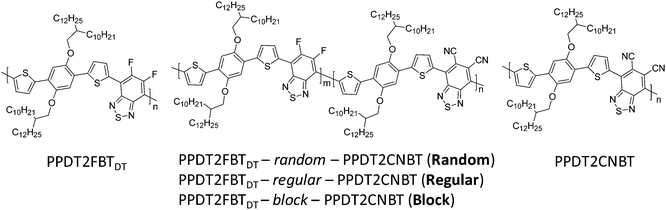
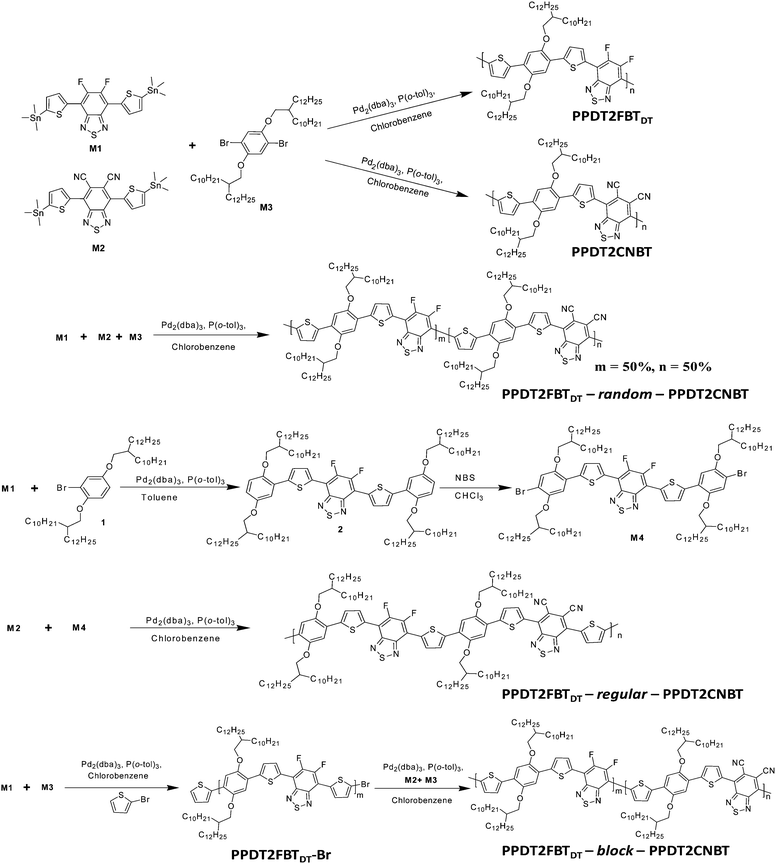
Synthesis and photovoltaic properties of three different types of terpolymers†
Mohammad Afsar
Uddin‡
ab,
Na Gyeong
An‡
 c,
Yuxiang
Li
ab,
Seyeong
Song
c,
Hwasook
Ryu
a,
Jin Young
Kim
*c and
Han Young
Woo
*a
c,
Yuxiang
Li
ab,
Seyeong
Song
c,
Hwasook
Ryu
a,
Jin Young
Kim
*c and
Han Young
Woo
*a
aDepartment of Chemistry, Korea University, Seoul 136-713, Republic of Korea. E-mail: hywoo@korea.ac.kr
bDepartment of Cogno-Mechatronics Engineering, Pusan National University, Miryang 627-706, Republic of Korea
cDepartment of Energy Engineering, Ulsan National Institute of Science and Technology (UNIST), Ulsan 44919, Republic of Korea. E-mail: jykim@unist.ac.kr
First published on 19th January 2017
Abstract
Three types of photoactive terpolymers (Random, Regular and Block) were synthesized by incorporating two electron-deficient moieties and one electron-rich moiety based on two parent donor polymers, poly[(2,5-bis(2-decyltetradecyloxy)phenylene)-alt-(5,6-difluoro-4,7-di(thiophen-2-yl)benzo[c][1,2,5]thiadiazole)] (PPDT2FBTDT) and poly[(2,5-bis(2-decyltetradecyloxy)phenylene)-alt-(5,6-dicyano-4,7-di(thiophen-2-yl)benzo[c][1,2,5]thiadiazole)] (PPDT2CNBT). All three terpolymers showed broad light absorption in the range of 300–850 nm covering the absorption of two parent polymers. Their optical, electrochemical, morphological and photovoltaic properties were compared and investigated in detail. Power conversion efficiencies (PCEs) of 5.63, 4.45 and 3.13% were obtained for PPDT2FBTDT-random-PPDT2CNBT, PPDT2FBTDT-regular-PPDT2CNBT and PPDT2FBTDT-block-PPDT2CNBT based photovoltaic devices, respectively. Through intensive investigation on charge carrier mobility, photocurrent and light intensity dependence of JSC, the random structure was proved to be the most effective molecular design in this series of terpolymers, showing weaker charge recombination and enhanced charge transport/extraction with well-distributed blend morphology. The fine modulation of different arrangements and compositions of the three monomers in a main chain is crucial for optimizing the terpolymeric structures as efficient photoactive materials.
Introduction
Recently, polymer solar cells (PSCs) have been attracting growing attention, demonstrating successfully their great potential as portable and flexible solar cells with several advantages such as room temperature solution processability on a plastic substrate and chemical versatility to fine-tune the electrical and optoelectronic properties.1–5 Typical bulk heterojunction (BHJ) PSCs consist of conjugated polymers as electron donor materials which function as major light harvesters while the fullerene derivatives (e.g., [6,6]-phenyl-C70 butyric acid methyl ester, PC70BM) act as electron acceptors.6–9 Molecular design via donor–acceptor (D–A) type alternating copolymers using intramolecular charge transfer (ICT) interactions is a straightforward and effective design strategy to tune the band gap of the resulting photovoltaic materials. Most of the highly efficient PSC devices have been achieved using D–A copolymer donor materials in conjunction with PC70BM derivatives.10–23 Unfortunately, most of the reported D–A type copolymers absorb a limited range of light and cover only a small portion of the solar spectrum, limiting photon harvesting. To resolve this issue, ternary blend devices where multiple donors (or acceptors) are used to harvest a broad range of UV-visible-near infrared light have been applied.24–31 A few successful examples have been reported to realize ternary systems. You et al. employed two donors in the ternary blend to form a polymer alloy and obtained a PCE of 10.5%.32 Recently, our group also reported ~10% PCE by incorporating a ternary component of poly[(2,5-bis(2-decyltetradecyloxy)phenylene)-alt-(5,6-dicyano-4,7-di(thiophen-2-yl)benzo[c][1,2,5]thiadiazole)] (PPDT2CNBT) with complementary absorption and energy levels and structural similarity into a binary blend of poly[(2,5-bis(2-hexyldecyloxy)phenylene)-alt-(5,6-difluoro-4,7-di(thiophen-2-yl)benzo[c][1,2,5]-thiadiazole)] (PPDT2FBT):PC70BM.33 However, the control of blend morphology of ternary systems is very difficult where the addition of a third component often disrupts optimized BHJ morphologies of the parent binary system and is likely to introduce charge recombination sites. Therefore, the increase in the short-circuit current density (JSC) and the fill factor (FF) is often far less than expected.Recently, terpolymers have emerged as an alternative molecular design strategy for photoactive polymers which comprise three components with two different donors and one acceptor (or one donor and two different acceptors) on their conjugated polymer backbones.34–49 Therefore, terpolymers allow broad light absorption via two different donor–acceptor ICT interactions with a distinct electronic structure. Several previous studies reported that random and regular terpolymers perform differently in BHJ PSC devices by forming different BHJ morphologies.35,44 Lee and coworkers synthesized a D1–A–D2–A terpolymer (PDTSTTBDT) incorporating dithieno[3,2-b:2′,3′-d]silole (DTS, D1), benzo[1,2-b:4,5-b]dithiophene (BDT, D2) and thieno[3,4-b]thiophene (TT, A) in a perfectly controlled manner and reported a remarkable enhancement of PCE in the regio-regular PDTSTTBDT-based PSCs due to the improved light absorption, effective polymer ordering, and high charge carrier mobility.35 Janssen et al. demonstrated successfully the potential of a random terpolymer of two electron deficient moieties and one electron rich unit (thienyl-substituted BDT), showing a very impressive PCE of ∼8%, higher than that of its binary parent polymers.36 Luo et al. also prepared a random terpolymer based on phenothiazine (PZT) and BDT cores in conjugation with an electron withdrawing benzo[c][1,2,5]thiadiazole (BT) unit and showed that the optimized terpolymer exhibits a longer photoluminescence (PL) lifetime (or exciton lifetime) and prominent charge transfer ability.37 You and coworkers synthesized regularly structured alternating and random terpolymers and these two terpolymers broadened the absorption and significantly reduced the aggregation signature compared to the parent D–A polymers.44 Although there have been several reports on terpolymeric PSCs, there have been no systematic previous approaches for the synthesis and characterization of different types of terpolymers (Random, Regular and Block) as active photoactive materials. Different arrangements of the donor–acceptor in a terpolymer backbone significantly influence their photophysical, electronic, morphological and resulting photovoltaic characteristics. A clear understanding of the molecular structure–property characteristics is necessary to further optimize the terpolymer-based PSC systems.
In this contribution, we report the design and synthesis of three types (Random, Regular and Block) of photovoltaic terpolymers based on the same constituting monomeric units of the two parent donor polymers, poly[(2,5-bis(2-decyltetradecyloxy)phenylene)-alt-(5,6-difluoro-4,7-di(thiophen-2-yl)benzo[c][1,2,5]thiadiazole)] (PPDT2FBTDT) and PPDT2CNBT. The three terpolymers showed broader light absorption than the two corresponding parent copolymers and their optical, electrochemical, morphological and photovoltaic properties were compared and investigated in detail.
Experimental section
Instruments and characterization
1H and 13C NMR spectra were recorded on a JEOL (JNM-AL300) FT NMR system operating at 300 MHz and 75 MHz, respectively. UV-Vis spectra were obtained using a JASCO V-630 spectrophotometer. The number- and weight-average molecular weights of polymers were determined by gel permeation chromatography (GPC) with o-dichlorobenzene as an eluent on an Agilent GPC 1200 series, relative to polystyrene standard. Cyclic voltammetry (CV) experiments were performed using a Versa STAT 3 analyzer. All CV measurements were carried out in 0.1 M tetrabutylammoniumtetrafluoroborate (Bu4NBF4) in acetonitrile with a conventional three-electrode configuration employing a platinum wire as a counter electrode, a platinum electrode coated with a thin polymer film as a working electrode, and a Ag/Ag+ electrode as a reference electrode (scan rate: 50 mV s−1). A dimension microscope (Veeco, USA) running with a Nanoscope V controller was used to obtain atomic force microscopy (AFM) surface images of polymer thin films. AFM images were measured in a high resolution tapping mode under ambient conditions. Silicon cantilevers (Bruker) with a resonant frequency of 300 kHz were used with a rotated tip to provide more symmetric representation of features over 200 nm. Grazing incidence wide angle X-ray scattering (GIWAXS) measurements were conducted at the PLS-II 9A U-SAXS beamline of Pohang Accelerator Laboratory, Pohang, Korea. Samples for GIWAXS measurements were prepared by spin-coating pristine polymers and polymer:PC70BM blend solutions on top of a silicon wafer. The interchain orientation was compared by measuring the (010) scattering intensity ratio (Iz/Ixy) in the z and xy directions for pristine and blend films.PSC fabrication and characterization
ITO coated glass substrates were washed with detergent and ultrasonicated in distilled water, acetone, and isopropyl alcohol for 10 min and dried overnight in an oven at 100 °C. Poly(3,4-ethylenedioxythiophene):polystyrene sulfonic acid (PEDOT:PSS) was deposited by spin-coating at 4000 rpm for 40 s on top of the ITO substrate and thermally annealed for 10 min at 140 °C in air. The samples were then transferred to a N2 filled glove box. The mixed solutions of polymer (1–1.5 wt%):PC70BM (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 (w/w) in chlorobenzene (CB)) were spin-coated on top of the PEDOT:PSS film. Subsequently, Al (∼100 nm) was thermally evaporated under high vacuum (<10−6 Torr), yielding devices with an active area of 13 mm2. Current density–voltage (J–V) characteristics of PSCs were measured using a Keithley 2365A source measurement unit. PSCs were characterized under AM 1.5 G conditions with an intensity of 100 mW cm−2 which was calibrated with a standard silicon photodiode (PV Measurements, Inc.). Incident photon to current efficiency (IPCE) spectra were measured using a QEX7 quantum efficiency measurement system (PV Measurements, Inc.) under ambient conditions. Spectral mismatch factors were found to be less than 9%. For space charge limited current (SCLC) measurements, hole-only (ITO/PEDOT:PSS/polymer:PC70BM/Au) and electron-only (FTO/polymer:PC70BM/Al; FTO, fluorine-doped tin oxide) devices were fabricated, respectively, under the same conditions for optimized devices. The charge mobilities were calculated by fitting the J–V characteristics of the single carrier diodes according to the Mott–Gurney relationship, JSCLC = (9/8) × (εrεoμ) × (Va2/L3), where εr is the dielectric constant of the material, εo is the vacuum permittivity, μ is the hole or electron mobility, L is the film thickness, and Va is the applied voltage. The film thickness was measured using a surface profiler (P6, KLA Tencor, USA).
1.5 (w/w) in chlorobenzene (CB)) were spin-coated on top of the PEDOT:PSS film. Subsequently, Al (∼100 nm) was thermally evaporated under high vacuum (<10−6 Torr), yielding devices with an active area of 13 mm2. Current density–voltage (J–V) characteristics of PSCs were measured using a Keithley 2365A source measurement unit. PSCs were characterized under AM 1.5 G conditions with an intensity of 100 mW cm−2 which was calibrated with a standard silicon photodiode (PV Measurements, Inc.). Incident photon to current efficiency (IPCE) spectra were measured using a QEX7 quantum efficiency measurement system (PV Measurements, Inc.) under ambient conditions. Spectral mismatch factors were found to be less than 9%. For space charge limited current (SCLC) measurements, hole-only (ITO/PEDOT:PSS/polymer:PC70BM/Au) and electron-only (FTO/polymer:PC70BM/Al; FTO, fluorine-doped tin oxide) devices were fabricated, respectively, under the same conditions for optimized devices. The charge mobilities were calculated by fitting the J–V characteristics of the single carrier diodes according to the Mott–Gurney relationship, JSCLC = (9/8) × (εrεoμ) × (Va2/L3), where εr is the dielectric constant of the material, εo is the vacuum permittivity, μ is the hole or electron mobility, L is the film thickness, and Va is the applied voltage. The film thickness was measured using a surface profiler (P6, KLA Tencor, USA).
Results and discussion
Design and synthesis of materials
Two parent polymeric structures PPDT2FBTDT and PPDT2CNBT33 with complementary light absorption were considered to synthesize three different types of terpolymers and investigate the structure–property relationships for terpolymer based PSCs. To attach the same side-chains on both the structures, the longer alkyl chain 2-decyltetradecyl (DT) groups were incorporated into the same backbone of PPDT2FBT (with 2-hexyldecyl side chains) which was reported previously.13 The final polymer structures and synthetic routes to the monomer and final polymers are described in Schemes 1 and 2. The detailed synthetic procedures can be found in the ESI.†1-Bromo-2,5-bis(2-decyltetradecyloxy)benzene (1),50 4,7-bis(5-trimethylstannylthiophen-2-yl)-5,6-difluoro-2,1,3-benzothiadiazole (M1),13 4,7-bis(5-trimethylstannylthiophen-2-yl)-5,6-dicyano-2,1,3-benzothiadiazole (M2),33 1,4-dibromo-2,5-bis(2-decyltetradecyloxy)benzene (M3),33 PPDT2FBTDT and PPDT2CNBT33 were synthesized by following the previous reports. Monomer M4 was synthesized by the Stille cross-coupling reaction between compound 1 and M1 and successive bromination with N-bromosuccinimide in 65% yield. The random terpolymer (PPDT2FBTDT-random-PPDT2CNBT, Random) was prepared via the Stille cross-coupling of M1, M2 and M3 (mole ratio of 0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5
0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) in chlorobenzene with tris(dibenzylideneacetone)dipalladium(0) (3 mol%) and tri(o-tolyl)phosphine (8 mol%) as catalysts using a microwave reactor. A Regular terpolymer (PPDT2FBTDT-regular-PPDT2CNBT, Regular) was synthesized by the Stille cross-coupling reaction of M2 with M4 (molar ratio of 1
1) in chlorobenzene with tris(dibenzylideneacetone)dipalladium(0) (3 mol%) and tri(o-tolyl)phosphine (8 mol%) as catalysts using a microwave reactor. A Regular terpolymer (PPDT2FBTDT-regular-PPDT2CNBT, Regular) was synthesized by the Stille cross-coupling reaction of M2 with M4 (molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). A Block copolymer (or terpolymer) (PPDT2FBTDT-block-PPDT2CNBT, Block) was synthesized using an end-capping method where a two-step synthesis approach involving the Stille cross-coupling polymerization of M1 and M3 for the preparation of Br-terminated PPDT2FBTDT by end capping with excessive 2-bromothiophene followed by the Stille cross-coupling polycondensation of M2 and M3 for preparation of the PPDT2CNBT block was used.51 The PPDT2FBTDT-block-PPDT2CNBT formation was roughly estimated by measuring the increase in the molecular weight before and after incorporating the PPDT2CNBT block by GPC analysis (Mn: 20 → 28 KDa). However, there must be a chance to form the PPDT2CNBT homopolymer as well as the block copolymer. We could not exclude the possibility of formation of PPDT2CNBT during the synthesis of PPDT2FBTDT-block-PPDT2CNBT at the present stage. The polymerization yield was 65–73% and the number-average molecular weight was measured to be 28–34 KDa by GPC (Table 1).
1). A Block copolymer (or terpolymer) (PPDT2FBTDT-block-PPDT2CNBT, Block) was synthesized using an end-capping method where a two-step synthesis approach involving the Stille cross-coupling polymerization of M1 and M3 for the preparation of Br-terminated PPDT2FBTDT by end capping with excessive 2-bromothiophene followed by the Stille cross-coupling polycondensation of M2 and M3 for preparation of the PPDT2CNBT block was used.51 The PPDT2FBTDT-block-PPDT2CNBT formation was roughly estimated by measuring the increase in the molecular weight before and after incorporating the PPDT2CNBT block by GPC analysis (Mn: 20 → 28 KDa). However, there must be a chance to form the PPDT2CNBT homopolymer as well as the block copolymer. We could not exclude the possibility of formation of PPDT2CNBT during the synthesis of PPDT2FBTDT-block-PPDT2CNBT at the present stage. The polymerization yield was 65–73% and the number-average molecular weight was measured to be 28–34 KDa by GPC (Table 1).
| Polymer | M n (Mw)a [kg mol−1] | λ abs (in solution) (nm) | λ abs (in film) (nm) | λ onset (nm) | E optg (eV) | HOMOb (eV) | LUMOc (eV) |
|---|---|---|---|---|---|---|---|
| a Number-average molecular weight (Mn) and weight-average molecular weight (Mw) determined by GPC with o-dichlorobenzene as the eluent at 80 °C. b HOMO level was estimated from the tangential onset of oxidation (Eox) by cyclic voltammetry. HOMO (eV) = −(Eox − E1/2(Fc/Fc+) + 4.8). c LUMO level was estimated from the HOMO value and the corresponding optical band gap. | |||||||
| PPDT2FBTDT | 31 (62) | 638 | 649 | 714 | 1.74 | −5.37 | −3.63 |
| Random | 30 (77) | 643 | 653 | 843 | 1.47 | −5.56 | −4.09 |
| Regular | 34 (79) | 664 | 714 | 836 | 1.48 | −5.60 | −4.12 |
| Block | 28 (73) | 630 | 643 | 860 | 1.44 | −5.43 | −3.99 |
| PPDT2CNBT | 28 (62) | 685 | 737 | 838 | 1.48 | −5.67 | −4.19 |
Optical and electrochemical properties
UV-Vis absorption spectra of three terpolymers and their parent D–A copolymers (PPDT2FBTDT and PPDT2CNBT) in chloroform and in films are shown in Fig. 1(a) and (b). Random, Regular and Block show broad absorption covering a range of 350–850 nm, where a peak at ∼400 nm corresponds to π–π* transition and the longer wavelength absorption is related to ICT interaction. The absorption of the three terpolymers covers the sum of absorption of the two parent copolymers PPDT2FBTDT and PPDT2CNBT. A comparison of the spectra in films and in solution indicates a clear red-shift in films for all polymers, suggesting enhanced intermolecular π–π interactions.47,49 However, the detailed peak shape and structure are different for the three terpolymers. Random and Regular show similar spectra in chloroform, but the spectra in films show a clearly different absorption maximum wavelength (λabs). In Block, the relatively weak absorption at ∼750 nm (originating from PPDT2CNBT absorption) is probably due to the shorter chain length of the PPDT2CNBT block in Block, which is consistent with the measured GPC data before and after incorporation of the PPDT2CNBT block.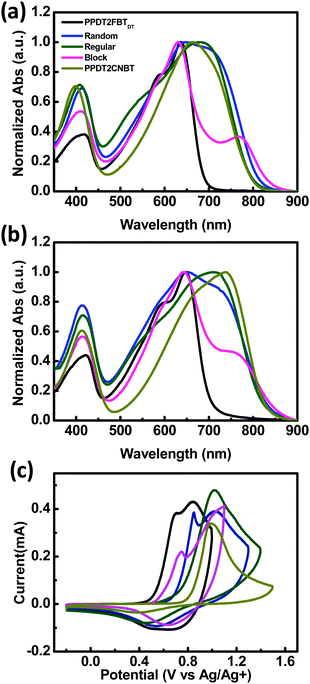 | ||
| Fig. 1 Normalized UV-Vis absorption spectra in chloroform (a) and in the film (b) and cyclic voltammograms (c) of polymers. | ||
The highest occupied molecular orbital (HOMO) energy levels of the polymers were determined by CV measurements and the lowest unoccupied molecular orbital (LUMO) energy levels were estimated from the optical band gap and the HOMO energy level. The HOMO and LUMO energy levels of polymers are summarized in Table 1, and their cyclic voltammograms are shown in Fig. 1(c). In the anodic scan, the oxidation onsets relative to an internal standard, ferrocene (Fc+/Fc), were determined and the corresponding HOMO levels were determined to be −5.37, −5.56, −5.60, −5.43, and −5.67 eV for PPDT2FBTDT, Random, Regular, Block, and PPDT2CNBT, respectively. The HOMO levels of the three terpolymers are between those of PPDT2FBTDT and PPDT2CNBT.
Photovoltaic properties
The photovoltaic devices were fabricated using a conventional architecture of ITO/PEDOT:PSS/polymer:PC70BM/Al. A series of devices were tested by changing the blend ratio, thickness of the active layer, solvent and processing additives. Finally, the optimized BHJ PSCs using PPDT2FBTDT, Random, Regular, Block and PPDT2CNBT as a donor and PC70BM as an acceptor were fabricated by spin-coating the blend solutions (polymer:PC70BM = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
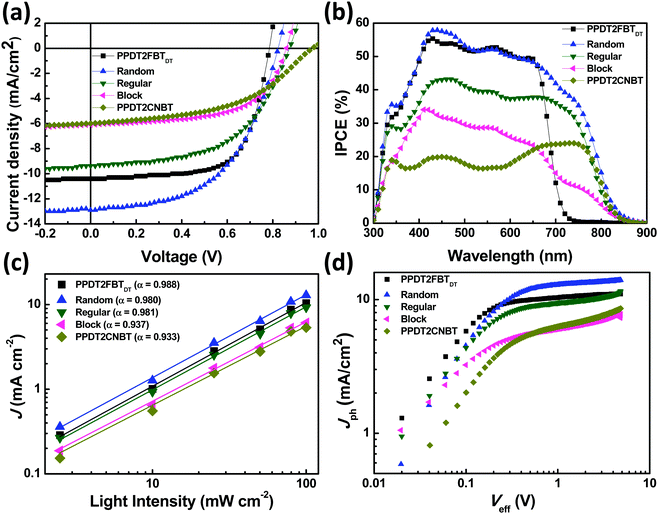
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 (w/w)) in chlorobenzene (CB) solvent and 3 vol% diphenyl ether (DPE) as a processing additive. J–V characteristics and the corresponding IPCE spectra of the devices for the five polymers are shown in Fig. 2(a) and (b) and the resulting photovoltaic parameters (open-circuit voltage (VOC), JSC, and FF) are summarized in Table 2.
1.5 (w/w)) in chlorobenzene (CB) solvent and 3 vol% diphenyl ether (DPE) as a processing additive. J–V characteristics and the corresponding IPCE spectra of the devices for the five polymers are shown in Fig. 2(a) and (b) and the resulting photovoltaic parameters (open-circuit voltage (VOC), JSC, and FF) are summarized in Table 2.
| Polymer | J SC [mA cm−2] | Cal. JSC [mA cm−2] | V OC [V] | FF | PCE [%] |
|---|---|---|---|---|---|
| PPDT2FBTDT | 10.40 | 9.92 | 0.79 | 0.67 | 5.45 |
| Random | 12.90 | 12.69 | 0.82 | 0.53 | 5.63 |
| Regular | 9.38 | 9.81 | 0.87 | 0.54 | 4.45 |
| Block | 6.10 | 6.25 | 0.86 | 0.60 | 3.13 |
| PPDT2CNBT | 5.99 | 5.50 | 0.97 | 0.48 | 2.80 |
The observed VOCs are 0.79, 0.82, 0.87, 0.86, and 0.97 V for the binary blends of PPDT2FBTDT, Random, Regular, Block and PPDT2CNBT with PC70BM, respectively. Replacement of fluorine (F) with nitrile (CN) groups on the benzothiadiazole (BT) units hence induces the increase in VOC for devices of Random, Regular and Block compared to the PPDT2FBTDT device. However, the extent of enhancement is different for each polymer, derived from the specific arrangement of two different BT units (difluoro and dicyano substituted). The resulting PCE was measured to be 5.45, 5.63, 4.45, 3.13 and 2.80% for PPDT2FBTDT, Random, Regular, Block and PPDT2CNBT devices, respectively.
The IPCE responses to the incident irradiance cover a broad range of 300–850 nm for all polymers except for PPDT2FBTDT, which are well matched with their UV-Vis absorption spectra. All JSCs obtained from the J–V characterization agree well with the calculated JSCs integrated from IPCE spectra (within 9% error). Random shows the highest quantum efficiency with a maximum of 60% near 450 nm. The IPCE spectrum of Block shows a low current generation over 700 nm. The spectral characteristic suggests the poor photovoltaic performance of PPDT2CNBT compared to PPDT2FBTDT. In addition, the smaller chain length of the PPDT2CNBT block in Block may also contribute to the smaller IPCE over 700 nm.
Charge transport and extraction analysis
Analysis of vertical charge transport in polymer:PC70BM films provides a detailed understanding of photovoltaic characteristics. The electron and hole mobilities (μe and μh) were measured using the SCLC technique.52 Detailed electron-only and hole-only device fabrication and measurement conditions are described in the Experimental section. J–V curves of electron-only and hole-only diodes are plotted in Fig. S1 (ESI†) and the calculated μe and μh with mobility ratios (μe/μh) are summarized in Table S1 (ESI†). The measured μes of polymers are in the range of 8.67 × 10−4–2.37 × 10−3 cm2 V−1 s−1, where Block showed relatively lower electron mobility than the other polymers. μh of 3.56 × 10−5 to 1.15 × 10−4 cm2 V−1 s−1 was measured and the resulting μe/μh values are in the range of 10.4–33.7. μe/μh for PPDT2FBTDT is 10.4 and that for PPDT2CNBT is 33.7.To gain insight into the charge carrier transport and recombination characteristics of the PSC devices of the 5 different polymers, we also measured the light intensity dependent JSC at various illumination light intensities in the range of 2.5–100 mW cm−2, from which information regarding bimolecular recombination and space charge accumulation can be obtained. The JSC curve can be fitted according to a power-law, eqn (1), where I is the light intensity and α indicates a fitting parameter or the slope of a logarithmic plot of JSCversus light intensity.53,54
| JSC ∝ Iα | (1) |
To characterize the charge generation and extraction properties, we studied the photocurrent (Jph) versus effective voltage (Veff) characteristics. The Jph is defined as Jph = JL − JD, where JL and JD are the current density under illumination (100 mW cm−2) and in the dark, respectively. Veff is defined as V0 − Va, where V0 is the built-in voltage, which corresponds to the voltage at Jph = 0, and Va is the applied bias. Fig. 2(d) displays a logarithmic relationship of Jph with respect to Veff for the binary blends of five polymers. The Random blend shows the highest Jph whereas Block and PPDT2CNBT exhibited substantially low Jph. With increasing Veff (>1 V), the photocurrent becomes gradually saturated for PPDT2FBTDT, Random and Regular binary blends, but Block and PPDT2CNBT blends show no saturation region, which shows a good agreement with the above mobility and light intensity dependent JSC data. The saturated photocurrent density (Jsat) is approximately given by Jsat = qLGmax, where q is the elementary charge, L is the thickness of the active layer, and Gmax is the maximum photo-induced carrier generation rate per unit volume. When the excitons are photo-generated, only a part can be dissociated into the free charge carriers, which can be estimated from charge dissociation probability (PD). The PD value is calculated as JSC/Jsat, where JSC = qLGmaxPD under short circuit conditions.55 The PD values are roughly estimated to be 94.5, 97.7 and 94.0%, for PPDT2FBTDT, Random and Regular, respectively. In addition, the charge extraction probability (PE) indicates the extraction efficiency of the separated charge carriers at the electrodes. The PE can be defined as JMP/Jsat, where JMP is the current density under maximum power conditions.56 The higher PE was calculated to be 81.5% for PPDT2FBTDT and 74.9% for Random, compared to Regular (PE of 63.5%).
According to the calculated PD and PE values, the terpolymers show relatively good charge separation/generation, but the charge extraction is probably hindered with significant recombination especially for the Block system. PPDT2FBTDT and Random devices showed higher charge extraction than other terpolymers with suppressed recombination, which may induce a higher JSC (>10 mA cm−2) and FF in the PSCs.
Morphological analysis
To explore intermolecular packing and orientation in pristine polymer and polymer:PC70BM blend films, GIWAXS characteristics were investigated (Fig. 3). Detailed GIWAXS line-cuts and packing parameters are also summarized in Fig. S2 and Table S2 (ESI†). As shown in Fig. 3, five pristine polymers show multi-diffractions, indicating the bimodal molecular packing along both in-plane (qxy) and out-of-plane (qz) directions. Clear (010) π–π stacking peaks were also observed in the out-of-plane direction for all polymers with a d-spacing of 3.6–3.7 Å (Table S2, ESI†). The π–π stacking was found to be more ordered in the qz direction for PPDT2FBTDT and Random compared to Regular, Block and PPDT2CNBT where the hump-shaped (010) scatterings were observed due to randomly packed inter-chain structures. This pronounced face-on π–π stacking in PPDT2FBTDT and Random supports efficient charge transport in the vertical direction in PSC devices. By blending with PC70BM (Fig. 3(f–j)), the strong (010) scattering was also observed in the qz direction for PPDT2FBTDT and Random, but the (010) peak intensity was measured to decrease substantially for other terpolymers and PPDT2CNBT. In addition, a broad hump peak in all blend films was observed originating from PC70BM aggregates at q = 1.2–1.4 Å−1. Furthermore, the orientation ratio was also estimated by comparing the maximum intensity ratio (Iz/Ixy) of the π–π stacking (010) scatterings along the qz and qxy directions for both pristine and blend films. The PPDT2FBTDT and Random pristine films showed the highest Iz/Ixy ratio (maximum value over ∼4). It is noted that both PPDT2FBTDT and Random tend to arrange strongly in the face-on fashion relative to other polymers. The orientation ratio, Iz/Ixy, in the blend films was also calculated similarly: 2.7 for Random, 2.3 for PPDT2FBTDT, and 1–1.5 for the other three polymers, respectively. A dominant face-on orientation is considered to be advantageous for efficient charge transport in a vertical direction in diode structures such as photovoltaics.57–59 The relative inter-chain orientation in the blends is well consistent with the measured JSC values: 12.90 mA cm−2 and 10.40 mA cm−2 for Random and PPDT2FBTDT based PSCs, respectively.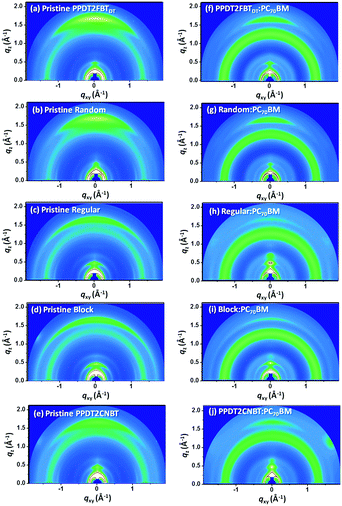 | ||
| Fig. 3 2D GIWAXS images of (a–e) pristine polymer films and (f–j) polymer:PC70BM blend films. The blend films were prepared under the same conditions for the optimal PSC devices. | ||
The surface film morphology of polymer:PC70BM BHJ blends on top of a glass/ITO/PEDOT:PSS substrate was also studied by tapping mode AFM. Height and phase images with root mean square (RMS) roughness are shown for binary blends of five polymers in Fig. 4. Random exhibited a smooth and homogeneous surface with the smallest RMS roughness of 2.32 nm. While PPDT2FBTDT and Regular blends displayed intermediate roughness (3.39–3.64 nm), Block and PPDT2CNBT possessed the roughest surface with RMS roughness of 6.11 and 4.29 nm, respectively. The surface roughness was significantly influenced by the different manners of arrangements of the three monomers in the terpolymeric structures. In Fig. 4(f–j), phase images also revealed a similar trend. The detailed inter-chain ordering, surface morphology and the charge transport/recombination characteristics show good agreement with the higher photovoltaic properties (i.e., JSC and FF) of Random and PPDT2FBTDT polymers.
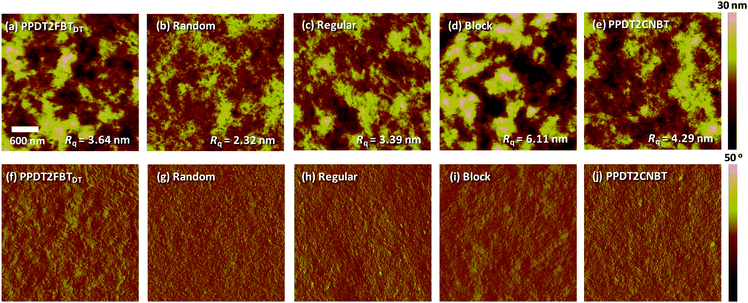 | ||
| Fig. 4 AFM (a–e) height and (f–j) their corresponding phase images of polymer:PC70BM blend films. Each film was prepared on a glass/ITO/PEDOT:PSS substrate. The size of all images is 3 μm × 3 μm. | ||
Conclusions
In summary, we synthesized a series of terpolymers (Random, Regular and Block) based on dithienylphenylene, difluorobenzothiadiazole and dicyanobenzothiadiazole moieties, and studied their optical, electrochemical, morphological, electrical and photovoltaic properties. The terpolymers show a broad light absorption covering all absorption ranges of parent two copolymers (PPDT2FBTDT and PPDT2CNBT). The fine structures in the absorption, film morphology, electronic structure and the resulting electrical properties are significantly dependent on different manners of arrangements of three moieties in a polymeric backbone. A combination of SCLC modeling, photocurrent and light intensity dependence of JSC data made possible the quantitative analysis of the difference in charge generation, transport and extraction in different terpolymer blends. Through thin film characterization using GIWAXS and AFM, the morphological properties of each polymer were found to be closely correlated to photovoltaic properties. Through these intensive investigations, the random terpolymer (Random) was proved to be the most effective molecular design in this series of terpolymers to show a weaker charge recombination and enhanced charge transport/extraction (compared to other terpolymers), showing well-distributed blend morphology with the highest PCE of 5.63%. Further optimization of terpolymer structures (by changing the composition, etc.) may reveal the great potential of terpolymers as an efficient light harvesting material compared to D–A copolymers.Acknowledgements
This work was supported by the National Research Foundation (NRF) of Korea (2015R1A2A1A15055605, 2015M1A2A2057506, 2015R1D1A1A09056905, and 20100020209).References
- N. Sariciftci, L. Smilowitz, A. J. Heeger and F. Wudl, Science, 1992, 258, 1474–1476 CAS.
- G. Yu, J. Gao, J. C. Hummelen, F. Wudl and A. J. Heeger, Science, 1995, 270, 1789–1791 CAS.
- M. C. Scharber and N. S. Sariciftci, Prog. Polym. Sci., 2013, 38, 1929–1940 CrossRef CAS PubMed.
- J. S. Wu, S. W. Cheng, Y. J. Cheng and C. S. Hsu, Chem. Soc. Rev., 2015, 44, 1113–1154 RSC.
- Y. Lin, Y. Li and X. Zhan, Chem. Soc. Rev., 2012, 41, 4245–4272 RSC.
- C. Cabanetos, A. E. Labban, J. A. Bartelt, J. D. Douglas, W. R. Mateker, J. M. J. Fréchet, M. D. McGehee and P. M. Beaujuge, J. Am. Chem. Soc., 2013, 135, 4656–4659 CrossRef CAS PubMed.
- Z. He, C. Zhong, S. Su, M. Xu, H. Wu and Y. Cao, Nat. Photonics, 2012, 6, 591–595 Search PubMed.
- L. Yang, J. R. Tumbleston, H. Zhou, H. Ade and W. You, Energy Environ. Sci., 2013, 6, 316–326 CAS.
- B. C. Thompson and J. M. J. Fréchet, Angew. Chem., Int. Ed., 2008, 47, 58–77 CrossRef CAS PubMed.
- Y. Liu, J. Zhao, Z. Li, C. Mu, W. Ma, H. Hu, K. Jiang, H. Lin, H. Ade and H. Yan, Nat. Commun., 2014, 5, 5293 CrossRef CAS PubMed.
- Y.-J. Cheng, S.-H. Yang and C.-S. Hsu, Chem. Rev., 2009, 109, 5868–5923 CrossRef CAS PubMed.
- P. M. Beaujuge and J. M. J. Fréchet, J. Am. Chem. Soc., 2011, 133, 20009–20029 CrossRef CAS PubMed.
- T. L. Nguyen, H. Choi, S.-J. Ko, M. A. Uddin, B. Walker, S. Yum, J.-E. Jeong, M. H. Yun, T. J. Shin, S. Hwang, J. Y. Kim and H. Y. Woo, Energy Environ. Sci., 2014, 7, 3040–3051 CAS.
- G. Li, R. Zhu and Y. Yang, Nat. Photonics, 2012, 6, 153–161 CrossRef CAS.
- Y. Li, Acc. Chem. Res., 2012, 45, 723–733 CrossRef CAS PubMed.
- H. Zhou, L. Yang and W. You, Macromolecules, 2012, 45, 607–632 CrossRef CAS.
- X. Guo, M. Baumgarten and K. Müllen, Prog. Polym. Sci., 2013, 38, 1832–1908 CrossRef CAS.
- J. You, L. Dou, Z. Hong, G. Li and Y. Yang, Prog. Polym. Sci., 2013, 38, 1909–1928 CrossRef CAS.
- K. Müllen and W. Pisula, J. Am. Chem. Soc., 2015, 137, 9503–9505 CrossRef PubMed.
- L. Liu, G. Zhang, B. He, S. Liu, C. Duan and F. Huang, Mater. Chem. Front., 2017 10.1039/c6qm00130k.
- W. Lee, H. Choi, S. Hwang, J. Y. Kim and H. Y. Woo, Chem. – Eur. J., 2012, 18, 2551–2558 CrossRef CAS PubMed.
- M. A. Uddin, T. H. Lee, S. Xu, S. Y. Park, T. Kim, S. Song, T. L. Nguyen, S.-J. Ko, S. Hwang, J. Y. Kim and H. Y. Woo, Chem. Mater., 2015, 27, 5997–6007 CrossRef CAS.
- Y. Li, S.-J. Ko, S. Y. Park, H. Choi, T. L. Nguyen, M. A. Uddin, T. Kim, S. Hwang, J. Y. Kim and H. Y. Woo, J. Mater. Chem. A, 2016, 4, 9967–9976 CAS.
- J. Y. Kim, K. Lee, N. E. Coates, D. Moses, T.-Q. Nguyen, M. Dante and A. J. Heeger, Science, 2007, 317, 222–225 CrossRef CAS PubMed.
- H. Zhou, Y. Zhang, C.-K. Mai, S. D. Collins, G. C. Bazan, T.-Q. Nguyen and A. J. Heeger, Adv. Mater., 2015, 27, 1767–1773 CrossRef CAS PubMed.
- J. Zhang, Y. Zhang, J. Fang, K. Lu, Z. Wang, W. Ma and Z. Wei, J. Am. Chem. Soc., 2015, 137, 8176–8183 CrossRef CAS PubMed.
- J. You, L. Dou, K. Yoshimura, T. Kato, K. Ohya, T. Moriarty, K. Emery, C.-C. Chen, J. Gao, G. Li and Y. Yang, Nat. Commun., 2013, 4, 1446 CrossRef PubMed.
- Q. An, F. Zhang, J. Zhang, W. Tang, Z. Deng and B. Hu, Energy Environ. Sci., 2016, 9, 281–322 Search PubMed.
- S.-J. Ko, W. Lee, H. Choi, B. Walker, S. Yum, S. Kim, T. J. Shin, H. Y. Woo and J. Y. Kim, Adv. Energy Mater., 2015, 5, 1401687 CrossRef.
- L. Xiao, K. Gao, Y. Zhang, X. Chen, L. Hou, Y. Cao and X. Peng, J. Mater. Chem. A, 2016, 4, 5288–5293 CAS.
- L. Lu, T. Xu, W. Chen, E. S. Landry and L. Yu, Nat. Photonics, 2014, 8, 716–722 CrossRef CAS.
- J. Zhang, Y. Zhang, J. Fang, K. Lu, Z. Wang, W. Ma and Z. Wei, J. Am. Chem. Soc., 2015, 137, 8176–8183 CrossRef CAS PubMed.
- T. H. Lee, M. A. Uddin, C. Zhong, S.-J. Ko, B. Walker, T. Kim, Y. J. Yoon, S. Y. Park, A. J. Heeger, H. Y. Woo and J. Y. Kim, Adv. Energy Mater., 2016, 1600637 CrossRef.
- B. Fan, X. Xue, X. Meng, X. Sun, L. Huo, W. Ma and Y. Sun, J. Mater. Chem. A, 2016, 4, 13930–13937 CAS.
- H. Heo, H. Kim, D. Lee, S. Jang, L. Ban, B. Lim, J. Lee and Y. Lee, Macromolecules, 2016, 49, 3328–3335 CrossRef CAS.
- C. Duan, K. Gao, J. J. van Franeker, F. Liu, M. M. Wienk and R. A. J. Janssen, J. Am. Chem. Soc., 2016, 138, 10782–10785 CrossRef CAS PubMed.
- Q. Li, X. Jin, Y. Song, Q. Zhang, Z. Xu, Z. Chen, Y. Cheng and X. Luo, ACS Appl. Mater. Interfaces, 2015, 7, 17408–17415 Search PubMed.
- W. Li, K. H. Hendriks, A. Furlan, A. Zhang, M. M. Wienk and R. A. Janssen, Chem. Commun., 2015, 51, 4290–4293 RSC.
- M. L. Keshtov, S. A. Kuklin, D. Y. Godovsky, A. R. Khokhlov, R. Kurchania, F. C. Chen, E. N. Koukaras and G. D. Sharma, J. Polym. Sci., Part A: Polym. Chem., 2016, 54, 155–168 CrossRef CAS.
- Y. Lee and E. D. Gomez, Macromolecules, 2015, 48, 7385–7395 CrossRef CAS.
- C. Guo, Y. Lee, Y.-H. Lin, J. Strzalka, C. Wang, A. Hexemer, C. Jaye, D. A. Fischer, R. Verduzco, Q. Wang and E. D. Gomez, Macromolecules, 2016, 49, 4599–4608 CrossRef CAS.
- P. D. Homyak, Y. Liu, J. D. Harris, F. Liu, K. R. Carter, T. P. Russell and E. B. Coughlin, Macromolecules, 2016, 49, 3028–3037 CrossRef CAS.
- Y.-H. Lin, K. A. Smith, C. N. Kempf and R. Verduzco, Polym. Chem., 2013, 4, 229–232 RSC.
- Q. Zhang, M. A. Kelly, A. Hunt, H. Ade and W. You, Macromolecules, 2016, 49, 2533–2540 CrossRef CAS.
- E. Y. Ko, G. E. Park, D. H. Lee, H. A. Um, J. Shin, M. Ju Cho and D. H. Choi, ACS Appl. Mater. Interfaces, 2015, 7, 28303–28310 Search PubMed.
- K. Nakabayashi and H. Mori, Materials, 2014, 7, 3274–3290 CrossRef CAS.
- T. E. Kang, H.-H. Cho, H. J. Kim, W. Lee, H. Kang and B. J. Kim, Macromolecules, 2013, 46, 6806–6813 CrossRef CAS.
- J. Qi, J. Han, X. Zhou, D. Yang, J. Zhang, W. Qiao, D. Ma and Z. Y. Wang, Macromolecules, 2015, 48, 3941–3948 CrossRef CAS.
- K.-H. Kim, H. Yu, H. Kang, D. J. Kang, C.-H. Cho, H.-H. Cho, J. H. Oh and B. J. Kim, J. Mater. Chem. A, 2013, 1, 14538–14547 CAS.
- A. Yokoyama, A. Kato, R. Miyakoshi and T. Yokozawa, Macromolecules, 2008, 41, 7271–7273 CrossRef CAS.
- J. W. Mok, Y.-H. Lin, K. G. Yager, A. D. Mohite, W. Nie, S. B. Darling, Y. Lee, E. Gomez, D. Gosztola, R. D. Schaller and R. Verduzco, Adv. Funct. Mater., 2015, 25, 5578–5585 CrossRef CAS.
- V. D. Mihailetchi, J. Wildeman and P. W. M. Blom, Phys. Rev. Lett., 2005, 94, 126602 CrossRef CAS PubMed.
- L. J. A. Koster, V. D. Mihailetchi, H. Xie and P. W. M. Blom, Appl. Phys. Lett., 2005, 87, 203502 CrossRef.
- C. M. Proctor, M. Kuik and T. Q. Nguyen, Prog. Polym. Sci., 2013, 38, 1941–1960 CrossRef CAS.
- A. K. K. Kyaw, D. H. Wang, D. Wynands, J. Zhang, T.-Q. Nguyen, G. C. Bazan and A. J. Heeger, Nano Lett., 2013, 13, 3796–3801 CrossRef CAS PubMed.
- G. Kim, S. Song, J. Lee, T. Kim, T. H. Lee, B. Walker, J. Y. Kim and C. Yang, Adv. Energy Mater., 2015, 5, 1500844 CrossRef.
- J. A. Bartelt, Z. M. Beiley, E. T. Hoke, W. R. Mateker, J. D. Douglas, B. A. Collins, J. R. Tumbleston, K. R. Graham, A. Amassian, H. Ade, J. M. J. Fréchet, M. F. Toney and M. D. McGehee, Adv. Energy Mater., 2013, 3, 364–374 CrossRef CAS.
- J. Rivnay, R. Steyrleuthner, L. H. Jimison, A. Casadei, Z. Chen, M. F. Toney, A. Facchetti, D. Neher and A. Salleo, Macromolecules, 2011, 44, 5246–5255 CrossRef CAS.
- Y. Yang, W. Chen, L. T. Dou, W. H. Chang, H. S. Duan, B. Li, G. Bob and Y. Yang, Nat. Photonics, 2015, 9, 190–198 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: Synthesis and characterization of intermediates, SCLC measurements, GIWAXS line-cut profiles and packing parameters. See DOI: 10.1039/c6qm00267f |
| ‡ These authors contributed equally. |
| This journal is © the Partner Organisations 2017 |