Size-selective adsorption of anionic dyes induced by the layer space in layered double hydroxide hollow microspheres†
Peipei
Huang
ab,
Jian
Liu
ab,
Fang
Wei
ab,
Yanan
Zhu
ab,
Xiaoshi
Wang
ab,
Changyan
Cao
*ab and
Weiguo
Song
 *ab
*ab
aBeijing National Laboratory for Molecular Sciences (BNLMS) & Key Laboratory of Molecular Nanostructure and Nanotechnology, CAS Research/Education Center for Excellence in Molecular Sciences, Institute of Chemistry, Chinese Academy of Sciences, Beijing, 100190, People's Republic of China. E-mail: cycao@iccas.ac.cn; wsong@iccas.ac.cn
bUniversity of Chinese Academy of Sciences, Beijing 100049, People's Republic of China
First published on 22nd March 2017
Abstract
Flower-like cobalt aluminum layered double hydroxide (CoAl-LDH) hollow microspheres were synthesized via a one-step solvothermal method without any template. An Ostwald ripening mechanism was proposed for the formation of hollow nanostructures. These flower-like CoAl-LDH hollow microspheres had a high surface area and exhibited excellent selectivity for anionic dyes. The limited space between LDH layers offered size selectivity for adsorbate molecules. For the small molecule methyl orange, the maximum adsorption capacity reached 816.0 mg g−1 under ambient conditions, while for larger molecules such as Eosin B, the adsorption capacity was only 95.1 mg g−1. All these features make the flower-like CoAl-LDH hollow microspheres an excellent adsorbent in water remediation.
Introduction
Fabricating hierarchical materials with a controllable structure and multifunctional properties is a rational approach for useful materials. Due to their high surface area, effective exposure of active sites, desirable mechanical strength and easy recovery, three-dimensional hierarchical micro-/nanostructure materials that are composed of nanometer-sized building blocks have attracted much attention in contaminant adsorption.1–4 Generally, hierarchical hollow micro-/nanostructure materials are prepared using hard-template or soft-template methods.5–9 However, they have many disadvantages, such as complex steps, difficulty in template removal, and aggregation during the removal of templates. Therefore, preparing hierarchical hollow micro-/nanostructure materials by template-free approaches has been explored.Layered double hydroxides (LDHs) have been demonstrated as effective adsorbents to remove organic dyes and heavy metal anions, showing advantages of low cost, thermal stabilities and excellent adsorption capacities.10–15 The general formula of LDHs can be expressed as [MII1−xMIIIx(OH)2]x+[Ax/nn−]·mH2O, where MII and MIII are di- and trivalent metal cations in the octahedral positions of brucite-like layers, yielding excessive positive charge and An− represents an n-valent anion which balances the positive charges on the layers.16,17 Various morphologies of LDHs have been reported, such as hierarchical flower-like,18,19 sandwich-like,20,21 core–shell structures,22,23 hollow sphere-like,24 nanoscrolls,25 macroporous26 and so on. Song et al. prepared 3D flower-like MgAl-LDHs via a solvothermal method and demonstrated that the main adsorption mechanism for As(V)/F− removal was anion exchange.18 He and Yang et al. reported that double-shelled carbon/layered double hydroxide hollow microspheres were prepared via a facile process with the aid of SiO2 templates and melamine molecules, which exhibited excellent electrochemical performance.24 However, nearly all of them are solid structures or formed with the help of templates. Although there have been many publications on hollow structural materials synthesized using template-free methods, such as Fe2O3,27 CeO2,28 MgO,29 TiO2,30 Sb2S3,31 FeOOH,32 Ni(OH)2,33 PtPdCu nanoalloys34 and so on, simple synthesis of hierarchical flower-like LDH hollow spheres using template-free methods is rare.
Another interesting thing worth mentioning is that the interlayer space limits the access to the LDH layers. Thus, shape selectivity or size selectivity is possible for LDH materials during catalysis or adsorption. Such size selectivity is frequently reported on microporous materials such as zeolite.35–37 Can organic dyes with different molecule sizes be intercalated into LDH interlayers, while the interlayer space imposes certain selectivity on the size of the molecules? Such size selectivity has not been fully explored in water treatment.
In this study, we produced hierarchical flower-like cobalt aluminum layered double hydroxide (CoAl-LDH) hollow microspheres via a one-pot solvothermal method without any template. A formation mechanism based on Ostwald ripening has been proposed for the formation of hollow nanostructures and fluoride ions played a decisive role. Due to their positively charged surface and hollow structure, the flower-like CoAl-LDH hollow microspheres showed excellent and fast selective adsorption properties for anionic dyes. Moreover, the limited space between LDH layers offered size selectivity for adsorbate molecules. For small molecule methyl orange, the maximum adsorption capacity was 816.0 mg g−1 under ambient conditions, while for larger molecules such as Eosin B, the adsorption capacity was only 95.1 mg g−1.
Experimental section
Materials
Analytical-grade cobaltous nitrate hexahydrate (Co(NO3)2·6H2O), aluminum nitrate nonahydrate (Al(NO3)3·9H2O), urea, ammonium fluoride, and anhydrous methanol were purchased from Beijing Chemicals Co. (Beijing, China). All chemicals were used without further purification. The deionized water used in all of the experiments was prepared using Milli-Q water by a Milli-Q system (Millipore, Bedford, MA).Synthesis of flower-like CoAl-LDH hollow microspheres
The samples were prepared using a modified method according to a previous report.18 12 mmol Co(NO3)2·6H2O, 6 mmol Al(NO3)3·9H2O, 42 mmol urea and 0.2 g NH4F were dissolved in 60 mL anhydrous methanol at room temperature. After stirring for 30 min, the clear solution was transferred into a 100 mL autoclave, and then the temperature was increased to 150 °C and kept for 18 h. A pink solid precipitate was collected by centrifugation and washed several times with water and ethanol, respectively. Finally, the product was dried in a vacuum oven at room temperature overnight.Characterization
The microscopic features of the samples were characterized by scanning electron microscopy (SEM, JSM-6701F) and transmission electron microscopy (TEM, JEOL JEM-2100F, 200 kV). X-Ray powder diffraction (XRD) patterns were collected on an X-ray diffractometer (Rigaku D/max-2500 diffractometer with Cu-Kα radiation, λ = 0.154056 nm) at 40 kV and 200 mA. The surface area of the products was measured using the Brunauer–Emmett–Teller (BET) method using N2 adsorption and desorption isotherms on an Autosorb-1 analyzer at 78.3 K. The concentration of dyes in the solution was determined using a Shimadzu UV-2550 spectrophotometer.Adsorption experiments
All the experiments were performed at 298 K. In the selectivity study of anionic dye molecule adsorption on an adsorbent sample, the initial anionic and cationic dye molecule concentrations were both 20 mg L−1. The experiments were performed using a 250 mL conical flask containing 100 mL mixture solutions including 50 mL of anionic dye molecule solution and 50 mL of cationic dye molecule solution, and the adsorbent dose was 20 mg. At predetermined time intervals, stirring was interrupted while 10 mL of the supernatant solutions were pipetted and centrifuged for determining the remaining dye molecule concentrations.For the equilibrium adsorption isotherm study, the adsorption experiments were performed in a series of 25 mL conical flasks equipped with frosted glass stoppers, with 6 mg adsorbents and 10 mL dye aqueous solution of different concentrations. After stirring for 6 h, the solutions were separated from the suspensions by centrifugation. The adsorption capacity of the adsorbents was measured by the difference between the initial and remaining concentrations of dye molecules. The amount of special adsorbate ions adsorbed per unit weight of the adsorbent at equilibrium (mg g−1), qe, was calculated according to the following equation:
| qe = (C0 − Ce)V/m |
In the kinetic study of anionic dye adsorption on the adsorbent sample, the initial anionic dye concentration was 100 mg L−1. The experiments were performed using a 250 mL conical flask containing 100 mL of anionic dye solution and the adsorbent dose was 60 mg in the kinetic study. At predetermined time intervals, stirring was interrupted while 10 mL of the supernatant solutions were pipetted and centrifuged for determining the remaining anionic dye molecule concentrations.
Results and discussion
Characterization of flower-like CoAl-LDH hollow microspheres
Fig. 1a shows the typical SEM image of as-prepared CoAl-LDHs. They were composed of many uniform, three-dimensional flower-like microspheres with a diameter of ca. 1 μm. The flower-like microspheres were built of many nanosheets with thicknesses of around 20–30 nm and connected to each other to form a highly open structure and no stand-alone plate-like LDHs could be seen. Especially, the hollow structures can be identified by several broken spheres (pointed by the yellow line in Fig. 1a). A TEM image further confirmed the hierarchical flower-like hollow structures (Fig. 1b). The typical X-ray diffraction pattern of the as-prepared CoAl-LDHs is shown in Fig. 1c. The diffraction peaks were in good agreement with the characteristics of LDH-type materials reported in the literature.38–40 No other diffraction peaks can be found, indicating the purity of the as-prepared CoAl-LDHs. The interlayer distance of CoAl-LDHs can be determined as 0.863 nm based on the (003) diffraction peak, which is attributed to nitrate ions in the interlayer.41,42 The surface area of the flower-like CoAl-LDH hollow microspheres was investigated using N2 adsorption–desorption measurements (Fig. 1d). They showed typical IV isotherms with H3-type hysteresis loops (P/P0 > 0.4), indicating the presence of mesopores. These mesopores may come from interconnected CoAl-LDH nanosheets. The Brunauer–Emmett–Teller (BET) surface area of flower-like CoAl-LDH hollow microspheres was estimated to be 167 m2 g−1.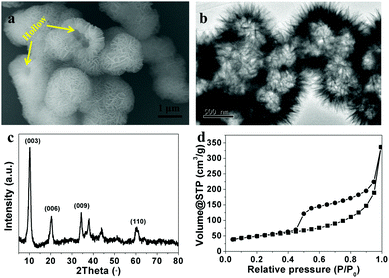 | ||
| Fig. 1 (a) SEM image, (b) TEM image, (c) XRD patterns and (d) nitrogen adsorption and desorption isotherms of flower-like CoAl-LDH hollow microspheres. | ||
To investigate the formation process of these interesting hierarchical flower-like hollow structures, samples prepared at different reaction times were collected and characterized by TEM. As shown in Fig. 2a, the solid flower-like structures with an average diameter of ca. 500 nm were obtained at the early stage of the reaction (50 min), in which the nanosheets connected to each other to form a rugged surface. When the reaction time was 1 h, the hollow morphology began to appear (Fig. 2b), and the cavities became more obvious when the time reached 2 h (Fig. 2c). In addition, the total size of a sphere was increased to ca. 1 μm. Further prolonging the reaction time to 18 h, the well-defined flower-like hollow microspheres with a hierarchical shell were well preserved (Fig. 2d), which implied that the flower-like hollow frameworks were stable.
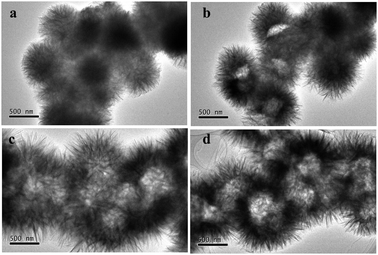 | ||
| Fig. 2 TEM images of the morphological evolution during the time-dependent experiments: (a) 50 min, (b) 1 h, (c) 2 h and (d) 18 h. | ||
Based on the above experimental observations, a plausible formation mechanism based on Ostwald ripening of the hierarchical flower-like CoAl-LDH hollow microspheres is proposed (Scheme 1). Firstly, ions of raw materials precipitated and self-assembled into solid flower-like spheres (step (I)). Then, the inner core of the solid flower-like spheres gradually began to form a void with the growth of nanosheets on the outer shell (step (II)). Finally, the hierarchical flower-like hollow spheres were obtained (step (III)). The growth of nanosheets on the surface of the initially formed microspheres and the slight diameter increase of the microspheres with prolonged solvothermal time confirm the so-called inside-out Ostwald-ripening process.26,32 In fact, the XRD peaks of the flower-like CoAl-LDH hollow microspheres became more and more obvious with elongation of the reaction time, which was also in agreement with the inside-out Ostwald ripening process (Fig. S1, ESI†).29,32
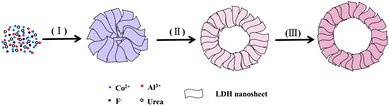 | ||
| Scheme 1 Schematic illustration of the formation process of hierarchical flower-like CoAl-LDH hollow microspheres. | ||
The additive NH4F also played an important role in the morphology of the hollow structures. Controlled samples were obtained by adjusting the mass of added NH4F to 0 g, 0.4 g and 0.8 g, respectively, with other reaction conditions unchanged. Detailed TEM images are shown in Fig. S2 (ESI†). Only flower-like solid spheres were obtained without the addition of NH4F (Fig. S2a, ESI†). Upon increasing the amount of NH4F to 0.2 g, uniform hierarchical flower-like hollow microspheres were acquired (Fig. S2b, ESI†). Upon further increasing the amount of NH4F to 0.4 g, the morphology of hollow spheres almost disappeared and the sample showed smaller diameter flower-like structures (Fig. S2c, ESI†). As the amount of NH4F was increased to 0.8 g, the morphologies of the sample became non-uniform and complex (Fig. S2d, ESI†). The corresponding SEM images are shown in Fig. S3 (ESI†). In order to obtain uniform flower-like CoAl-LDH hollow microspheres, the amount of NH4F was fixed at 0.2 g. In addition, when NH4F is replaced with the same amount of NaF, flower-like hollow spheres can still be obtained (Fig. S4a and b, ESI†). While the same amount of NH4Cl led to only irregular flower-like solid spheres (Fig. S4c and d, ESI†). This result demonstrated that fluoride played a crucial role in the formation of hollow structures. Fluoride ions not only induce the formation of hollow structures, but also promote the crystallization of CoAl-LDHs (Fig. S5, ESI†).
Adsorption properties of flower-like CoAl-LDH hollow microspheres
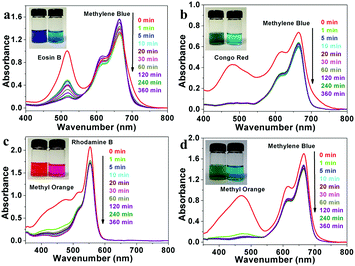
In general, there are two kinds of adsorption mechanisms for organic dyes with adsorbents: electrostatic adsorption and ion exchange.43,44 Due to the electrostatic effect, LDHs with positively charged layers are prone to attract anionic ions, resulting in superior adsorption properties towards anionic ions compared to cationic ions. Thus, a series of controlled adsorption experiments were performed to evaluate the adsorption performance for different kinds of dyes with as-prepared flower-like CoAl-LDH hollow microspheres.The organic dyes used in this work include four anionic dyes (Methyl Orange (MO), Coomassie Brilliant Blue (G-250) (CBB), Congo Red (CR) and Eosin B (EB)), and two another cationic dyes (Methylene Blue (MB) and Rhodamine B (RB)). As shown in Fig. 3, the adsorption peaks of MO, CBB and CR molecules in the absorption spectra almost disappeared within 1 min at the initial dye concentration of 10 mg L−1. However, the adsorption peaks of cationic molecules MB and RB remained unchanged. Similarly, when flower-like CoAl-LDH hollow microspheres were added into the binary mixtures of CBB & RB, CR & RB and EB & RB, respectively, all the absorption peaks of anionic dyes disappeared quickly, while the characteristic absorption peaks of cationic dyes remained unchanged (Fig. S6, ESI†). The photographs showed the changes in color of the mixed solutions: the solutions showed mixed color of each dyes before selective adsorption, the solution's color changed to pure blue MB or pink RB almost within 1 minute after adsorption (Fig. 3 and Fig. S6 (inset), ESI†). These results demonstrate excellent selective removal of anionic dyes over cationic dyes due to the electrostatic effect.
Then, the adsorption kinetic curve and isotherm of the flower-like CoAl-LDH hollow microspheres for anionic dyes were studied. As shown in Fig. S7 (ESI†), the kinetics of anionic dye molecule adsorption on flower-like CoAl-LDH hollow microspheres were rather fast. Most anionic dye molecules could be removed within 1 h. Especially, MO and CBB can be thoroughly removed within 10 minutes. All the above adsorption kinetic experimental data can be fitted well with a pseudo-second-order rate kinetic model, which indicated that the rate-controlling step of all the four dye molecules during adsorption is chemical adsorption. Fig. 4 shows the adsorption isotherms with a series of different initial concentrations of anionic dyes. It can be seen that the adsorption data for all the tested dyes were fitted better with the Langmuir model than with the Freundlich model (Fig. S8 and Table S1, ESI†). The maximum adsorption capacities of flower-like CoAl-LDH hollow microspheres for Methyl Orange, Coomassie Brilliant Blue, Congo Red, and Eosin B were 816.0 (2.49), 654.8 (0.767), 178.3 (0.256) and 95.1 (0.152) mg g−1 (mmol g−1), respectively.
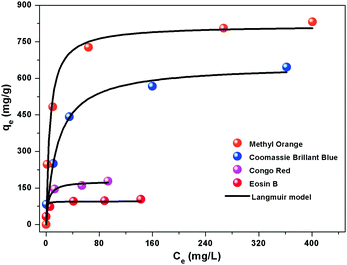 | ||
| Fig. 4 Adsorption performance of Methyl Orange, Coomassie Brilliant Blue, Congo Red and Eosin B on the flower-like CoAl-LDH hollow microspheres. | ||
Compared with other adsorbents reported previously in Table S2 (ESI†), the flower-like CoAl-LDH hollow microspheres showed good potential for MO removal from aqueous solution. The fast adsorption rate and large adsorption capacities for MO on flower-like CoAl-LDH hollow microspheres can be mainly ascribed to the high surface area and mesoporous structure, which can not only greatly increase the adsorbent–adsorbate contact area, but also provide abundant adsorption sites and efficient ion transport channels.45–48
However, the adsorption capacities of flower-like CoAl-LDH hollow microspheres for CBB, CR and EB were obviously lower than that of MO. Surface adsorption and intercalation are two kinds of mechanisms accounting for the adsorption process with LDHs. Because the size of MO molecules is smaller than those of CBB, CR and EB, we speculate that the discrepancy in adsorption capacities among the four anionic dyes can be ascribed to the molecule size. That is, MO can be intercalated into the interlayer due to the smaller size, while CBB, CR and EB are too large to intercalate into the LDH interlayers.
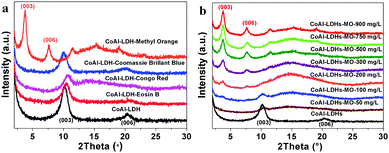
In order to certify the above speculation, samples saturated with the above four dyes were obtained. X-ray diffraction is a useful characteristic to discriminate the structure change of LDHs before and after adsorption. As shown in Fig. 5a, compared with the pristine LDH, the characteristic (003) and (006) diffraction peaks of the hollow LDH spheres that are saturated with MO molecules shifted from 10.3° to 3.7°, while samples saturated with CBB, CR and EB molecules showed no such change. For MO saturated LDH samples, the characteristic peaks shifted to smaller angles after adsorption (Fig. 5b), indicating that the interlayer distance increased after MO intercalation. Further increasing the concentration of MO, the layered characteristic peak (a 2θ position of 10.3°) gradually decreased and eventually disappeared after adsorption. At the same time, another new peak representing MO ions as the interlayer anions at a lower diffraction angle (a 2 position of 3.7°) appeared.
According to the information on these four anionic dye molecules (Fig. S9 and Table S3, ESI†), Methyl Orange has the smallest size and cross-section area so the relatively small interlayer space of CoAl-LDHs provides good accessibility for MO molecules but not the other three dyes. That is to say, only MO molecules can be intercalated into the interlayer, while CBB, CR and EB cannot be intercalated into the layer of the LDHs. This is a nice example of shape/size selectivity for layered materials.
Conclusions
Three-dimensional flower-like cobalt aluminum layered double hydroxide hollow microspheres were synthesized using a one-step solvothermal method without any template. A formation mechanism based on Ostwald ripening has been proposed for the formation of hollow nanostructures and fluoride ions played a decisive role. The flower-like CoAl-LDH hollow microspheres had a high surface area and showed excellent adsorption properties for methyl orange with a maximum capacity of 816.0 mg g−1. In addition, the limited space between the LDH layers offered size selectivity for adsorbate molecules. The facile synthesis method and excellent selective adsorption properties for organic anions make the flower-like cobalt aluminum layered double hydroxide hollow microspheres a potential adsorbent in water remediation.Acknowledgements
We thank the financial support from the National Natural Science Youth Foundation of China (NSFC 51402305), the Chinese Academy of Sciences (XDA09030203) and the Youth Innovation Promotion Association of CAS (2017049).References
- H. B. Yao, H. Y. Fang, X. H. Wang and S. H. Yu, Chem. Soc. Rev., 2011, 40, 3764–3785 RSC.
- W. Zhou and L. Guo, Chem. Soc. Rev., 2015, 44, 6697–6707 RSC.
- Q. N. Liu, Z. Q. Sun, Y. H. Dou, J. H. Kim and S. X. Dou, J. Mater. Chem. A, 2015, 3, 11688–11699 CAS.
- X. Li, J. G. Yu and M. Jaroniec, Chem. Soc. Rev., 2016, 45, 2603–2636 RSC.
- J. Hu, M. Chen, X. S. Fang and L. M. Wu, Chem. Soc. Rev., 2011, 40, 5472–5491 RSC.
- J. Y. Wang, H. J. Tang, H. Wang, R. B. Yu and D. Wang, Mater. Chem. Front., 2017, 1, 414–430 RSC.
- Y. Q. Zhang, G. C. Jia, H. W. Wang, B. Ouyang, R. S. Rawat and H. J. Fan, Mater. Chem. Front. 10.1039/c6qm00168h.
- Z. Y. Zhuang, H. Chen, Z. Lin and Z. Dang, Environ. Sci.: Nano, 2016, 3, 1254–1258 RSC.
- J. Qi, X. Y. Lai, J. Y. Wang, H. J. Tang, H. Ren, Y. Yang, Q. Jin, L. J. Zhang, R. B. Yu, G. H. Ma, Z. G. Su, H. J. Zhao and D. Wang, Chem. Soc. Rev., 2015, 44, 6749–6773 RSC.
- K. Mandel, A. Drenkova-Tuhtan, F. Hutter, C. Gellermann, H. Steinmetz and G. Sextl, J. Mater. Chem. A, 2013, 1, 1840–1848 CAS.
- S. L. Wang, C. H. Liu, M. K. Wang, Y. H. Chuang and P. N. Chiang, Appl. Clay Sci., 2009, 43, 79–85 CrossRef CAS.
- T. Wen, X. L. Wu, X. L. Tan, X. K. Wang and A. W. Xu, ACS Appl. Mater. Interfaces, 2013, 5, 3304–3311 CAS.
- Y. F. Xu, Y. C. Dai, J. Z. Zhou, Z. P. Xu, G. R. Qian and G. Q. Lu, J. Mater. Chem., 2010, 20, 4684–4691 RSC.
- J. B. Zhou, Y. Cheng, J. G. Yu and G. Liu, J. Mater. Chem., 2011, 21, 19353–19361 RSC.
- E. Gardner, K. M. Huntoon and T. J. Pinnavaia, Adv. Mater., 2001, 13, 1263–1266 CrossRef CAS.
- K. H. Goh, T.-T. Lim and Z. L. Dong, Water Res., 2008, 42, 1343–1368 CrossRef CAS PubMed.
- B. Li and J. He, J. Phys. Chem. C, 2008, 112, 10909–10917 CAS.
- P. P. Huang, C. Y. Cao, F. Wei, Y. B. Sun and W. G. Song, RSC Adv., 2015, 5, 10412–10417 RSC.
- X. Y. Yu, T. Luo, Y. Jia, R. X. Xu, C. Gao, Y. X. Zhang, J. H. Liu and X. J. Huang, Nanoscale, 2012, 4, 3466–3474 RSC.
- P. P. Huang, C. Y. Cao, Y. B. Sun, S. L. Yang, F. Wei and W. G. Song, J. Mater. Chem. A, 2015, 3, 10858–10863 CAS.
- Z. J. Huang, P. X. Wu, B. N. Gong, Y. P. Fang and N. W. Zhu, J. Mater. Chem. A, 2014, 2, 5534 CAS.
- M. F. Shao, F. Y. Ning, Y. F. Zhao, J. W. Zhao, M. Wei, D. G. Evans and X. Duan, Chem. Mater., 2012, 24, 1192–1197 CrossRef CAS.
- C. P. Chen, C. F. H. Byles, J.-C. Buffet, N. H. Rees, Y. Wu and D. O'Hare, Chem. Sci., 2016, 7, 1457–1461 RSC.
- J. Xu, F. He, S. L. Gai, S. H. Zhang, L. Li and P. P. Yang, Nanoscale, 2014, 6, 10887–10895 RSC.
- W. Y. Lv, M. Du, W. J. Ye and Q. Zheng, J. Mater. Chem. A, 2015, 3, 23395–23402 CAS.
- E. Géraud, S. Rafqah, M. Sarakha, C. Forano, V. Prevot and F. Leroux, Chem. Mater., 2008, 20, 1116–1125 CrossRef.
- W. Cheng, K. B. Tang, Y. X. Qi, J. Sheng and Z. P. Liu, J. Mater. Chem., 2010, 20, 1799–1805 RSC.
- C. Y. Cao, Z. M. Cui, C. Q. Chen, W. G. Song and W. Cai, J. Phys. Chem. C, 2010, 114, 9865–9870 CAS.
- S. L. Yang, P. P. Huang, L. Peng, C. Y. Cao, Y. N. Zhu, F. Wei, Y. B. Sun and W. G. Song, J. Mater. Chem. A, 2016, 4, 400–406 CAS.
- H. X. Li, Z. F. Bian, J. Zhu, D. Q. Zhang, G. S. Li, Y. N. Huo, H. Li and Y. F. Lu, J. Am. Chem. Soc., 2007, 129, 8406–8407 CrossRef CAS PubMed.
- X. B. Cao, L. Gu, L. J. Zhuge, W. J. Gao, W. C. Wang and S. F. Wu, Adv. Funct. Mater., 2006, 16, 896–902 CrossRef CAS.
- B. Wang, H. B. Wu, L. Yu, R. Xu, T. T. Lim and X. W. Lou, Adv. Mater., 2012, 24, 1111–1116 CrossRef CAS PubMed.
- Y. Wang, Q. S. Zhu and H. G. Zhang, Chem. Commun., 2005, 5231–5233 RSC.
- J. Lan, K. Wang, Q. Yuan and X. Wang, Mater. Chem. Front. 10.1039/c6qm00277c.
- U. Olsbye, S. Svelle, M. Bjørgen, P. Beato, T. V. W. Janssens, F. Joensen, S. Bordiga and K. P. Lillerud, Angew. Chem., Int. Ed., 2012, 51, 5810–5831 CrossRef CAS PubMed.
- Z. M. Cui, Q. Liu, W. G. Song and L. J. Wan, Angew. Chem., Int. Ed., 2006, 45, 6512–6515 CrossRef CAS PubMed.
- F. F. Wei, J. Liu, Q. Y. Zhang, Y. T. Zhang, X. Zhang, C. Y. Cao and W. G. Song, RSC Adv., 2016, 6, 89499–89502 RSC.
- J. Yang, C. Yu, X. M. Fan, Z. Ling, J. S. Qiu and Y. Gogotsi, J. Mater. Chem. A, 2013, 1, 1963–1968 CAS.
- J. G. Wang, Y. Wei and J. Yu, Appl. Clay Sci., 2013, 72, 37–43 CrossRef CAS.
- Z. P. Xu and G. Q. Lu, Chem. Mater., 2005, 17, 1055–1062 CrossRef CAS.
- S. Mandal, D. Tichit, D. A. Lerner and N. Marcotte, Langmuir, 2009, 25, 10980–10986 CrossRef CAS PubMed.
- G. Darmograi, B. Prelot, G. Layrac, D. Tichit, G. Martin-Gassin, F. Salles and J. Zajac, J. Phys. Chem. C, 2015, 119, 23388–23397 CAS.
- B. Chen, Y. Liu, S. J. Chen, X. S. Zhao, W. L. Yue and X. J. Pan, Environ. Sci.: Nano, 2016, 3, 670–681 RSC.
- B. Q. Song, X. L. Wang, Y. T. Zhang, X. S. Wu, H. S. Liu, K. Z. Shao and Z. M. Su, Chem. Commun., 2015, 51, 9515–9518 RSC.
- B. Li, F. Li, S. Y. Bai, Z. J. Wang, L. C. Sun, Q. L. Yang and C. Li, Energy Environ. Sci., 2012, 5, 8229–8233 CAS.
- Z. M. Cui, Z. Chen, C. Y. Cao, L. Jiang and W. G. Song, Chem. Commun., 2013, 49, 2332–2334 RSC.
- S. Y. Zhu, S. H. Jiao, Z. W. Liu, G. S. Pang and S. H. Feng, Environ. Sci.: Nano, 2014, 1, 172–180 RSC.
- C. Y. Cao, P. Li, J. Qu, Z. F. Dou, W. S. Yan, J. F. Zhu, Z. Y. Wu and W. G. Song, J. Mater. Chem., 2012, 22, 19898–19903 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: XRD patterns, SEM and TEM images of controlled samples, adsorption kinetic curves and isotherm curves of anionic dyes, tables summarizing the isotherm model parameters, comparison of adsorption capacities for methyl orange and information about anionic dyes. See DOI: 10.1039/c7qm00079k |
| This journal is © the Partner Organisations 2017 |