynyl)-N-tosylanilines 4–6
AgNO3 (0.217 g, 1.27 mmol) and NBS (0.790 g, 4.44 mmol) were added to a rt solution of the corresponding N-(3-bromoprop-2-ynyl)-N-tosylaniline (3.17 mmol) in dry acetone (40 mL). The reaction mixture was stirred overnight, the solvent was evaporated and the residue was diluted with EtOAc (50 mL). The organic phase was washed with brine (25 mL), dried, filtered and concentrated in vacuo. The residue was purified by flash chromatography on silica gel to afford, after concentration and high vacuum drying, the corresponding N-(3-bromoprop-2-ynyl)-N-tosylaniline.General procedure for sequential IMHA-cross coupling reactions of N-(3-bromo-2-propynyl)-N-tosylanilines with triorganoindium reagents (Table 3, entries 4–10 and 13–20)
In a Schlenk tube filled with argon a solution of InBr3 or InI3 (0.021 mmol) and the corresponding N-(3-bromo-2-propynyl)-N-tosylaniline (0.415 mmol) in toluene (5 mL) was heated at 100 °C until the starting material had been consumed (TLC). Then, the palladium catalyst (0.021 mmol) and the solution of R3In (70 mol%, 0.291 mmol, ∼0.1 M in dry THF) were added and the mixture was heated at 80 °C until the starting material had been consumed. The reaction was quenched by the addition of a few drops of MeOH and the mixture was concentrated in vacuo. EtOAc (10 mL) and H2O (10 mL) were added. The aqueous phase was extracted with EtOAc (3 × 10 mL) and the combined organic phases were washed with brine (15 mL), dried, filtered and concentrated in vacuo. The residue was purified by flash chromatography to afford, after concentration and high vacuum drying, the corresponding IMHA-cross-coupling product.Acknowledgements
We are grateful to the Spanish Ministerio de Economía y Competividad (CTQ2015-68369-P), Xunta de Galicia (GRC2014/042) and EDRF funds for financial support.Notes and references
- (a) Y. Hayashi, Chem. Sci., 2016, 7, 866–880 RSC; (b) X. Zeng, Chem. Rev., 2013, 113, 6864–6900 CrossRef CAS PubMed; (c) C. Vaxelaire, P. Winter and M. Christmann, Angew. Chem., Int. Ed., 2011, 50, 3605–3607 CrossRef CAS PubMed; (d) A. Burke, V. Marinho and O. Furtado, Curr. Org. Synth., 2010, 7, 94–119 CrossRef CAS.
- (a) J. Tsoung, J. Panteleev, M. Tesch and M. Lautens, Org. Lett., 2014, 16, 110–113 CrossRef CAS PubMed; (b) A. A. Friedman, J. Panteleev, J. Tsoung, V. Huynh and M. Lautens, Angew. Chem., Int. Ed., 2013, 52, 9755–9758 CrossRef CAS PubMed; (c) L. Zhang, L. Sonaglia, J. Stacey and M. Lautens, Org. Lett., 2013, 15, 2128–2131 CrossRef CAS PubMed; (d) M. M. Hansmann, A. S. K. Hashmi and M. Lautens, Org. Lett., 2013, 15, 3226–3229 CrossRef CAS PubMed; (e) N. T. Patil, V. S. Shinde and B. Gajula, Org. Biomol. Chem., 2012, 10, 211–224 RSC; (f) J. M. Lee, Y. Na, H. Han and S. Chang, Chem. Soc. Rev., 2004, 33, 302–312 RSC.
- For selected examples, see: (a) P. García-Domínguez and C. Nevado, J. Am. Chem. Soc., 2016, 138, 3266–3269 CrossRef PubMed; (b) T. L. Lohr and T. J. Marks, Nat. Chem., 2015, 7, 477–482 CrossRef CAS PubMed; (c) C. S. Higman, M. P. de Araujo and D. E. Fogg, Catal. Sci. Technol., 2016, 6, 2077–2084 RSC; (d) M. H. Pérez-Temprano, J. A. Casares and P. Espinet, Chem. – Eur. J., 2012, 18, 1864–1884 CrossRef PubMed; (e) N. Jeong, S. D. Seo and A. J. Y. Shin, J. Am. Chem. Soc., 2000, 122, 10220–10221 CrossRef CAS; (f) Y. Nishibayashi, M. Yoshikawa, Y. Inada, M. D. Milton, M. Hidai and S. Uemura, Angew. Chem., Int. Ed., 2003, 42, 2681–2684 CrossRef CAS PubMed; (g) M. D. Milton, Y. Inada, Y. Nishibayashi and S. Uemura, Chem. Commun., 2004, 2712–2713 RSC; (h) B. Zimmermann, J. Herwig and M. Beller, Angew. Chem., Int. Ed., 1999, 38, 2372–2375 CrossRef CAS.
- L. Alonso-Marañón, M. M. Martínez, L. A. Sarandeses and J. Pérez Sestelo, Org. Biomol. Chem., 2015, 13, 379–387 Search PubMed.
- M. G. Menkir and S.-L. Lee, Org. Biomol. Chem., 2016, 14, 6508–6516 CAS.
- B. C. Ranu, Eur. J. Org. Chem., 2000, 2347–2356 CrossRef CAS.
- P. Morán-Poladura, E. Rubio and J. M. González, Beilstein J. Org. Chem., 2013, 9, 2120–2128 CrossRef PubMed.
- For representative references, see: (a) I. Pérez, J. Pérez Sestelo and L. A. Sarandeses, Org. Lett., 1999, 1, 1267–1269 CrossRef; (b) I. Pérez, J. Pérez Sestelo and L. A. Sarandeses, J. Am. Chem. Soc., 2001, 123, 4155–4160 CrossRef; (c) D. Rodríguez, J. Pérez Sestelo and L. A. Sarandeses, J. Org. Chem., 2003, 68, 2518–2520 CrossRef PubMed; (d) D. Rodríguez, J. Pérez Sestelo and L. A. Sarandeses, J. Org. Chem., 2004, 69, 8136–8139 CrossRef PubMed; (e) R. Riveiros, D. Rodríguez, J. Pérez Sestelo and L. A. Sarandeses, Org. Lett., 2006, 8, 1403–1406 CrossRef CAS PubMed; (f) J. Caeiro, J. Pérez Sestelo and L. A. Sarandeses, Chem. – Eur. J., 2008, 14, 741–746 CrossRef CAS PubMed; (g) R. Riveiros, L. Saya, J. Pérez Sestelo and L. A. Sarandeses, Eur. J. Org. Chem., 2008, 1959–1966 CrossRef CAS.
- (a) B. Martín-Matute, C. Nevado, D. J. Cárdenas and A. M. Echavarren, J. Am. Chem. Soc., 2003, 125, 5757–5766 CrossRef PubMed; (b) S. J. Pastine, S. W. Youn and D. Sames, Tetrahedron, 2003, 59, 8859–8868 CrossRef CAS; (c) C. Nevado and A. M. Echavarren, Chem. – Eur. J., 2005, 11, 3155–3164 CrossRef CAS PubMed; (d) R. S. Menon, A. D. Findlay, A. C. Bissember and M. G. Banwell, J. Org. Chem., 2009, 74, 8901–8903 CrossRef CAS PubMed; (e) K. Komeyama, R. Igawa and K. Takaki, Chem. Commun., 2010, 46, 1748–1750 RSC; (f) K. C. Majumdar, R. K. Nandi, S. Ganai and A. Taher, Synlett, 2011, 116–120 CrossRef CAS; (g) P. Morán-Poladura, S. Suárez-Pantiga, M. Piedrafita, E. Rubio and J. M. González, J. Organomet. Chem., 2011, 696, 12–15 CrossRef; (h) H. Murase, K. Senda, M. Senoo, T. Hata and H. Urabe, Chem. – Eur. J., 2014, 20, 317–322 CrossRef CAS PubMed; (i) Y. Sun, P. Gu, Y. Gao, Q. Xua and M. Shi, Chem. Commun., 2016, 52, 6942–6945 RSC.
- (a) E. Vessally, L. Edjlali, A. Hosseinian, A. Bekhradnia and M. D. Esrafili, RSC Adv., 2016, 6, 49730–49746 RSC; (b) H. Hussain, A. Al-Harrasi, A. Al-Rawahi, I. R. Green and S. Gibbons, Chem. Rev., 2014, 114, 10369–10428 CrossRef CAS PubMed; (c) R. Pratap and V. J. Ram, Chem. Rev., 2014, 114, 10476–10526 CrossRef CAS PubMed; (d) C. Bhanja, S. Jena, S. Nayak and S. Mohapatra, Beilstein J. Org. Chem., 2012, 8, 1668–1694 CrossRef CAS PubMed; (e) J. Barluenga, F. Rodríguez and F. J. Fañanás, Chem. – Asian J., 2009, 4, 1036–1048 CrossRef CAS PubMed.
- (a) Z.-L. Shen, S.-Y. Wang, Y.-K. Chok, Y.-H. Xu and T.-P. Loh, Chem. Rev., 2013, 113, 271–401 CrossRef CAS PubMed. For additional references: (b) J. A. Moral, S.-J. Moon, S. Rodríguez-Torres and T. G. Minehan, Org. Lett., 2009, 11, 3734–3737 CrossRef CAS PubMed; (c) Z.-L. Shen, K. K. K. Goh, Y.-S. Yang, Y.-C. Lai, C. H. A. Wong, H.-L. Cheong and T.-P. Loh, Angew. Chem., Int. Ed., 2011, 50, 511–514 CrossRef CAS PubMed; (d) L. Adak and N. Yoshikai, J. Org. Chem., 2011, 76, 7563–7568 CrossRef CAS PubMed; (e) S. Bernhardt, Z.-L. Shen and P. Knochel, Chem. – Eur. J., 2013, 19, 828–833 CrossRef CAS PubMed; (f) D. Lee, T. Ryu, Y. Park and P. H. Lee, Org. Lett., 2014, 16, 1144–1147 CrossRef CAS PubMed; (g) S. Thapa, S. K. Gurung, D. A. Dickie and R. Giri, Angew. Chem., Int. Ed., 2014, 53, 11620–11624 CrossRef CAS PubMed.
- (a) V. Papoian and T. Minehan, J. Org. Chem., 2008, 73, 7376–7379 CrossRef CAS PubMed; (b) M. M. Martínez, C. Pérez-Caaveiro, M. Peña-López, L. A. Sarandeses and J. Pérez Sestelo, Org. Biomol. Chem., 2012, 10, 9045–9051 RSC; (c) A. Criado, M. Vilas-Varela, A. Cobas, D. Pérez, D. Peña and E. Guitián, J. Org. Chem., 2013, 78, 12637–12649 CrossRef CAS PubMed; (d) Á. Mosquera, M. I. Férnandez, M. Canle López, J. Pérez Sestelo and L. A. Sarandeses, Chem. – Eur. J., 2014, 20, 14524–14530 CrossRef PubMed; (e) C. Pérez-Caaveiro, J. Pérez Sestelo, M. M. Martínez and L. A. Sarandeses, J. Org. Chem., 2014, 79, 9586–9593 CrossRef PubMed.
- The indium-catalyzed IMHA of compounds 5 and 6 afforded the 4-bromo-1,2-dihydroquinolines 8 and 9 in 88% and 75% isolated yields, respectively.
Footnote |
| † Electronic supplementary information (ESI) available: General methods, characterization data and copies of the 1H, 13C NMR and DEPT-135 spectra for all compounds prepared. See DOI: 10.1039/c6qo00721j |
| This journal is © the Partner Organisations 2017 |