Application of ionic liquids for dissolving cellulose and fabricating cellulose-based materials: state of the art and future trends
Jinming
Zhang
 a,
Jin
Wu
a,
Jian
Yu
*a,
Xiaoyu
Zhang
b,
Jiasong
He
a and
Jun
Zhang
*a
a,
Jin
Wu
a,
Jian
Yu
*a,
Xiaoyu
Zhang
b,
Jiasong
He
a and
Jun
Zhang
*a
aBeijing National Laboratory for Molecular Sciences, CAS Key Laboratory of Engineering Plastics, Institute of Chemistry, Chinese Academy of Sciences (CAS), Beijing, 100190, China. E-mail: yuj@iccas.ac.cn; jzhang@iccas.ac.cn
bShandong Henglian New Materials Co. Ltd., Weifang, 261061, Shandong, China
First published on 16th January 2017
Abstract
Cellulose, a well-known fascinating biopolymer, has been considered to be a sustainable feedstock of energy sources and chemical engineering in the future. However, due to its highly ordered structure and strong hydrogen bonding network, cellulose is neither meltable nor soluble in conventional solvents, which limits the extent of its application. Therefore, the search for powerful and eco-friendly solvents for cellulose processing has been a key issue in this field for decades. More recently, certain ionic liquids (ILs) have been found to be able to efficiently dissolve cellulose, providing a new and versatile platform for cellulose processing and functionalization. A series of cellulose-based materials, such as films, fibers, gels and composites, have been produced readily with the aid of ILs. This review article highlights recent advances in the field of dissolution and processing of cellulose with ILs. It is hoped that this review work will stimulate a wide range of research studies and collaborations, leading to significant progress in this area.
Introduction
Cellulose, the major polysaccharide produced by plant photosynthesis, is the most abundant biorenewable and biodegradable resource on earth, with an estimated annual yield of 1.5 × 1012 tons. Since its isolation in the nineteenth century, cellulose has been widely used in our daily life, such as in paper, cloth, film, coating, tissues, medicines and polymeric materials. The effective and environmentally friendly utilization of cellulose not only reduces dependence on fossil resources but also protects our environment. However, there is an inherent obstacle to preventing the processing, derivatization and conversion of cellulose, because cellulose is neither meltable nor soluble in conventional solvents because of its highly ordered structure and strong hydrogen-bonding network. To date, the industrial production of regenerated cellulose materials (cellophane and rayon) is dominated by the traditional viscose process, which requires hazardous chemicals like CS2 and causes heavy pollution due to H2S, SO2, strong base and sulphuric acid.In order to resolve this problem, several new solvent systems have been developed for dissolving and processing cellulose. They are N,N-dimethylacetamide/lithium chloride (DMAc/LiCl), N,N-dimethylformamide/dinitrogen tetroxide (DMF/N2O4), dimethyl sulfoxide/tetrabutylammonium fluoride (DMSO/TBAF), N-methylmorpholine-N-oxide (NMMO), molten salt hydrates (LiClO4·3H2O, LiSCN·2H2O, etc.), aqueous solutions of alkali hydroxide (NaOH/H2O, NaOH/urea/H2O, LiOH/thiourea/H2O, etc.), and aqueous solutions of metal complexes (ZnCl2, [Cu(NH3)4](OH)2, etc.). However, most of these solvent systems suffer from certain fatal drawbacks, such as instability, toxicity, high cost, low dissolving capability, difficulty in solvent recovery or harsh processing conditions, thus limiting their applications on an industrial scale. Therefore, the design of environmentally friendly, effective, stable and cheap solvents with good recyclability and reusability is still of prime importance for the dissolution and processing of cellulose.
Ionic liquids (ILs) are a novel class of organic molten salts having melting points below 100 °C. They have many attractive advantages, such as negligible vapour pressure, high thermal and chemical stabilities, high solvating capacities, structure tunability, easy recyclability and non-flammability, and thus show promise for widespread applications in industry. More inspiringly, recent studies have found that some ILs effectively dissolve cellulose up to a high concentration of 30–40 wt%. By using ILs as the solvents, a variety of cellulose-based materials, including fibers, films, hydrogels, aerogels, composites and derivatives have been fabricated successfully and efficiently. Among them, regenerated cellulose fibers and films with outstanding mechanical properties are being produced on the pilot scale. ILs have provided a new and versatile platform for the wide utilization of cellulose resources and creation of novel functional materials.
Herein, this review article aims to provide a relatively comprehensive summary of research progress on the dissolution and processing of cellulose with ILs. The dissolution capabilities of ILs, the dissolution mechanism of cellulose in ILs, preparative routes and property control of regenerated cellulose materials (fibers, films, gels and composites), and preparation of functional cellulose-based materials are discussed in detail as well.
Ionic liquids with good capability for dissolving cellulose
The initial report of using ILs for cellulose dissolution and processing can be traced back to 1934. In a US patent, Graenacher1 reported that molten N-alkylpyridinium chloride could dissolve cellulose, thus allowing for efficient chemical modification of cellulose. Unfortunately, this did not attract significant attention, mainly due to the high melting points (118–120 °C) of the pyridinium salts.In 2002, Swatloski et al.2 discovered that dialkylimidazolium-based ILs, with melting points below 100 °C, could dissolve cellulose. Among the room-temperature ILs (RTILs) they studied, the most successful ionic liquid was 1-butyl-3-methylimidazolium chloride (BmimCl) with the greatest dissolving capability of up to 10 wt% concentration of cellulose by heating, and to 25 wt% by microwave-assisted heating.
Also in 2002, our group found that 1-allyl-3-methylimidazolium chloride (AmimCl) and 1-ethyl-3-methylimidazolium acetate (EmimAc) were powerful solvents for cellulose.3 Compared with BmimCl, AmimCl and EmimAc have lower melting points and viscosities, and higher dissolving capability. By using AmimCl or EmimAc as the solvent, a solution containing up to 30 wt% cellulose with a degree of polymerization (DP) as high as 650 was readily prepared in only 30 min. Then, if the cellulose/IL solutions were coagulated in water, regenerated cellulose fibers or transparent cellulose films with high tensile strength (138 MPa) were obtained.4 If the cellulose/IL solution was mixed with acetic anhydride, homogeneous acetylation was successfully accomplished without using any catalyst. Consequently, cellulose acetates (CA) with a controllable degree of substitution (DS) values were synthesized rapidly and simply.5
Since then, intensive research studies have been carried out in the field of dissolving cellulose with imidazolium-based ILs. Researchers from different groups6–15 systematically investigated the dissolving capabilities of the ILs with carboxylate-based or alkylphosphate-based anions, such as [HCOO], [CH3(CH2)0–3COO], [PhCOO], [HSCH2COO] and [(MeO)(R)PO2], and found that these ILs dissolved cellulose efficiently under relatively low temperatures, even at room temperature. The good dissolving capabilities of these ILs were directly correlated with their strong hydrogen bond basicity and lower viscosity. In contrast, ILs containing anions with weak hydrogen bond basicity, such as [BF4], [PF6], [N(CN)2], [MeSO4], [HSO4] and [Tf2N], were non-solvents for cellulose.2,16 On the other hand, the chemical structure of imidazolium cations also had an important influence on cellulose dissolution.17–24 In general, when the chain length of alkyl groups or the symmetry of cations increased, the dissolution rate of cellulose in ILs decreased, because of the increase of viscosity or/and the decrease of hydrogen bond acidity. The [Emim], [Amim] and [Bmim] cations are the most favorite and efficient cations in ILs for dissolving cellulose.
The pyridinium-based ionic liquids are capable of dissolving cellulose as well. Heinze et al.25 and Sashina et al.26 pointed out that 1-butyl-3-methylpyridinium chloride (BmPyCl) was more effective than BmimCl. BmPyCl could dissolve cellulose with a DP of 593 to reach a 37 wt% concentration at 105 °C. However, significant depolymerization of cellulose occurred during the dissolving process. Miyata and Miyafuji27 also found that cellulose was completely dissolved while depolymerized simultaneously in 1-ethylpyridinium chloride. Therefore, alkylpyridinium-based ILs are commonly used in the dissolution and conversion of cellulose.
At the beginning, the cheap quaternary ammonium-based ILs were regarded as poor solvents for cellulose.12,25,28 However, after pairing with carboxylate anions (formate or acetate anions), the corresponding ammonium-based ILs became effective solvents to dissolve cellulose.11,29–35 For example, Köhler et al. claimed that triethylmethylammonium formate and tributylmethylammonium formate dissolved cellulose at 85 °C within 15 min.32 In order to utilize inexpensive, readily accessible and stable ammonium chloride salts, Kostag et al. investigated the influence of the alkyl chain length on the dissolving capability of ammonium chloride.36,37 They found that pure molten tetraalkylammonium chlorides with one long alkyl chain could dissolve up to 15 wt% of microcrystalline cellulose. Furthermore, the dissolution of cellulose was accelerated by the addition of polar and aprotic organic solvents (DMAc, DMSO and acetone) as the co-solvents.
The morpholinium-based ILs are less toxic and have higher electrochemical stability than the commonly used imidazolium, pyridinium and tetraalkylammonium-based ILs. It was found that 4-allyl-4-methylmorpholinium acetate ([AMMorp]Ac),38 4-benzyl-4-methylmorpholinium acetate39 and 4-benzyl-4-methylmorpholinium formate39 dissolved cellulose readily without any pre-processing of original cellulose. For example, [AMMorp]Ac could dissolve cellulose with a DP of 789 to a 30 wt% concentration at 120 °C in 20 min.
The cholinium-based ionic liquids are cheap, nontoxic, biodegradable and available on a very large scale. Most of the cholinium-based ILs dissolved lignin efficiently and selectively (with solubilities of 140–220 mg of lignin per g of IL).40–42 However, cellulose showed little and even no solubility in all these ILs, independent of the anions. After the combination of choline chloride ([Ch]Cl) with metal chlorides (e.g., ZnCl2, SnCl2), or hydrogen-bond donors (urea, carboxylic acids, or polyols, etc.), deep eutectic solvents were formed.43–45 Unfortunately, in [Ch]Cl/urea (molar ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) or [Ch]Cl/ZnCl2 (molar ratio 1
2) or [Ch]Cl/ZnCl2 (molar ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2), cellulose was sparingly soluble, and even almost no solubilisation occurred. Zhang et al.46 chose tributylmethylammonium chloride ([TBMA]Cl) as an additive, and found that the addition of 5–15 wt% of [TBMA]Cl to choline acetate ([Ch]Ac) enabled the complete dissolution of 2–6 wt% cellulose at 110 °C within 5–10 min.
2), cellulose was sparingly soluble, and even almost no solubilisation occurred. Zhang et al.46 chose tributylmethylammonium chloride ([TBMA]Cl) as an additive, and found that the addition of 5–15 wt% of [TBMA]Cl to choline acetate ([Ch]Ac) enabled the complete dissolution of 2–6 wt% cellulose at 110 °C within 5–10 min.
In 2006, a new class of distillable acid–base conjugated ILs was made by combining a range of superbases (1,1,3,3-tetramethylguanidine (TMG), 1,5-diazabicyclo-[4.3.0]non-5-ene (DBN), 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU), etc.) with organoacids.47–58 These ILs not only rapidly dissolved cellulose to high concentrations, but also could be recycled via distillation with the recovery and purity over 99%. By using [DBNH]Ac as the solvent, Sixta and co-workers50–53 successfully fabricated cellulose fibers, resulting in properties equal to or better than Lyocell fibers. In addition, the DBU, in conjunction with or without alcohol (such as methanol and ethylene glycol) after CO2 capture in DMSO, created switchable ILs, which were capable of dissolving up to 15 wt% of cellulose at 25–40 °C.54–58 In these switchable ILs, the highly efficient acylation of cellulose was also accomplished.
From the above-mentioned literature, it is apparent that a significant number of ILs dissolving cellulose are known to date. These ILs consist of imidazolium, pyridinium, ammonium-based cations and halide, carboxylate, alkylphosphate-based anions, as shown in Fig. 1A. It is reported that the dissolving capability of ILs follows the order of imidazolium-based ILs > pyridinium-based ILs > ammonium-based ILs, and carboxylate-based ILs > alkylphosphate-based ILs > halide-based Ils.25,59–62 Among them, the most favorite and typical ILs are AmimCl, BmimCl and EmimAc. Via two high-throughput screening tests, Zavrel et al.63 indicated that EmimAc was the most efficient for dissolving cellulose, and AmimCl for dissolving wood chips. More recently, by combining a quantitative structure–activity relationship model with a mixed integer nonlinear programming method, Mai et al.64 predicted that methoxymethyl-allyl-bimethyl-phosphonium methoxyacetate IL was optimal for dissolving cellulose.
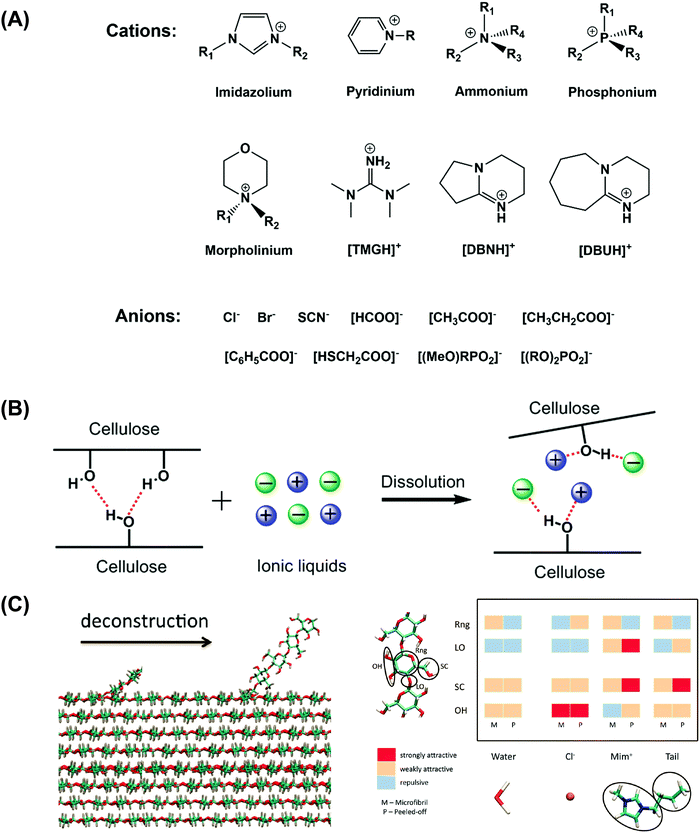 | ||
| Fig. 1 (A) The cation and anion structures of typical ionic liquids for dissolving cellulose. (B) The dissolution mechanism of cellulose in ionic liquids.4,88,89 (C) Simulation model of cellulose deconstruction. Reprinted with permission from ref. 96. Copyright 2011 American Chemical Society. | ||
The addition of polar and aprotic co-solvents (DMAc, DMSO, DMF, etc.) to ILs would enhance their solvating power,65,66 because these co-solvents could accelerate mass transport by decreasing the solvent viscosity without having a significant influence on the specific interactions between cations and anions or between ILs and cellulose.67–70 Other methods were found to significantly increase the dissolution of cellulose, such as microwave-assisted dissolution,2,71 sonication-assisted dissolution,72,73 and the addition of lithium salts.14,74,75 In addition, the dissolution of cellulose was greatly affected by the source of cellulose, the water content of ILs and cellulose, and the temperature used for the dissolution.
Dissolution mechanism of cellulose in ionic liquids
It is well known that the disruption of the hydrogen-bonding network in cellulose is crucial to dissolve cellulose. The anions in ILs play a predominant role by the formation of strong hydrogen bonds with hydrogen atoms of hydroxyls in cellulose. However, the role of the cations in ILs remains debatable.Swatloski et al.2 speculated that the high chloride concentration and its activity in BmimCl was the main reason for dissolving cellulose in ILs. Then, via NMR relaxation and molecular dynamics studies, Remsing et al.76,77 and Youngs et al.78–80 expected to prove this conjecture. The results suggested that the interaction between the IL cation and cellulose was negligible. However, soon afterwards, some of other molecular simulation studies found that the cation played a minor role in the solvation of cellulose,62,81–87 such as van der Waals interaction, electrostatic interaction, and hydrogen bonding interaction.
In 2005, our group proposed a possible mechanism of cellulose dissolution in ILs, as shown in Fig. 1B.4 The synergistic effect of anions and cations in ILs was the major force for cellulose dissolution. The role of the cations in the dissolution of cellulose was non-negligible. Subsequently, after NMR studies, we proved the formation of the hydrogen bonding interactions between hydroxyls in cellulose and both anions and cations in ILs.88,89 The relatively small anions favored the formation of hydrogen bonds with hydrogen atoms of hydroxyls, and the aromatic protons in the imidazolium cation, especially H2, preferred to associate with oxygen atoms of hydroxyls with less steric hindrance. More experimental and theoretical evidence was provided recently, and is in agreement with our proposed mechanism.90–104 For example, after free-energy simulation and coarse-grain analysis, Cho et al.96 found that the hydrogen-bonding interactions between anions and cations with glucose units contributed significantly in their dissolution (Fig. 1C). Through a series of dissolution tests and NMR studies, Xu et al.70,93 pointed out that the cation of ILs was related to the breakage of hydrogen bonds in cellulose. Okushita et al.103 and Chang et al.104 confirmed that the cation of ILs was associated with the collapse of hydrogen bonds in cellulose by infrared spectroscopy and solid-state NMR studies.
In a word, just like in other solvents for cellulose, the dissolution mechanism of cellulose in ILs is still incompletely understood. The effective combination of more experimental and theoretical studies is needed urgently to reveal a universal mechanism.
Cellulose materials regenerated from cellulose/IL solutions
Regenerated cellulose fibers
In principle, all kinds of ILs, which can dissolve cellulose to obtain a viscous solution, can be used to prepare regenerated cellulose fibers. Thus, many attempts for cellulose spinning have been made by using dialkylimidazolium-based ILs such as AmimCl, BmimCl, EmimCl, EmimAc, BmimAc and [Emim][(EtO)2PO2],105 and ammonium-based ILs such as TBAA/DMSO35 and [DBNH]Ac.106–109 In 2005, we began to study the dry-jet wet spinning process of cellulose/AmimCl solution on an industrial test scale. After the filtration of cellulose/AmimCl solutions, extrusion through a spinneret containing 36 holes, drawing in air and coagulation in water were conducted, followed by washing, drying on a hot roll, and finally winding the resultant fibers on a paper tube. By minimizing the degradation of cellulose and optimizing the air gap, cellulose fibers with an average tensile strength of 45 cN tex−1 were prepared from 5 wt% cellulose/AmimCl solution (Fig. 2B). Such a tensile strength was comparable to that of Tencell (∼40 cN tex−1), and far exceeded the standard of normal viscose fibers (18.5 cN tex−1).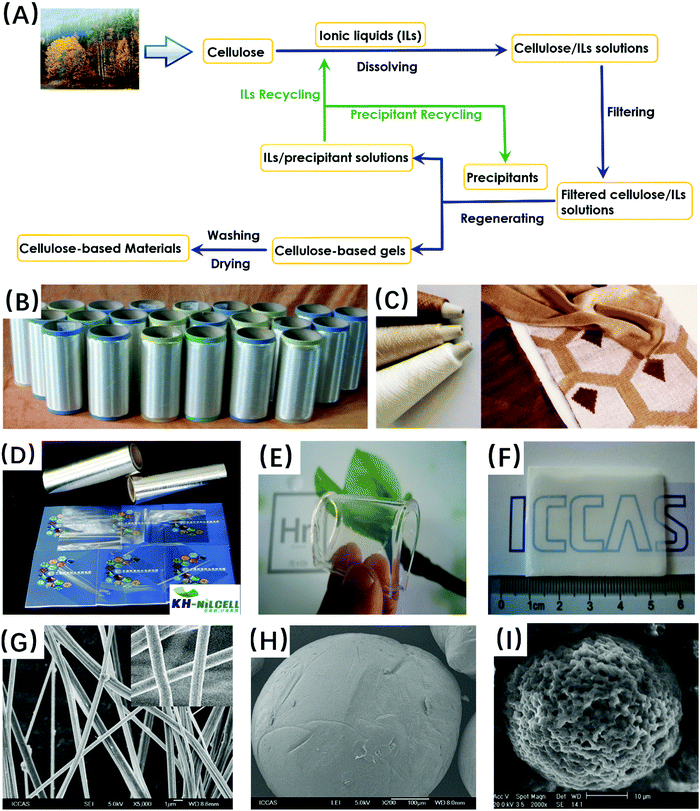 | ||
| Fig. 2 (A) Schematic of the production process of regenerated cellulose materials with ionic liquids. (B) Regenerated cellulose fibers obtained in a pilot scale. (C) Yarns and final knitted garments made of fibers spun from bleached pulp (white), cardboard (beige), and pulp with additional lignin added (brown). Reprinted with permission from ref. 53. Copyright 2016 The Royal Society of Chemistry. (D) Cellulose films fabricated in an industrial test scale. (E) Flexible cellulose hydrogel. (F) Transparent cellulose aerogel. Reprinted with permission from ref. 161. Copyright 2016 American Chemical Society. (G) Cellulose nanofibers fabricated via an electrospinning process. (H) Homogeneous cellulose microbeads. (I) Porous cellulose microbeads. Reprinted with permission from ref. 287. Copyright 2010 Elservier. | ||
Kosan et al.110 compared 5 kinds of ILs with NMMO in a cellulose spinning study, and found that the tensile strength of all fibers from the IL regeneration process was superior to the Lyceoll fiber (NMMO process). Jiang et al.111 obtained the best cellulose fibers with the tensile strength of 42.1 cN tex−1 from BmimCl solutions. Olsson112 reported that in EmimAc, the highest tensile strength of cellulose fibers was 35 cN tex−1. In a series of studies of cellulose solution spinning with [DBNH]Ac, the tensile strength of cellulose fibers reached 70 cN tex−1 (Fig. 2C).53,108
In the dry–wet spinning process, many parameters, such as the DP of cellulose, the concentration of the spinning dope, and the drawing ratio in the air gap, had an impact on the mechanical properties of fibers. Furthermore, Michud et al.108 indicated that the distribution of cellulose chains and the dynamic modulus of the spinning dope had a significant influence on the spinnability. Sixta et al.107 and Sun et al.113 found that the dope concentration and the stretching rate affected the fiber properties. Hauru et al.106 reported that the increase of the temperature of the coagulation bath led to the decrease of fiber tenacity.
The wet spinning process could be employed to fabricate cellulose fibers, when the viscosity of cellulose solutions decreased using some methods, such as increasing spinning temperature,114 decreasing dope concentration,105,115 and using co-solvents.35,112 The strength of wet spinning fibers was as high as 20–30 cN tex−1,35,116 which was similar to that of viscose fibers and lower than that of dry-jet wet spinning fibers. In addition, the fibrillation of fibers that occurred during the dry-jet wet spinning process was avoidable in the wet spinning process.105
Regenerated cellulose films
As a material from biomass resources, regenerated cellulose films have attracted much attention for different applications, such as green packaging, printable labels, biomedical materials, electronic devices, and optical products. So far, commercially regenerated cellulose films are still manufactured by the viscose process, which was invented more than 100 years ago and is well known for its low efficiency, high pollution, and high energy consumption.In the past decade, efforts have been made in both academia and industry to search for simpler and more environmentally benign methods. Among the cellulose direct solvents that have been used to prepare regenerated cellulose films are ionic liquids, and in particular, those containing 1-alkyl-3-methylimidazolium cations, such as AmimCl,117–119 BmimCl,113,120–125 EmimAc,117,126–133 BmimAc,134 and 1-allyl-3-methylimidazolium perchlorate.135 Besides these neat cellulose films, cellulose blends and cellulose composites were also formed into films for various applications, as shown in the following sections.
The regenerated cellulose films are obtained by casting or extruding cellulose solutions and then transferring to the coagulation bath for regeneration. Spin-coating was used to fabricate thin films of cellulose from its ionic liquid solutions.129 Generally, the regenerated cellulose films display a homogeneous and compact structure due to the complete dissolution of cellulose in these ionic liquids.117,119,126 The obtained films were further oxidized to produce efficient hemostasis materials.135 Active enzymes could be incorporated into cellulose films during the processing.125 Attributed to the high dissolving ability of ILs to three major components of lignocelluloses, i.e., cellulose, hemicelluloses, and lignin, regenerated lignocellulose films are also fabricated by dissolving wood in ionic liquids.133 It was found that a small amount of water in the cellulose solution facilitated the increase of the mechanical properties of regenerated cellulose films.128 The water absorbed in the film showed a significant plasticizing effect on the films.130 Adding appropriate amounts of aprotic polar solvents, such as DMSO, decreased the viscosity of cellulose solutions without precipitation of the polymer. Therefore, thin films with thickness as thin as 10 nm were fabricated via spin-coating.129
During the dissolution and regeneration processes, cellulose I in original cellulose was commonly transformed to cellulose II with a decreased crystallinity degree in the regenerated cellulose films.113,117–132 The crystallinity index and crystal orientation of cellulose films were also lower than those of regenerated cellulose fibers under the same drawing speed.113 On the other hand, the crystallinity degree of films could be tuned by changing the coagulation bath and drying conditions.131
In recent years, a new technique based on ionic liquids, KH-NILCELL™, has been jointly developed by ICCAS and Shandong Henglian New Materials Co. Ltd. The process is characterized by the use of AmimCl as a direct and powerful solvent to dissolve cellulose, and the use of water to coagulate cellulose into films. These obtained cellulose films, as shown in Fig. 2D, are smooth, transparent, and glossy, and the chain degradation of regenerated cellulose is negligible due to the mild dissolution and regeneration conditions. Therefore, these films have higher strengths and elongation at break than the commercially regenerated cellulose films with the same cellulose feedstock. In addition, it has been calculated from the results of a pilot plant that, compared with the viscose process, the new KH-NILCELL™ process has an economic benefit. More importantly, due to the excellent recycling of ILs, the new KH-NILCELL™ process is a completely closed loop, in which all the solvent (AmimCl) and the precipitating agent (water) are recycled and reused, and there is zero-waste. Thus, the new process also has environmental benefits in the preparation of biodegradable cellulose films, as an attractive alternative to the petrochemical derived materials.
Cellulose gels and aerogels
Cellulose dissolved in ionic liquids was gelated into the shapes of fibers,136,137 films,138,139 and beads,140–146 in which solid cellulose networks filled with liquids were constructed, mostly by coagulation with water and alcohols.147 Heating–cooling processes,139,148–153 chemical and irradiation crosslinking,138,154,155 and longtime aging156,157 were also used for gelation. Generally, the obtained cellulose gels were immersed in a series of solvents to remove ionic liquids and exchange the liquid phase to obtain hydrogels or organogels (Fig. 2E). For cellulose-based gel electrolytes, ionic liquids were kept in the gels to enhance the electrochemical properties.152,153If the liquid in these gels was replaced with air while the solid network was maintained by using special drying techniques, a highly porous material, namely aerogel, was obtained.158 Since the beginning of the 21st century, cellulose aerogel has received increasing and considerable attention due to its biological and sustainable origin. On the other hand, cellulose aerogels exhibit unusual properties of highly porous materials, such as large specific surface area, high porosity, low density, and low thermal conductivity. Relative to inorganic aerogels, cellulose aerogels were generally less brittle, even flexible and easily compressed to larger strains of more than 50% without disintegration.23,159–161
For the preparation of regenerated cellulose aerogels, the dissolution–regeneration route is most commonly adopted with the development of direct solvents for cellulose, including the above-mentioned promising green ionic liquids.162 AmimCl148,149,161–171 and BmimCl159,160,172–177 are the two most used ionic liquids. DMSO was mixed with ionic liquids as the co-solvent.177,178 Ionic liquids are also used to dissolve wood165,172 and waste newspapers169 to prepare lignocellulosic aerogels.
During the preparation of aerogels, cellulose was regenerated from its solution in ionic liquids in the form of wet gels, which were further dried by a special process to remove the liquid from the gels while their 3D network skeleton was maintained. Both supercritical fluid drying159–178 and freeze-drying techniques148,149,166–177 were widely used for removing solvents. In general, regenerated cellulose aerogels prepared by supercritical fluid drying had a nanofibrillar network structure with open-pores (Fig. 2F),161–172 while those prepared by freeze-drying were composed of networks of thin cellulose sheets.165,176
Regenerated cellulose aerogels could be further modified with functional materials to modulate their properties, such as increasing hydrophobicity,164 antibacterial activity,166 and flame retardancy,171 and to extend their applications, such as protein adsorption,174 photo-catalysis,175 oil adsorption,169 drug loading,148,149 and urea adsorption.176 Nanosized fillers, such as reduced graphene oxide, nanosilica, nano-TiO2 and quantum dots, were mixed with cellulose in ionic liquid solutions to obtain highly tough,160 thermal super-insulating,178 photocatalytically active,165 or photoluminescent (Fig. 3)23 cellulose nanocomposite aerogels.
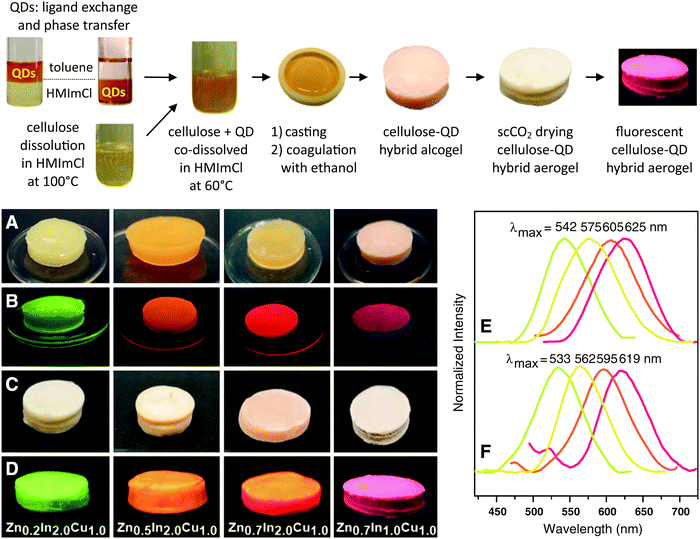 | ||
| Fig. 3 Cellulose-(ZnS)x(CuInS2)1−x@ZnS quantum dots hybrid gels. Reprinted with permission from ref. 23. Copyright 2013 Springer. | ||
Cellulose blends
Ionic liquids are also used for blending cellulose with other components to bring additional functionalities and vary the morphology and properties of materials.In order to prepare cellulose blend films and fibers, ionic liquids were used as common solvents to dissolve cellulose and polysaccharides, such as chitosan (Fig. 4E–J),166,179–181,289 chitin,152,153,157,182 xylan,144,172,183 agarose,140 carrageenans,140,150 glucomannan,184 cyclodextrins,185,186 and starch,156,187 or to dissolve proteins, such as silk,188,189 wool,190 keratin,151 and collagen.145,146,191 Homogeneous cellulose blends were often obtained with improved mechanical properties and thermal stability due to the strong intermolecular hydrogen bonding interaction between cellulose and these biopolymers.179–184,189–195 Phase separation occurred in cellulose/starch blend films, because of no specific interactions between these two polysaccharides.187 Moreover, these blends combined the unique properties of each component, for example antibacterial ability, pollutant adsorption, water retention, and bioactivity. The cellulose blends could be used in tissue engineering applications.185,188
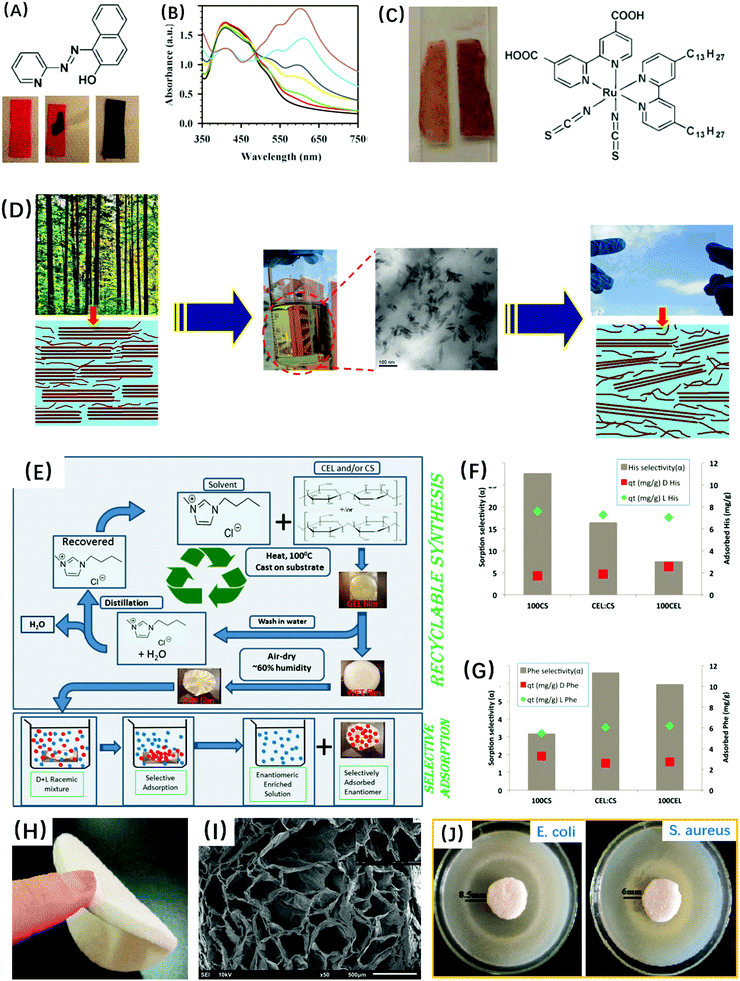 | ||
| Fig. 4 (A–C) Cellulose/probe molecules (1-(2-pyridylazo)-2-naphthol and N621) composite films as the Hg2+ sensor. Reprinted with permission from ref. 291. Copyright 2007 The Royal Society of Chemistry. (D) All-cellulose nanocomposite films prepared via a selective dissolution process. Reprinted with permission from ref. 247 and 292. Copyright 2011 and 2016 American Chemical Society. (E–G) Cellulose/chitosan composite film for separating the amino acids. Reprinted with permission from ref. 289. Copyright 2014 American Chemical Society. (H–J) Chitosan/cellulose composite sponges with good antimicrobial activities. Reprinted with permission from ref. 166. Copyright 2014 Springer. | ||
Cellulose and lignin,144,172,196 both extracted from wood, could be dissolved in some specific ionic liquids to fabricate wood-mimetic fibers, hydrogel beads, and aerogels. The addition of lignin increased the carbonization yield of blend fibers196 and the activity for lipase immobilization of blend hydrogels.143,144 And the presence of lignin influenced the gelation and porous structure of the resultant aerogels.172 Cellulose was also blended with synthetic polymers to increase the functionalities of cellulose.148,192–200
After the blend preparation, the solvent could be recycled with high yields for reuse.181–195 Therefore, using ionic liquids to develop novel cellulose blend materials is a green processing method.
Cellulose composites
Compounding cellulose with organic or inorganic fillers to prepare cellulose composites is another effective route to offering great improvements in a wide range of physical properties and strong potential in more widespread practical applications for cellulose materials. In particular, for widely used nanoparticles, the performances of obtained cellulose nanocomposites can be improved with lower filler loadings.As for inorganic fillers, zero-dimensional nanoparticles,178,200–211 one-dimensional nanotubes,212–215 and two-dimensional nanosheets160,216–227 were embedded in cellulose by adding fillers into cellulose/ionic liquid solutions directly or by forming fillers in situ.228,229
Strong interface interactions between cellulose and organic or inorganic components would be positive for the good dispersion of fillers. These interactions were typically formed by hydrogen bonding between the hydroxyl groups of cellulose and the functional groups of fillers. And these fillers included multi-wall carbon nanotube (MWCNT) (Fig. 5A–C and 6),136,212,293 LAPONITE®,216 montmorillonite,219,220 exfoliated graphite nanosheets,223,225 graphene,160 Fe3O4 (Fig. 5D–F),237,288 zeolite,230 sepiolite,218 halloysite,213,215 silica (Fig. 5I–K),231,290 leather fibers,232 and chitosan nanoparticles.233 Subsequently, the presence of fillers significantly enhanced the thermo-mechanical properties of cellulose composites, such as thermal stability, char yield, tensile strength and modulus without loss of ductility, as compared with the pure regenerated cellulose materials. Furthermore, some nano-sized fillers with a two-dimensional flat219,223,225 or one-dimensional nanotube structure213,215 improved the gas barrier properties and reduced the water absorption and diffusivity of cellulose nanocomposites.
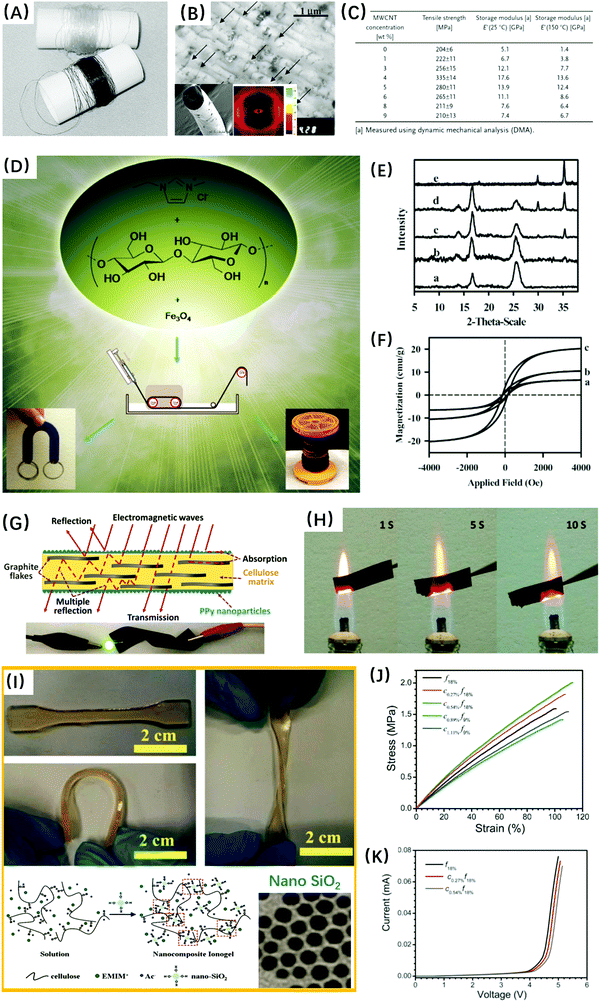 | ||
| Fig. 5 (A–C) Cellulose/MWCNT composite fibers with excellent mechanical properties. Reprinted with permission from ref. 212. Copyright 2007 Wiley. (D–F) Magnetic cellulose/Fe3O4 fibers. Reprinted with permission from ref. 288. Copyright 2008 The Royal Society of Chemistry. (G and H) Conductive cellulose/graphite/polypyrrole composite films with excellent electromagnetic interference shielding and flame resistance effects. Reprinted with permission from ref. 222. Copyright 2015 American Chemical Society. (I–K) Cellulose/nano-SiO2/EMIMAc bionanocomposite ionogels with high tensile strength and ionic conductivity. Reprinted with permission from ref. 290. Copyright 2013 The Royal Society of Chemistry. | ||
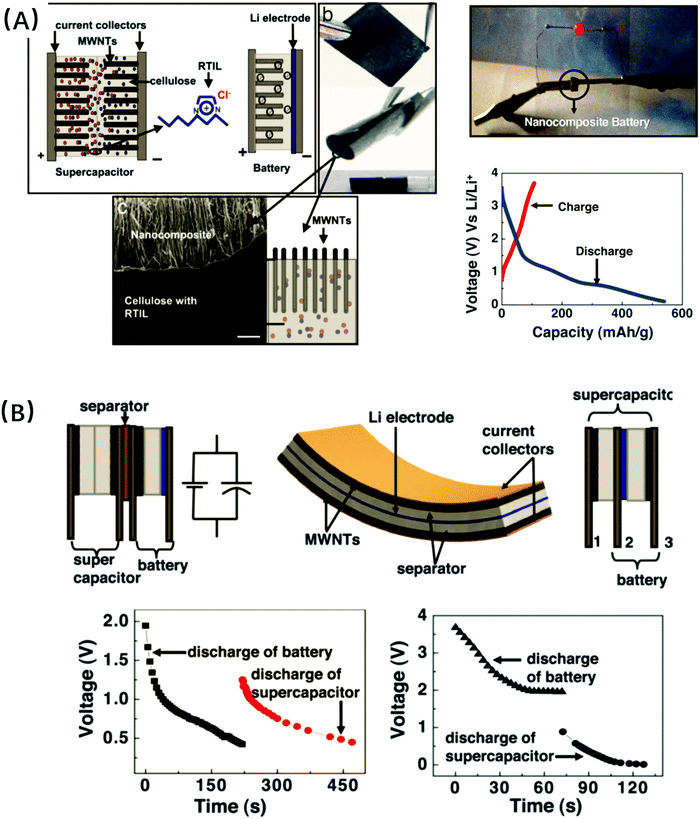 | ||
| Fig. 6 Fabrication of the nanocomposite paper units for batteries and supercapacitors. (A) Nanocomposite paper batteries; (B) supercapacitor-battery hybrid energy devices. Reprinted with permission from ref. 293. Copyright 2007 PNAS. | ||
As effective solvents for cellulose, ionic liquids provide a viable access for the preparation of composite materials based on regenerated cellulose. The ionic liquids used for the preparation of cellulose composites consisted of cations of 1-alkyl-3-methylimidazolium, in particular, combined with anions of chloride,200–248 acetate,224,225,249–251 tetrafluoroborate,209,228 and bis(trifluoromethylsulfonyl)imide (Fig. 7).294 AmimCl, BmimCl, and EmimAc were the most commonly used ionic liquids. In some cases, microwaves208–210,228 or DMSO136,178,236 and DMAc217 were used to assist the dissolution of cellulose.
 | ||
| Fig. 7 Cellulose-based ionogels for paper electronics. Reprinted with permission from ref. 294. Copyright 2014 Wiley. | ||
Cellulose composites have been prepared in various forms, such as films,215–227,230–252 fibers or electrospun fibers,200–205,212,214,220,248 beads,238,243,250 scaffolds,211,235,245 hydrogels,141,170 aerogels,178 and ionogels.290,294 And cellulose solutions in ionic liquids were used to finish the surface of polyethylene terephthalate fibers to achieve durable functionalization.253
Recently, all-cellulose composites, in which both the matrix and the dispersed phase were cellulose and completely compatible with each other like mono-component composite materials, have attracted great attention234–256,292,295 because they are fully bio-based and are fully biodegradable. These composites were generally obtained by partially dissolving or in situ welding of cellulose in ionic liquids, as shown in Fig. 4D. Therefore, the reinforcement of undissolved cellulose I crystals and the good interfacial interaction between the crystal and the regenerated cellulose matrix resulted in improved thermal and mechanical performances, and even optical transparency.
Functional materials
Ionic liquids have acted as environmentally benign reaction media for homogeneous esterification,138,257–267 silylation,178,268–270 and various polymerization processes271–281 to cellulose because of their non-volatility, non-flammability, chemically inertness, and recyclability. Therefore, intense efforts have been devoted to covalently bond functional moieties to impart hydrophobicity,164,206,268,269 flame retardancy,171,267 interaction to specific substances,264,270,277,280 pH-responsibility,278 fluorescence properties,138,260 gas permselectivity,262 chiral recognition ability,266 temperature sensibility,274,279etc.Functional cellulose materials were also produced from cellulose solutions in ionic liquids via efficient and green physical modifications by adding functional components and modulating the material morphology.254–256,286 These materials have potential applications in electrical136,199,282 and electrochemical devices,152,153,240,283 magnetically responsive devices,252,288 adsorption of organic pollutants181,186 and heavy metal ions,145,146,174,284 photocatalysis,170,175,203 enzyme immobilization,125,143,198,243 sensing and analysis,254,285 ultrafiltration membranes,202 antimicrobial fibers and films,179,200,204 drug load,139 tissue engineering,188,211,245,256 and other biomedical fields.166,208,248
Summary and perspective
In summary, ILs have demonstrated their huge potential for the dissolution and processing of cellulose over the past 14 years. With the significant progress in the fundamental research, we need to focus our attention on their potential industrial applications. Taking advantage of these recyclable and “green” ILs, many efforts have to be made to accomplish the eco-friendly utilization of biorenewable cellulose resources, and to overcome the shortcomings of the traditional cellulose industry, such as heavy pollution and high energy consumption. Before the large-scale utilization of ILs in the cellulose industry, several urgent topics need to be investigated: (1) the development of cheaper and more efficient recycling techniques for ILs; (2) the design of next generation ILs with higher dissolving capability, lower price and more safety; (3) the control of the structure, morphology and performances of regenerated cellulose materials; and (4) the utilization of low-cost agricultural and industrial cellulose resources.Acknowledgements
This work was supported by the National Science Foundation of China (No. 51425307, 51573196, 51273206, 21374126, and 21174151), the Program of Taishan Industry Leading Talents (Shandong Province), and the Innovative Research Team Program of Beijing Academy of Science and Technology (No. IG2016 05 N/C1).References
-
C. Graenacher, US Pat., 1943176, 1934 Search PubMed
.
- R. P. Swatloski, S. K. Spear, J. D. Holbrey and R. D. Rogers, J. Am. Chem. Soc., 2002, 124, 4974–4975 CrossRef CAS PubMed
.
-
J. Zhang, Q. Ren and J. S. He, CN Pat., ZL02155945, 2002 Search PubMed
.
- H. Zhang, J. Wu, J. Zhang and J. S. He, Macromolecules, 2005, 38, 8272–8277 CrossRef CAS
.
- J. Wu, J. Zhang, H. Zhang, J. S. He, Q. Ren and M. Guo, Biomacromolecules, 2004, 5, 266–268 CrossRef CAS PubMed
.
- Y. Fukaya, A. Sugimoto and H. Ohno, Biomacromolecules, 2006, 7, 3295–3297 CrossRef CAS PubMed
.
- H. Ohno and Y. Fukaya, Chem. Lett., 2009, 38, 2–7 CrossRef CAS
.
- Y. Fukaya, K. Hayashi, M. Wada and H. Ohno, Green Chem., 2008, 10, 44–46 RSC
.
- Y. Fukaya, A. Tsukamoto, K. Kuroda and H. Ohno, Chem. Commun., 2011, 47, 1994–1996 RSC
.
- M. Hummel, C. Froschauer, G. Laus, T. Roder, H. Kopacka, L. K. J. Hauru, H. K. Weber, H. Sixta and H. Schottenberger, Green Chem., 2011, 13, 2507–2517 RSC
.
- H. Zhao, G. A. Baker, Z. Y. Song, O. Olubajo, T. Crittle and D. Peters, Green Chem., 2008, 10, 696–705 RSC
.
- B. Zhao, L. Greiner and W. Leitner, RSC Adv., 2012, 2, 2476–2479 RSC
.
- D. S. Zhao, H. Li, J. Zhang, L. L. Fu, M. S. Liu, J. T. Fu and P. B. Ren, Carbohydr. Polym., 2012, 87, 1490–1494 CrossRef CAS
.
- A. R. Xu, J. J. Wang and H. Y. Wang, Green Chem., 2010, 12, 268–275 RSC
.
- Y. J. Zhang, A. R. Xu, B. L. Lu, Z. Y. Li and J. J. Wang, Carbohydr. Polym., 2015, 117, 666–672 CrossRef CAS PubMed
.
- A. Brandt, M. J. Ray, T. Q. To, D. J. Leak, R. J. Murphy and T. Welton, Green Chem., 2011, 13, 2489–2499 RSC
.
- S. Barthel and T. Heinze, Green Chem., 2006, 8, 301–306 RSC
.
- T. Erdmenger, C. Haensch, R. Hoogenboom and U. S. Schubert, Macromol. Biosci., 2007, 7, 440–445 CrossRef CAS PubMed
.
- D. T. Liu, K. F. Xia, W. H. Cai, R. D. Yang, L. Q. Wang and B. Wang, Carbohydr. Polym., 2012, 87, 1058–1064 CrossRef CAS
.
- B. R. Caes, J. B. Binder, J. J. Blank and R. T. Raines, Green Chem., 2011, 13, 2719–2722 RSC
.
- W. J. Xiao, Q. Chen, Y. Wu, T. H. Wu and L. Z. Dai, Carbohydr. Polym., 2011, 83, 233–238 CrossRef CAS
.
- A. S. Amarasekara and O. S. Owereh, Ind. Eng. Chem. Res., 2009, 48, 10152–10155 CrossRef CAS
.
- H. Q. Wang, Z. Q. Shao, M. Bacher, F. Liebner and T. Rosenau, Cellulose, 2013, 20, 3007–3024 CrossRef CAS PubMed
.
- S. Possidonio, L. C. Fidale and O. A. E. Seoud, J. Polym. Sci., Part A: Polym. Chem., 2010, 48, 134–143 CrossRef CAS
.
- T. Heinze, K. Schwikal and S. Barthel, Macromol. Biosci., 2005, 5, 520–525 CrossRef CAS PubMed
.
- E. S. Sashina, D. A. Kashirskii, M. Zaborski and S. Jankowski, Russ. J. Gen. Chem., 2012, 82, 1994–1998 CrossRef CAS
.
- A. Miyata and H. Miyafuji, J. Wood Sci., 2014, 60, 438–445 CrossRef CAS
.
- A. Pinkert, K. N. Marsh and S. S. Pang, Ind. Eng. Chem. Res., 2010, 49, 11809–11813 CrossRef CAS
.
- Z. J. Chen, S. M. Liu, Z. P. Li, Q. H. Zhang and Y. Q. Deng, New J. Chem., 2011, 35, 1596–1606 RSC
.
- S. K. Tang, G. A. Baker, S. Ravula, J. E. Jones and H. Zhao, Green Chem., 2012, 14, 2922–2932 RSC
.
- J. Grasvik, B. Eliasson and J. P. Mikkola, J. Mol. Struct., 2012, 1028, 156–163 CrossRef
.
- S. Kohler, T. Liebert and T. Heinze, Macromol. Biosci., 2009, 9, 836–841 CrossRef PubMed
.
- J. Pernak, R. Kordala, B. Markiewicz, F. Walkiewicz, M. Poplawski, A. Fabianska, S. Jankowski and M. Lozynski, RSC Adv., 2012, 2, 8429–8438 RSC
.
- J. J. Miao, H. B. Sun, Y. Q. Yu, X. L. Song and L. P. Zhang, RSC Adv., 2014, 4, 36721–36724 RSC
.
- H. B. Sun, J. J. Miao, Y. Q. Yu and L. P. Zhang, Appl. Phys. A: Mater. Sci. Process., 2015, 119, 539–546 CrossRef CAS
.
- M. Kostag, T. Liebert, O. A. El Seoud and T. Heinze, Macromol. Rapid Commun., 2013, 34, 1580–1584 CrossRef CAS PubMed
.
- M. Kostag, T. Liebert and T. Heinze, Macromol. Rapid Commun., 2014, 35, 1419–1422 CrossRef CAS PubMed
.
- D. G. Raut, O. Sundman, W. Q. Su, P. Virtanen, Y. Sugano, K. Kordas and J. P. Mikkola, Carbohydr. Polym., 2015, 130, 18–25 CrossRef CAS PubMed
.
- J. Pernak, N. Borucka, F. Walkiewicz, B. Markiewicz, P. Fochtman, S. Stolte, S. Steudte and P. Stepnowski, Green Chem., 2011, 13, 2901–2910 RSC
.
- N. Muhammad, Z. Man, M. A. Bustam, M. I. A. Mutalib, C. D. Wilfred and S. Rafiq, Appl. Biochem. Biotechnol., 2011, 165, 998–1009 CrossRef CAS PubMed
.
- X. D. Hou, T. J. Smith, N. Li and M. H. Zong, Biotechnol. Bioeng., 2012, 109, 2484–2493 CrossRef CAS PubMed
.
- Q. P. Liu, X. D. Hou, N. Li and M. H. Zong, Green Chem., 2012, 14, 304–307 RSC
.
- A. P. Abbott, T. J. Bell, S. Handa and B. Stoddart, Green Chem., 2005, 7, 705–707 RSC
.
- A. P. Abbott, T. J. Bell, S. Handa and B. Stoddart, Green Chem., 2006, 8, 784–786 RSC
.
- M. Francisco, A. van den Bruinhorst and M. C. Kroon, Green Chem., 2012, 14, 2153–2157 RSC
.
- Q. H. Zhang, M. Benoit, K. D. Vigier, J. Barrault and F. Jerome, Chem. – Eur. J., 2012, 18, 1043–1046 CrossRef CAS PubMed
.
-
D. Giovanni, S. Laszlo, M. Klemens and S. Veit, PCT Pat., WO2008043837, 2006 Search PubMed
.
- A. W. T. King, J. Asikkala, I. Mutikainen, P. Jarvi and I. Kilpelainen, Angew. Chem., Int. Ed., 2011, 50, 6301–6305 CrossRef CAS PubMed
.
- P. D. de Maria, J. Chem. Technol. Biotechnol., 2014, 89, 11–18 CrossRef
.
- L. K. J. Hauru, M. Hummel, A. W. T. King, I. Kilpelainen and H. Sixta, Biomacromolecules, 2012, 13, 2896–2905 CrossRef CAS PubMed
.
- A. Parviainen, A. W. T. King, I. Mutikainen, M. Hummel, C. Selg, L. K. J. Hauru, H. Sixta and I. Kilpelainen, ChemSusChem, 2013, 6, 2161–2169 CrossRef CAS PubMed
.
- L. K. J. Hauru, M. Hummel, K. Nieminen, A. Michud and H. Sixta, Soft Matter, 2016, 12, 1487–1495 RSC
.
- Y. Ma, M. Hummel, M. Maattanen, A. Sarkilahti, A. Harlin and H. Sixta, Green Chem., 2016, 18, 858–866 RSC
.
- Q. H. Zhang, N. S. Oztekin, J. Barrault, K. D. Vigier and F. Jerome, ChemSusChem, 2013, 6, 593–596 CrossRef CAS PubMed
.
- J. Sun, W. G. Cheng, Z. F. Yang, J. Q. Wang, T. T. Xu, J. Y. Xin and S. J. Zhang, Green Chem., 2014, 16, 3071–3078 RSC
.
- H. B. Xie, X. Yu, Y. L. Yang and Z. K. Zhao, Green Chem., 2014, 16, 2422–2427 RSC
.
- Y. L. Yang, H. B. Xie and E. H. Liu, Green Chem., 2014, 16, 3018–3023 RSC
.
- Y. L. Yang, L. C. Song, C. Peng, E. H. Liu and H. B. Xie, Green Chem., 2015, 17, 2758–2763 RSC
.
- J. Vitz, T. Erdmenger, C. Haensch and U. S. Schubert, Green Chem., 2009, 11, 417–424 RSC
.
- A. Pinkert, K. N. Marsh, S. S. Pang and M. P. Staiger, Chem. Rev., 2009, 109, 6712–6728 CrossRef CAS PubMed
.
- H. Wang, G. Gurau and R. D. Rogers, Chem. Soc. Rev., 2012, 41, 1519–1537 RSC
.
- H. F. N. de Oliveira and R. Rinaldi, ChemSusChem, 2015, 8, 1577–1584 CrossRef PubMed
.
- M. Zavrel, D. Bross, M. Funke, J. Buchs and A. C. Spiess, Bioresour. Technol., 2009, 100, 2580–2587 CrossRef CAS PubMed
.
- N. L. Mai and Y. M. Koo, ACS Sustainable Chem. Eng., 2016, 4, 541–547 CrossRef CAS
.
- R. Rinaldi, Chem. Commun., 2011, 47, 511–513 RSC
.
- L. Z. Lin, H. Yamaguchi and A. Suzuki, RSC Adv., 2013, 3, 14379–14384 RSC
.
- J. M. Andanson, E. Bordes, J. Devemy, F. Leroux, A. A. H. Padua and M. F. C. Gomes, Green Chem., 2014, 16, 2528–2538 RSC
.
- S. Velioglu, X. Yao, J. Devemy, M. G. Ahunbay, S. B. Tantekin-Ersolmaz, A. Dequidt, M. F. C. Gomes and A. A. H. Padua, J. Phys. Chem. B, 2014, 118, 14860–14869 CAS
.
- J. M. Andanson, A. A. H. Padua and M. F. C. Gomes, Chem. Commun., 2015, 51, 4485–4487 RSC
.
- A. R. Xu, L. L. Cao and B. J. Wang, Carbohydr. Polym., 2015, 125, 249–254 CrossRef CAS PubMed
.
- S. H. Ha, L. M. Ngoc, G. M. An and Y. M. Koo, Bioresour. Technol., 2011, 102, 1214–1219 CrossRef CAS PubMed
.
- J. P. Mikkola, A. Kirilin, J. C. Tuuf, A. Pranovich, B. Holmbom, L. M. Kustov, D. Y. Murzin and T. Salmi, Green Chem., 2007, 9, 1229–1237 RSC
.
- W. Lan, C. F. Liu, F. X. Yue, R. C. Sun and J. F. Kennedy, Carbohydr. Polym., 2011, 86, 672–677 CrossRef CAS
.
- S. Agarwal, A. M. Hossain, Y. S. Choi, M. Cheong, H. G. Jang and J. S. Lee, Bull. Korean Chem. Soc., 2013, 34, 3771–3776 CrossRef CAS
.
- Z. Q. Pang, C. H. Dong and X. J. Pan, Cellulose, 2016, 23, 323–338 CrossRef CAS
.
- R. C. Remsing, R. P. Swatloski, R. D. Rogers and G. Moyna, Chem. Commun., 2006, 1271–1273, 10.1039/b600586c
.
- R. C. Remsing, G. Hernandez, R. P. Swatloski, W. W. Massefski, R. D. Rogers and G. Moyna, J. Phys. Chem. B, 2008, 112, 11071–11078 CrossRef CAS PubMed
.
- T. G. A. Youngs, J. D. Holbrey, M. Deetlefs, M. Nieuwenhuyzen, M. F. C. Gomes and C. Hardacre, ChemPhysChem, 2006, 7, 2279–2281 CrossRef CAS PubMed
.
- T. G. A. Youngs, C. Hardacre and J. D. Holbrey, J. Phys. Chem. B, 2007, 111, 13765–13774 CrossRef CAS PubMed
.
- T. G. A. Youngs, J. D. Holbrey, C. L. Mullan, S. E. Norman, M. C. Lagunas, C. D'Agostino, M. D. Mantle, L. F. Gladden, D. T. Bowron and C. Hardacre, Chem. Sci., 2011, 2, 1594–1605 RSC
.
- H. B. Liu, G. Cheng, M. Kent, V. Stavila, B. A. Simmons, K. L. Sale and S. Singh, J. Phys. Chem. B, 2012, 116, 8131–8138 CrossRef CAS PubMed
.
- A. Pinkert, J. Chem. Eng. Data, 2012, 57, 1338–1340 CrossRef CAS
.
- F. Huo, Z. P. Liu and W. C. Wang, J. Phys. Chem. B, 2013, 117, 11780–11792 CrossRef CAS PubMed
.
- R. S. Payal and S. Balasubramanian, Phys. Chem. Chem. Phys., 2014, 16, 17458–17465 RSC
.
- B. D. Rabideau, A. Agarwal and A. E. Ismail, J. Phys. Chem. B, 2014, 118, 1621–1629 CrossRef CAS PubMed
.
- E. Hassan, F. Mutelet and M. Bouroukba, Carbohydr. Polym., 2015, 127, 316–324 CrossRef CAS PubMed
.
- A. Benedetto and P. Ballone, ACS Sustainable Chem. Eng., 2016, 4, 392–412 CrossRef CAS
.
- J. M. Zhang, H. Zhang, J. Wu, J. Zhang, J. S. He and J. F. Xiang, Phys. Chem. Chem. Phys., 2010, 12, 1941–1947 RSC
.
- J. M. Zhang, H. Zhang, J. Wu, J. Zhang, J. S. He and J. F. Xiang, Phys. Chem. Chem. Phys., 2010, 12, 14829–14830 RSC
.
- Z. Liu, H. Wang, Z. X. Li, X. M. Lu, X. P. Zhang, S. J. Zhang and K. B. Zhou, Mater. Chem. Phys., 2011, 128, 220–227 CrossRef CAS
.
- R. S. Payal, R. Bharath, G. Periyasamy and S. Balasubramanian, J. Phys. Chem. B, 2012, 116, 833–840 CrossRef CAS PubMed
.
- J. Li, Y. Lu, D. J. Yang, Q. F. Sun, Y. X. Liu and H. J. Zhao, Biomacromolecules, 2011, 12, 1860–1867 CrossRef CAS PubMed
.
- B. L. Lu, A. R. Xu and J. J. Wang, Green Chem., 2014, 16, 1326–1335 RSC
.
- A. Stark, M. Sellin, B. Ondruschka and K. Massonne, Sci. China: Chem., 2012, 55, 1663–1670 CrossRef CAS
.
- A. Pinkert, K. N. Marsh and S. S. Pang, Ind. Eng. Chem. Res., 2010, 49, 11121–11130 CrossRef CAS
.
- H. M. Cho, A. S. Gross and J. W. Chu, J. Am. Chem. Soc., 2011, 133, 14033–14041 CrossRef CAS PubMed
.
- A. S. Gross, A. T. Bell and J. W. Chu, J. Phys. Chem. B, 2011, 115, 13433–13440 CrossRef CAS PubMed
.
- J. Muraoka, N. Kamiya and Y. Ito, J. Mol. Liq., 2013, 182, 76–78 CrossRef CAS
.
- C. S. Lovell, A. Walker, R. A. Damion, A. Radhi, S. F. Tanner, T. Budtova and M. E. Ries, Biomacromolecules, 2010, 11, 2927–2935 CrossRef CAS PubMed
.
- M. E. Ries, A. Radhi, A. S. Keating, O. Parker and T. Budtova, Biomacromolecules, 2014, 15, 609–617 CrossRef CAS PubMed
.
- X. F. Sun, Q. Q. Tian, Z. M. Xue, Y. W. Zhang and T. C. Mu, RSC Adv., 2014, 4, 30282–30291 RSC
.
- K. Kathirgamanathan, W. J. Grigsby, J. Al-Hakkak and N. R. Edmonds, Int. J. Polym. Sci., 2015, 958653 Search PubMed
.
- K. Okushita, E. Chikayama and J. Kikuchi, Biomacromolecules, 2012, 13, 1323–1330 CrossRef CAS PubMed
.
- H. C. Chang, R. L. Zhang and D. T. Hsu, Phys. Chem. Chem. Phys., 2015, 17, 27573–27578 RSC
.
- D. Ingildeev, F. Effenberger, K. Bredereck and F. Hermanutz, J. Appl. Polym. Sci., 2013, 128, 4141–4150 CrossRef CAS
.
- L. K. J. Hauru, M. Hummel, A. Michud and H. Sixta, Cellulose, 2014, 21, 4471–4481 CrossRef CAS
.
- H. Sixta, A. Michud, L. Hauru, S. Asaadi, Y. B. Ma, A. W. T. King, I. Kilpelainen and M. Hummel, Nord. Pulp Pap. Res. J., 2015, 30, 43–57 CAS
.
- A. Michud, M. Hummel and H. Sixta, Polymer, 2015, 75, 1–9 CrossRef CAS
.
- A. Parviainen, R. Wahlstrom, U. Liimatainen, T. Liitia, S. Rovio, J. K. J. Helminen, U. Hyvakko, A. W. T. King, A. Suurnakki and I. Kilpelainen, RSC Adv., 2015, 5, 69728–69737 RSC
.
- B. Kosan, C. Michels and F. Meister, Cellulose, 2008, 15, 59–66 CrossRef CAS
.
- G. S. Jiang, Y. Yuan, B. C. Wang, X. M. Yin, K. S. Mukuze, W. F. Huang, Y. M. Zhang and H. P. Wang, Cellulose, 2012, 19, 1075–1083 CrossRef CAS
.
- C. Olsson, A. Hedlund, A. Idstrom and G. Westman, J. Mater. Sci., 2014, 49, 3423–3433 CrossRef CAS
.
- L. F. Sun, J. Y. Chen, W. Jiang and V. Lynch, Carbohydr. Polym., 2015, 118, 150–155 CrossRef CAS PubMed
.
- S. J. Kim and J. Jang, Fibers Polym., 2013, 14, 909–914 CrossRef CAS
.
- J. H. Chen, Y. Guan, K. Wang, X. M. Zhang, F. Xu and R. C. Sun, Carbohydr. Polym., 2015, 128, 147–153 CrossRef CAS PubMed
.
- X. J. Li, N. K. Li, J. G. Xu, X. Q. Duan, Y. S. Sun and Q. Zhao, J. Appl. Polym. Sci., 2014, 131, 40225 Search PubMed
.
- Y. Cao, H. Q. Li, Y. Zhang, J. Zhang and J. S. He, J. Appl. Polym. Sci., 2010, 116, 547–554 CrossRef CAS
.
- K. O. Reddy, J. M. Zhang, J. Zhang and A. V. Rajulu, Carbohydr. Polym., 2014, 114, 537–545 CrossRef CAS PubMed
.
- J. H. Pang, X. Liu, X. M. Zhang, Y. Y. Wu and R. C. Sun, Materials, 2013, 6, 1270–1284 CrossRef CAS
.
- Q. J. Li, Z. Q. Zhao, Y. M. Yuan, J. X. Gong and J. F. Zhang, Chem. J. Chin. Univ., 2015, 36, 2590–2597 CAS
.
- J. Kadokawa, R. Endo, D. Hatanaka and K. Yamamoto, J. Polym. Environ., 2015, 23, 348–355 CrossRef CAS
.
- Y. Wang, Y. C. Ji, L. Wei, Q. H. Wang and H. Li, J. Macromol. Sci., Part B: Phys., 2015, 54, 571–580 CrossRef CAS
.
- H. Z. Chen, N. Wang and L. Y. Liu, J. Chem. Technol. Biotechnol., 2012, 87, 1634–1640 CrossRef CAS
.
- Z. W. Jin, S. Wang, J. Q. Wang and M. X. Zhao, J. Appl. Polym. Sci., 2012, 125, 704–709 CrossRef CAS
.
- M. B. Turner, S. K. Spear, J. D. Holbrey and R. D. Rogers, Biomacromolecules, 2004, 5, 1379–1384 CrossRef CAS PubMed
.
- J. H. Pang, M. Wu, Q. H. Zhang, X. Tan, F. Xu, X. M. Zhang and R. C. Sun, Carbohydr. Polym., 2015, 121, 71–78 CrossRef CAS PubMed
.
- S. Livazovic, Z. Li, A. R. Behzad, K. V. Peinemann and S. P. Nunes, J. Membr. Sci., 2015, 490, 282–293 CrossRef CAS
.
- D. Wawro, W. Steplewski, W. Madaj, A. Michud, M. Hummel and H. Sixta, Fibres Text. East. Eur., 2015, 23, 25–32 CrossRef CAS
.
- R. Kargl, T. Mohan, V. Ribitsch, B. Saake, J. Puls and K. S. Kleinschek, Nord. Pulp Pap. Res. J., 2015, 30, 6–13 CAS
.
- J. Sundberg, G. Toriz and P. Gatenholm, Polymer, 2013, 54, 6555–6560 CrossRef CAS
.
- A. Ostlund, A. Idstrom, C. Olsson, P. T. Larsson and L. Nordstierna, Cellulose, 2013, 20, 1657–1667 CrossRef
.
- X. Liu, J. H. Pang, X. M. Zhang, Y. Y. Wu and R. C. Sun, Cellulose, 2013, 20, 1391–1399 CrossRef CAS
.
- S. H. Kim, M. H. Kim, J. H. Kim, S. Park, H. Kim, K. Won and S. H. Lee, J. Appl. Polym. Sci., 2015, 132, 42109 CrossRef
.
- T. Huber, S. S. Pang and M. P. Staiger, Composites, Part A, 2012, 43, 1738–1745 CrossRef CAS
.
- W. L. Cheng, J. M. He, Y. D. Wu, C. Song, S. S. Xie, Y. D. Huang and B. Fu, Cellulose, 2013, 20, 2547–2558 CrossRef CAS
.
- P. Chen, Y. S. Yun, H. Bak, S. Y. Cho and H. J. Jin, Mol. Cryst. Liq. Cryst., 2010, 519, 169–178 CrossRef CAS
.
- M. Kimura, Y. Shinohara, J. Takizawa, S. Ren, K. Sagisaka, Y. D. Lin, Y. Hattori and J. P. Hinestroza, Sci. Rep., 2015, 5, 16266 CrossRef PubMed
.
- A. Hufendiek, A. Carlmark, M. A. R. Meier and C. Barner-Kowollik, ACS Macro Lett., 2016, 5, 139–143 CrossRef CAS
.
- C. D. Tran and T. M. Mututuvari, Langmuir, 2015, 31, 1516–1526 CrossRef CAS PubMed
.
- M. H. Kim, S. An, K. Won, H. J. Kim and S. H. Lee, J. Mol. Catal. B: Enzym., 2012, 75, 68–72 CrossRef CAS
.
- Z. Liu, H. S. Wang, C. Liu, Y. J. Jiang, G. Yu, X. D. Mu and X. Y. Wang, Chem. Commun., 2012, 48, 7350–7352 RSC
.
- S. Napso, D. M. Rein, R. Khalfin, O. Kleinerman and Y. Cohen, Colloids Surf., B, 2016, 137, 70–76 CrossRef CAS PubMed
.
- S. Park, S. H. Kim, J. H. Kim, H. Yu, H. J. Kim, Y. H. Yang, H. Kim, Y. H. Kim, S. H. Ha and S. H. Lee, J. Mol. Catal. B: Enzym., 2015, 119, 33–39 CrossRef CAS
.
- S. Park, S. H. Kim, K. Won, J. W. Choi, Y. H. Kim, H. J. Kim, Y. H. Yang and S. H. Lee, Carbohydr. Polym., 2015, 115, 223–229 CrossRef CAS PubMed
.
- J. L. Wang, M. H. Li, L. G. Wei, Y. C. Ma, K. L. Li and Y. C. Yu, Adv. Mater. Res., 2012, 535–537, 2365–2369 CrossRef CAS
.
- J. L. Wang, L. G. Wei, Y. C. Ma, K. L. Li, M. H. Li, Y. C. Yu, L. Wang and H. H. Qiu, Carbohydr. Polym., 2013, 98, 736–743 CrossRef CAS PubMed
.
- C. Y. Chang and L. N. Zhang, Carbohydr. Polym., 2011, 84, 40–53 CrossRef CAS
.
- X. Y. Hu, K. Hu, L. L. Zeng, M. M. Zhao and H. H. Huang, Carbohydr. Polym., 2010, 82, 62–68 CrossRef CAS
.
- X. Y. Hu, J. Wang and H. H. Huang, Cellulose, 2013, 20, 2923–2933 CrossRef CAS
.
- K. Prasad, Y. Kaneko and J. Kadokawa, Macromol. Biosci., 2009, 9, 376–382 CrossRef CAS PubMed
.
- M. Setoyama, K. Yamamoto and J. Kadokawa, J. Polym. Environ., 2014, 22, 298–303 CrossRef CAS
.
- S. Yamazaki, A. Takegawa, Y. Kaneko, J. Kadokawa, M. Yamagata and M. Ishikawa, Electrochem. Commun., 2009, 11, 68–70 CrossRef CAS
.
- S. Yamazaki, A. Takegawa, Y. Kaneko, J. Kadokawa, M. Yamagata and M. Ishikawa, J. Electrochem. Soc., 2010, 157, A203–A208 CrossRef CAS
.
- A. Kimura, N. Nagasawa and M. Taguchi, Radiat. Phys. Chem., 2014, 103, 216–221 CrossRef CAS
.
- L. X. Zhong, X. W. Peng, D. Yang and R. C. Sun, J. Agric. Food Chem., 2012, 60, 5621–5628 CrossRef CAS PubMed
.
- J. I. Kadokawa, M. A. Murakami, A. Takegawa and Y. Kaneko, Carbohydr. Polym., 2009, 75, 180–183 CrossRef CAS
.
- A. Takegawa, M. Murakami, Y. Kaneko and J. Kadokawa, Carbohydr. Polym., 2010, 79, 85–90 CrossRef CAS
.
- N. Husing and U. Schubert, Angew. Chem., Int. Ed., 1998, 37, 22–45 CrossRef CAS
.
- R. Sescousse, R. Gavillon and T. Budtova, Carbohydr. Polym., 2011, 83, 1766–1774 CrossRef CAS
.
- M. M. Xu, Q. B. Huang, X. H. Wang and R. C. Sun, Ind. Crops Prod., 2015, 70, 56–63 CrossRef CAS
.
- Q. Y. Mi, S. R. Ma, J. Yu, J. S. He and J. Zhang, ACS Sustainable Chem. Eng., 2016, 4, 656–660 CrossRef CAS
.
- S. R. Ma, Q. Y. Mi, J. Yu, J. S. He and J. Zhang, Prog. Chem., 2014, 26, 796–809 CAS
.
- C. Tsioptsias, A. Stefopoulos, I. Kokkinomalis, L. Papadopoulou and C. Panayiotou, Green Chem., 2008, 10, 965–971 RSC
.
- M. Granstrom, M. K. N. Paakko, H. Jin, E. Kolehmainen, I. Kilpelainen and O. Ikkala, Polym. Chem., 2011, 2, 1789–1796 RSC
.
- Y. Lu, Q. F. Sun, D. J. Yang, X. L. She, X. D. Yao, G. S. Zhu, Y. X. Liu, H. J. Zhao and J. Li, J. Mater. Chem., 2012, 22, 13548–13557 RSC
.
- F. B. Lv, C. X. Wang, P. Zhu and C. J. Zhang, Cellulose, 2014, 21, 4405–4418 CrossRef CAS
.
- L. K. Voon, S. C. Pang and S. F. Chin, Mater. Lett., 2016, 164, 264–266 CrossRef CAS
.
- Y. X. Lv, X. Y. Li, Q. Y. Mi, D. X. Wang, J. Yu and J. Zhang, Sci. Sin.: Chim., 2011, 41, 1331–1337 CrossRef
.
- C. D. Jin, S. J. Han, J. P. Li and Q. F. Sun, Carbohydr. Polym., 2015, 123, 150–156 CrossRef CAS PubMed
.
-
Y. Lu, Q. F. Sun, J. Li and Y. X. Liu, in Micro-Nano Technology Xv, ed. F. Tang, 2014, vol. 609–610, pp. 542–546 Search PubMed
.
- B. Yuan, Q. Y. Mi, J. Yu, R. Song, J. M. Zhang, J. S. He and J. Zhang, Sci. China: Chem., 2016, 59, 1335–1341 CrossRef CAS
.
- O. Aaltonen and O. Jauhiainen, Carbohydr. Polym., 2009, 75, 125–129 CrossRef CAS
.
- M. L. Deng, Q. Zhou, A. K. Du, J. van Kasteren and Y. Z. Wang, Mater. Lett., 2009, 63, 1851–1854 CrossRef CAS
.
- T. Oshima, T. Sakamoto, K. Ohe and Y. Baba, Carbohydr. Polym., 2014, 103, 62–69 CrossRef CAS PubMed
.
- Z. H. Zhou, X. X. Zhang, C. H. Lu, L. D. Lan and G. P. Yuan, RSC Adv., 2014, 4, 8966–8972 RSC
.
- X. Y. Liu, P. R. Chang, P. W. Zheng, D. P. Anderson and X. F. Ma, Cellulose, 2015, 22, 709–715 CrossRef CAS
.
- A. A. Shamsuri, D. K. Abdullah and R. Daik, Cellul. Chem. Technol., 2012, 46, 45–52 CAS
.
- A. Demilecamps, C. Beauger, C. Hildenbrand, A. Rigacci and T. Budtova, Carbohydr. Polym., 2015, 122, 293–300 CrossRef CAS PubMed
.
- Y. H. Zhou, X. L. Luo, L. L. Huang, S. Lin and L. H. Chen, J. Biobased Mater. Bioenergy, 2015, 9, 389–395 CrossRef CAS
.
- C. Stefanescu, W. H. Daly and I. I. Negulescu, Carbohydr. Polym., 2012, 87, 435–443 CrossRef CAS
.
- C. D. Tran, S. Duri, A. Delneri and M. Franko, J. Hazard. Mater., 2013, 252, 355–366 CrossRef PubMed
.
- K. Mundsinger, A. Muller, R. Beyer, F. Hermanutz and M. R. Buchmeiser, Carbohydr. Polym., 2015, 131, 34–40 CrossRef CAS PubMed
.
- J. Sundberg, G. Toriz and P. Gatenholm, Cellulose, 2015, 22, 1943–1953 CrossRef CAS
.
- Z. J. Yu, Y. Q. Jiang, W. W. Zou, J. J. Duan and X. P. Xiong, J. Polym. Sci., Part B: Polym. Phys., 2009, 47, 1686–1694 CrossRef CAS
.
- M. Soheilmoghaddam, G. Sharifzadeh, R. H. Pour, M. U. Wahit, W. T. Whye and X. Y. Lee, Mater. Lett., 2014, 135, 210–213 CrossRef CAS
.
- S. Duri and C. D. Tran, Langmuir, 2013, 29, 5037–5049 CrossRef CAS PubMed
.
- W. Q. Liu and T. Budtova, Polymer, 2012, 53, 5779–5787 CrossRef CAS
.
- N. Singh, S. S. Rahatekar, K. K. K. Koziol, T. H. S. Ng, A. J. Patil, S. Mann, A. P. Hollander and W. Kafienah, Biomacromolecules, 2013, 14, 1287–1298 CrossRef CAS PubMed
.
- S. M. Shang, L. Zhu and J. T. Fan, Carbohydr. Polym., 2011, 86, 462–468 CrossRef CAS
.
- N. Hameed and Q. P. Guo, Carbohydr. Polym., 2009, 78, 999–1004 CrossRef CAS
.
- M. Zhang, C. C. Ding, L. H. Chen and L. L. Huang, BioResources, 2014, 9, 756–771 CAS
.
- N. Hameed, R. Y. Xiong, N. V. Salim and Q. P. Guo, Cellulose, 2013, 20, 2517–2527 CrossRef CAS
.
- X. M. Zhang, J. Zhu and X. Q. Liu, Macromol. Res., 2012, 20, 703–708 CrossRef
.
- M. Soheilmoghaddam, M. U. Wahit and N. I. Akos, Mater. Lett., 2013, 111, 221–224 CrossRef CAS
.
- R. Y. Xiong, N. Hameed and Q. P. Guo, Carbohydr. Polym., 2012, 90, 575–582 CrossRef CAS PubMed
.
- Y. B. Ma, S. Asaadi, L. S. Johansson, P. Ahvenainen, M. Reza, M. Alekhina, L. Rautkari, A. Michud, L. Hauru, M. Hummel and H. Sixta, ChemSusChem, 2015, 8, 4030–4039 CrossRef CAS PubMed
.
- M. Bagheri, H. Rodriguez, R. P. Swatloski, S. K. Spear, D. T. Daly and R. D. Rogers, Biomacromolecules, 2008, 9, 381–387 CrossRef CAS PubMed
.
- M. B. Turner, S. K. Spear, J. D. Holbrey, D. T. Daly and R. D. Rogers, Biomacromolecules, 2005, 6, 2497–2502 CrossRef CAS PubMed
.
- X. T. Liang, B. Qu, J. R. Li, H. N. Xiao, B. H. He and L. Y. Qian, React. Funct. Polym., 2015, 86, 1–6 CrossRef CAS
.
- F. Wendler, B. Kosan, M. Krieg and F. Meister, Macromol. Symp., 2009, 280, 112–122 CrossRef CAS
.
- N. S. Venkataramanan, K. Matsui, H. Kawanami and Y. Ikushima, Green Chem., 2007, 9, 18–19 RSC
.
- D. Nevstrueva, A. Pihlajamaki and M. Manttari, Cellulose, 2015, 22, 3865–3876 CrossRef CAS
.
- A. Wittmar, H. Thierfeld, S. Kocher and M. Ulbricht, RSC Adv., 2015, 5, 35866–35873 RSC
.
- J. Y. Chen, L. F. Sun, W. Jiang and V. M. Lynch, J. Bioact. Compat. Polym., 2015, 30, 17–33 CrossRef
.
- H. Z. Song, Z. Q. Luo, C. Z. Wang, X. F. Hao and J. G. Gao, Carbohydr. Polym., 2013, 98, 161–167 CrossRef CAS PubMed
.
- A. S. Amarasekara and O. S. Owereh, Carbohydr. Polym., 2009, 78, 635–638 CrossRef CAS
.
- H. Z. Song and L. W. Zheng, Cellulose, 2013, 20, 1737–1746 CrossRef CAS
.
- M. G. Ma, Y. Y. Dong, L. H. Fu, S. M. Li and R. C. Sun, Carbohydr. Polym., 2013, 92, 1669–1676 CrossRef CAS PubMed
.
- M. G. Ma, S. J. Qing, S. M. Li, J. F. Zhu, L. H. Fu and R. C. Sun, Carbohydr. Polym., 2013, 91, 162–168 CrossRef CAS PubMed
.
- M. Bagheri and S. Rabieh, Cellulose, 2013, 20, 699–705 CrossRef CAS
.
- A. Salama, M. Neumann, C. Gunter and A. Taubert, Beilstein J. Nanotechnol., 2014, 5, 1553–1568 CrossRef PubMed
.
- H. Zhang, Z. G. Wang, Z. N. Zhang, J. Wu, J. Zhang and H. S. He, Adv. Mater., 2007, 19, 698–704 CrossRef CAS
.
- N. A. Hanid, M. U. Wahit, Q. P. Guo, S. Mahmoodian and M. Soheilmoghaddam, Carbohydr. Polym., 2014, 99, 91–97 CrossRef PubMed
.
- Z. Q. Luo, A. Q. Wang, C. Z. Wang, W. C. Qin, N. N. Zhao, H. Z. Song and J. G. Gao, J. Mater. Chem. A, 2014, 2, 7327–7336 CAS
.
- M. Soheilmoghaddam, M. U. Wahit, S. Mahmoudian and N. A. Hanid, Mater. Chem. Phys., 2013, 141, 936–943 CrossRef CAS
.
- W. T. Wu, Z. Dong, J. S. He, J. Yu and J. Zhang, J. Mater. Sci., 2016, 51, 4125–4133 CrossRef CAS
.
- I. Sen, Y. Seki, M. Sarikanat, L. Cetin, B. O. Gurses, O. Ozdemir, O. C. Yilmaz, K. Sever, E. Akar and O. Mermer, Composites, Part B, 2015, 69, 369–377 CrossRef CAS
.
- M. Soheilmoghaddam, M. U. Wahit, A. A. Yussuf, M. A. Al-Saleh and W. T. Whye, Polym. Test., 2014, 33, 121–130 CrossRef CAS
.
- S. Mahmoudian, M. U. Wahit, A. F. Ismail and A. A. Yussuf, Carbohydr. Polym., 2012, 88, 1251–1257 CrossRef CAS
.
- S. Mahmoudian, M. U. Wahit, A. F. Ismail, H. Balakrishnan and M. Imran, J. Mater. Sci., 2015, 50, 1228–1236 CrossRef CAS
.
- T. P. Zhang, X. T. Liu, M. Jiang, Y. X. Duan and J. M. Zhang, RSC Adv., 2015, 5, 76302–76308 RSC
.
- J. H. Chen, J. K. Xu, K. Wang, X. R. Qian and R. C. Sun, ACS Appl. Mater. Interfaces, 2015, 7, 15641–15648 CAS
.
- M. Soheilmoghaddam, P. Pasbakhsh, M. U. Wahit, H. C. Bidsorkhi, R. H. Pour, W. T. Whye and R. T. De Silva, Polymer, 2014, 55, 3130–3138 CrossRef CAS
.
- L. Hardelin and B. Hagstrom, J. Appl. Polym. Sci., 2015, 132, 41417 CrossRef
.
- S. Mahmoudian, M. U. Wahit, M. Imran, A. F. Ismail and H. Balakrishnan, J. Nanosci. Nanotechnol., 2012, 12, 5233–5239 CrossRef CAS PubMed
.
- C. Y. Yan, P. G. Ren, Z. P. Zhang, H. Wang and Z. M. Li, Cellulose, 2015, 22, 1243–1251 CrossRef CAS
.
- B. G. Wang, W. J. Lou, X. B. Wang and J. C. Hao, J. Mater. Chem., 2012, 22, 12859–12866 RSC
.
- F. Deng, L. H. Fu and M. G. Ma, Carbohydr. Polym., 2015, 121, 163–168 CrossRef CAS PubMed
.
- M. Mashkour, M. Tajvidi, F. Kimura, H. Yousefi and T. Kimura, ACS Appl. Mater. Interfaces, 2014, 6, 8165–8172 CAS
.
- M. Soheilmoghaddam, M. U. Wahit, W. T. Whye, N. I. Akos, R. H. Pour and A. A. Yussuf, Carbohydr. Polym., 2014, 106, 326–334 CrossRef CAS PubMed
.
- Q. Zhao, R. Yam, B. Q. Zhang, Y. K. Yang, X. J. Cheng and R. Li, Cellulose, 2009, 16, 217–226 CrossRef CAS
.
- G. M. Xia, V. Sadanand, B. Ashok, K. O. Reddy, J. Zhang and A. V. Rajulu, Int. J. Polym. Anal. Charact., 2015, 20, 693–703 CrossRef CAS
.
- F. Niroomand, A. Khosravani and H. Younesi, Cellulose, 2016, 23, 1311–1324 CrossRef CAS
.
- B. Adak and S. Mukhopadhyay, J. Appl. Polym. Sci., 2016, 133, 43398 CrossRef
.
- S. Zadegan, M. Hosainalipour, H. R. Rezaie, H. Ghassai and M. A. Shokrgozar, Mater. Sci. Eng., C, 2011, 31, 954–961 CrossRef CAS
.
- D. Lourdin, J. Peixinho, J. Breard, B. Cathala, E. Leroy and B. Duchemin, Cellulose, 2016, 23, 529–543 CrossRef CAS
.
- S. Peng, H. C. Meng, L. Zhou and J. Chang, J. Nanosci. Nanotechnol., 2014, 14, 7010–7014 CrossRef CAS PubMed
.
- S. Peng, H. C. Meng, Y. Ouyang and J. Chang, Ind. Eng. Chem. Res., 2014, 53, 2106–2113 CrossRef CAS
.
- M. Shibata, N. Teramoto, T. Nakamura and Y. Saitoh, Carbohydr. Polym., 2013, 98, 1532–1539 CrossRef CAS PubMed
.
- R. X. Ou, Y. J. Xie, X. P. Shen, F. P. Yuan, H. G. Wang and Q. W. Wang, J. Mater. Sci., 2012, 47, 5978–5986 CrossRef CAS
.
- A. Shakeri, A. P. Mathew and K. Oksman, J. Compos. Mater., 2012, 46, 1305–1311 CrossRef CAS
.
- W. Gindl-Altmutter, J. Keckes, J. Plackner, F. Liebner, K. Englund and M. P. Laborie, Compos. Sci. Technol., 2012, 72, 1304–1309 CrossRef CAS
.
- Z. Liu, H. S. Wang, B. Li, C. Liu, Y. J. Jiang, G. Yu and X. D. Mu, J. Mater. Chem., 2012, 22, 15085–15091 RSC
.
- F. Shi, D. Q. Lin, W. Phottraithip and S. J. Yao, J. Appl. Polym. Sci., 2011, 119, 3453–3461 CrossRef CAS
.
- C. Tsioptsias and C. Panayiotou, Carbohydr. Polym., 2008, 74, 99–105 CrossRef CAS
.
- H. Ma, B. Zhou, H. S. Li, Y. Q. Li and S. Y. Ou, Carbohydr. Polym., 2011, 84, 383–389 CrossRef CAS
.
- H. Yousefi, T. Nishino, M. Faezipour, G. Ebrahimi and A. Shakeri, Biomacromolecules, 2011, 12, 4080–4085 CrossRef CAS PubMed
.
- G. Viswanathan, S. Murugesan, V. Pushparaj, O. Nalamasu, P. M. Ajayan and R. J. Linhardt, Biomacromolecules, 2006, 7, 415–418 CrossRef CAS PubMed
.
- B. Tisserat, E. Larson, D. Gray, N. Dexter, C. Meunier, L. Moore and L. Haverhals, Int. J. Polym. Sci., 2015, 181097 Search PubMed
.
- M. Zhang, C. C. Ding, L. H. Chen, L. L. Huang and H. Y. Yang, J. Biobased Mater. Bioenergy, 2014, 8, 610–617 CrossRef CAS
.
- T. Huber, S. Bickerton, J. Mussig, S. S. Pang and M. P. Staiger, Carbohydr. Polym., 2012, 90, 730–733 CrossRef CAS PubMed
.
- R. P. Swatloski, J. D. Holbrey, J. L. Weston and R. D. Rogers, Chim. Oggi-Chem. Today, 2006, 24, 31–35 CAS
.
- T. Textor, L. Derksen and J. S. Gutmann, Carbohydr. Polym., 2016, 146, 139–147 CrossRef CAS PubMed
.
- K. F. Du, M. Yan, Q. Y. Wang and H. Song, J. Chromatogr. A, 2010, 1217, 1298–1304 CrossRef CAS PubMed
.
- H. L. Zhu, Z. Q. Fang, Z. Wang, J. Q. Dai, Y. G. Yao, F. Shen, C. Preston, W. X. Wu, P. Peng, N. Jang, Q. K. Yu, Z. F. Yu and L. B. Hu, ACS Nano, 2016, 10, 1369–1377 CrossRef CAS PubMed
.
- E. J. Shin, S. M. Choi, D. Singh, S. M. Zo, Y. H. Lee, J. H. Kim and S. S. Han, Cellulose, 2014, 21, 3515–3525 CrossRef CAS
.
- J. M. Zhang, W. W. Chen, Y. Feng, J. Wu, J. Yu, J. S. He and J. Zhang, Polym. Int., 2015, 64, 963–970 CrossRef CAS
.
- J. M. Zhang and J. Zhang, Acta Polym. Sin., 2010, 1376–1398 CrossRef CAS
.
- J. M. Zhang, J. Wu, Y. Cao, S. M. Sang, J. Zhang and J. S. He, Cellulose, 2009, 16, 299–308 CrossRef CAS
.
- J. Chen, J. M. Zhang, W. W. Chen, Y. Feng and J. Zhang, Acta Polym. Sin., 2013, 1235–1240 CAS
.
- Y. H. Luan, J. M. Zhang, M. S. Zhan, J. Wu, J. Zhang and J. S. He, Carbohydr. Polym., 2013, 92, 307–311 CrossRef CAS PubMed
.
- J. Chen, J. M. Zhang, Y. Feng, J. Wu, J. S. He and J. Zhang, J. Membr. Sci., 2014, 469, 507–514 CrossRef CAS
.
- P. Xiao, J. M. Zhang, Y. Feng, J. Wu, J. S. He and J. Zhang, Cellulose, 2014, 21, 2369–2378 CrossRef CAS
.
- Q. Q. Dai, J. L. Ren, W. Q. Kong, F. Peng and L. Meng, BioResources, 2015, 10, 3666–3681 CAS
.
- W. W. Chen, Y. Feng, M. Zhang, J. Wu, J. M. Zhang, X. Gao, J. S. He and J. Zhang, RSC Adv., 2015, 5, 58536–58542 RSC
.
- W. W. Chen, M. Zhang, Y. Feng, J. Wu, X. Gao, J. M. Zhang, J. S. He and J. Zhang, Polym. Int., 2015, 64, 1037–1044 CrossRef CAS
.
- Y. B. Zheng, J. Song, B. W. Cheng and X. L. Fang, Fibers Polym., 2016, 17, 1–8 CrossRef CAS
.
- Z. Qin, Y. Fang, Y. Wang, H. Li and J. Liang, Microporous Mesoporous Mater., 2015, 207, 78–83 CrossRef CAS
.
- J. J. Miao, Y. Q. Yu, Z. M. Jiang and L. P. Zhang, Cellulose, 2016, 23, 1209–1219 CrossRef CAS
.
- J. H. Pang, J. K. Liu, Q. H. Zhang, X. J. Jin, X. M. Zhang, J. Yang, F. Xu and R. C. Sun, Sci. Adv. Mater., 2016, 8, 1135–1144 CrossRef CAS
.
- C. H. Yan, J. M. Zhang, Y. X. Lv, J. Yu, J. Wu, J. Zhang and J. S. He, Biomacromolecules, 2009, 10, 2013–2018 CrossRef CAS PubMed
.
- Y. H. Luan, J. Wu, M. S. Zhan, J. M. Zhang, J. Zhang and J. S. He, Chem. J. Chin. Univ., 2012, 33, 2135–2137 CAS
.
- Y. H. Luan, J. Wu, M. S. Zhan, J. M. Zhang, J. Zhang and J. S. He, Cellulose, 2013, 20, 327–337 CrossRef CAS
.
- L. L. Yang, J. M. Zhang, J. S. He, J. Zhang and Z. H. Gan, Chin. J. Polym. Sci., 2015, 33, 1640–1649 CrossRef CAS
.
- L. L. Yang, J. M. Zhang, J. S. He, J. Zhang and Z. H. Gan, RSC Adv., 2016, 6, 17617–17623 RSC
.
- R. L. Wu, L. Y. Tian, W. Wang and X. L. Man, J. Appl. Polym. Sci., 2015, 132, 41830 Search PubMed
.
- L. Xu, X. Q. Lu and X. M. Cheng, RSC Adv., 2015, 5, 79022–79030 RSC
.
- E. J. Tang, K. D. Du, X. Y. Feng, M. Yuan, S. J. Liu and D. S. Zhao, Eur. Polym. J., 2015, 66, 228–235 CrossRef CAS
.
- Y. Hao, J. Peng, J. Q. Li, M. L. Zhai and G. S. Wei, Carbohydr. Polym., 2009, 77, 779–784 CrossRef CAS
.
- C. X. Lin, H. Y. Zhan, M. H. Liu, S. Y. Fu and L. A. Lucia, Langmuir, 2009, 25, 10116–10120 CrossRef CAS PubMed
.
- A. Takegawa, M. Murakami, Y. Kaneko and J. Kadokawa, Polym. Compos., 2009, 30, 1837–1841 CrossRef CAS
.
- F. Wang, J. H. Jeon, S. Park, C. D. Kee, S. J. Kim and I. K. Oh, Soft Matter, 2016, 12, 246–254 RSC
.
- M. H. Khanmirzaei, S. Ramesh and K. Ramesh, Sci. Rep., 2015, 5, 7 Search PubMed
.
- S. Kalidhasan, A. S. KrishnaKumar, V. Rajesh and N. Rajesh, J. Colloid Interface Sci., 2012, 367, 398–408 CrossRef CAS PubMed
.
- K. H. Liu, B. H. He, L. Y. Qian and J. R. Li, J. Appl. Polym. Sci., 2015, 132, 41639 Search PubMed
.
- S. S. Xu, J. Zhang, A. H. He, J. X. Li, H. Zhang and C. C. Han, Polymer, 2008, 49, 2911–2917 CrossRef CAS
.
- K. F. Du, M. Yan, Q. Y. Wang and H. Song, J. Chromatogr. A, 2010, 1217, 1298–1304 CrossRef CAS PubMed
.
- N. Sun, R. P. Swatloski, M. L. Maxim, M. Rahman, A. G. Harland, A. Haque, S. K. Spear, D. T. Daly and R. D. Rogers, J. Mater. Chem., 2008, 18, 283–290 RSC
.
- S. Duri and C. D. Tran, Langmuir, 2014, 30, 642–650 CrossRef CAS PubMed
.
- H. Z. Song, Z. Q. Luo, H. C. Zhao, S. S. Luo, X. J. Wu, J. G. Gao and Z. G. Wang, RSC Adv., 2013, 3, 11665 RSC
.
- J. H. Poplin, R. P. Swatloski, J. D. Holbrey, S. K. Spear, A. Metlen, M. Grätzel, M. K. Nazeeruddin and R. D. Rogers, Chem. Commun., 2007, 2025–2027 RSC
.
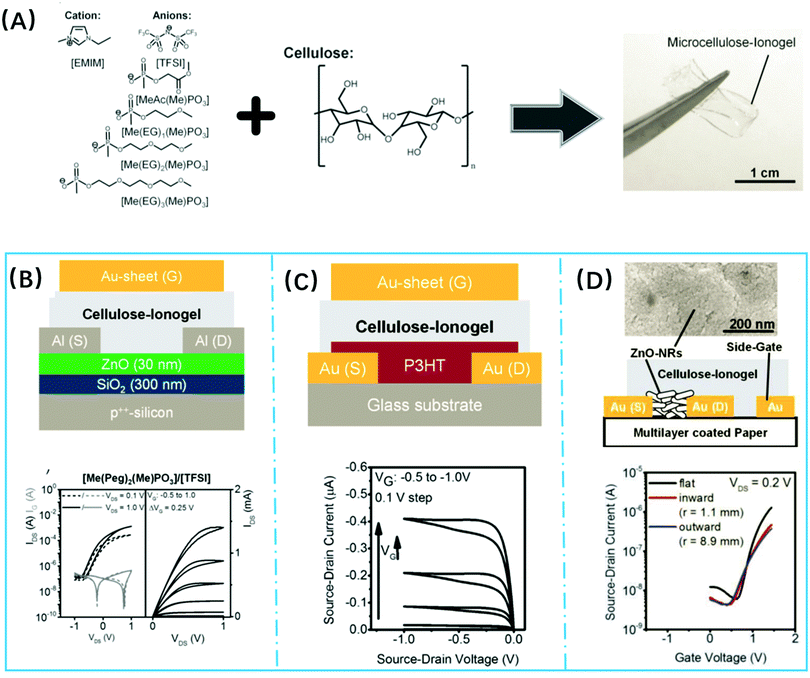
- J. M. Zhang, N. Luo, X. Y. Zhang, L. L. Xu, J. Wu, J. Yu, J. S. He and J. Zhang, ACS Sustainable Chem. Eng., 2016, 4, 4417–4423 CrossRef CAS
.
- V. L. Pushparaj, M. M. Shaijumon, A. Kumar, S. Murugesan, L. J. Ci, R. Vajtai, R. J. Linhardt, O. Nalamasu and P. M. Ajayan, Proc. Natl. Acad. Sci. U. S. A., 2007, 104, 13574–13577 CrossRef CAS PubMed
.
- S. Thiemann, S. J. Sachnov, F. Pettersson, R. Bollström, R. Österbacka, P. Wasserscheid and J. Zaumseil, Adv. Funct. Mater., 2014, 24, 625–634 CrossRef CAS
.
- N. Luo, Y. X. Lv, D. X. Wang, J. M. Zhang, J. Wu, J. S. He and J. Zhang, Chem. Commun., 2012, 48, 6283–6285 RSC
.
| This journal is © the Partner Organisations 2017 |