Graphene papers: smart architecture and specific functionalization for biomimetics, electrocatalytic sensing and energy storage
Minwei
Zhang†
a,
Chengyi
Hou†
ab,
Arnab
Halder
a,
Hongzhi
Wang
*b and
Qijin
Chi
 *a
*a
aDepartment of Chemistry, Technical University of Denmark, DK-2800, Kongens Lyngby, Denmark. E-mail: cq@kemi.dtu.dk
bState Key Laboratory for Modification of Chemical Fibers and Polymer Materials, College of Materials Science and Engineering, Donghua University, Shanghai 201620, P. R. China. E-mail: wanghz@dhu.edu.cn
First published on 29th September 2016
Abstract
Paper is an attractively assembled form of materials and has accompanied our daily life almost everywhere. Two-dimensional layered materials, especially graphene, have unique intrinsic structures to be exploited for smart architecture of macroscopic papers that are offering many newly emerging applications. Research advances in graphene based papers in the past few years have created a new category of composite materials. This review aims at offering an up-to-date comprehensive summary of graphene-supported papers, with the emphasis on smart assembly and purpose-driven specific functionalization for their critical applications associated with sensing, environmental and energy technologies. The contents of this review are based on a balance combination of our own studies and selected research studies done by worldwide academic groups. We first give a brief introduction to graphene as a versatile building block and to the current status of research studies on graphene papers. This is followed by addressing some crucial methods of how to prepare graphene papers. We then summarize multiple possibilities of functionalizing graphene papers, membranes or films. Finally, we evaluate some key applications of graphene papers in the areas of chemical/electrochemical sensors, biomimetics and energy storage devices, just before leading to our concluding remarks and perspectives.
1. Introduction
Graphene has attracted much attention from researchers, from physicists, chemists to material scientists in the past decade. It is a material structurally characterized as a two-dimensional (2D) sheet of carbon atoms arranged in a hexagonal configuration with atoms bonded by sp2 bonds. In 2004, Geim, Novoselov and co-workers at the University of Manchester first reported that graphene nanosheets exfoliated from bulk graphite using Scotch tape can stably exist and can be studied for their unique electronic and optical properties.1 Although an apparently rigid 2D material, graphene is not completely flat, but rather exhibits ripples that even provide the possibility for confining nanoparticles and molecules. In the case of graphene oxide (GO), and its reduced form (reduced graphene oxide, rGO), the structure is even more complicated. Owing to the oxygenated functional groups existing on both the basal planes and at their edges, GO (or rGO) has a hybrid structure comprised of a mixture of sp2 and sp3 hybridized carbon atoms.2,3Several methods have been developed for the preparation of graphene, such as peeling-off graphite, liquid phase exfoliation,4–6 chemical vapour deposition (CVD),7–9 wet-chemical reduction of GO,10–12 graphitization of silicon carbide,13 unzipping of carbon nanotubes,14,15 and bottom-up organic synthesis.16,17 These methods, all of which produce graphene with different size, shape, chemical composition, and environment, have different requirements for functionalization. Since 2004, graphene has been studied for its potential applications in electronics, energy storage and conversion (e.g. supercapacitors, batteries, fuel cells, and solar cells), and bioscience/biotechnologies because of its unique physicochemical properties such as high surface area (theoretically 2630 m2 g−1 for single-layer graphene sheets), excellent thermal and electrical conductivity, and robust mechanical strength.
The mass production of high-quality GO has been achieved by chemical oxidation and exfoliation of low-cost graphite, based on the modified Hummers' method, and a stable suspension of GO can be obtained by ultrasonic treatment of graphite oxide or GO in water, which has therefore become a widely-used precursor for the solution-processing synthesis of graphene based nanomaterials. The abundant hydroxyl and epoxy groups on the basal plane and the carboxyl groups at the sheet edges are normally introduced during the wet-chemical synthesis of GO. These functional groups not only facilitate the dispersion of GO sheets in water and polar organic solvents, but also provide chemically active sites for GO functionalization. GO and rGO are thus promising building blocks to construct 2D graphene films/papers/membranes, which are multi-layered composite structures formed by the ordered self-assembly or random stacking of GO (or rGO) platelets, to obtain the collective or enhanced optical, electrical, and mechanical properties.
2-D graphene films/papers/membranes are attractive candidates for novel applications and paper-like composites. It is difficult to use pristine graphene nanosheets as building blocks for macroscopic solution-processing assembly because of their low solubility in all solvents. The combined features of the planar structure and abundant processable groups of GO nanosheets allow the assembly of macroscopic architectures with hierarchical structures through various techniques. The resulting materials possess the advantages of being light weight, and having robust mechanical properties, and high electrical conductivity. Alternatively, graphene frameworks, with different macroscale shapes, large surface areas, and high porosity, are ideal scaffolds for the incorporation of various functional guest nanomaterials, such as metals, semiconductors, and polymers, which enrich their functionalities and broaden the possibilities for their practical applications. In this way, the synergy of the hierarchical structure and multifunctional components makes graphene-based macroscopic assemblies attractive candidates for a wide range of practical demanding applications.
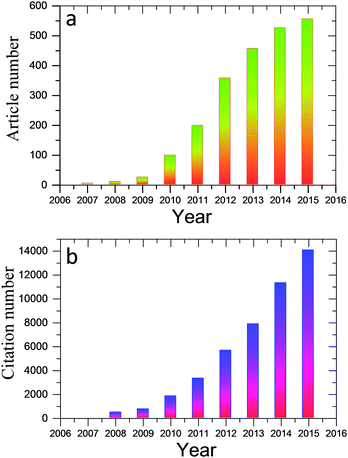
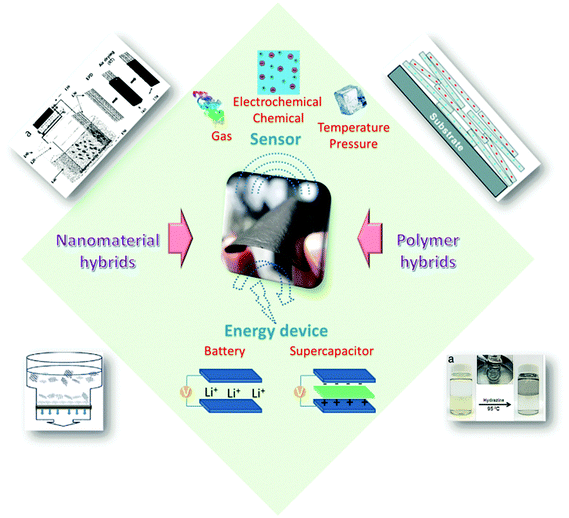
Much effort has been devoted to assembling these well-dispersed oxidized or chemically reduced (under controlled conditions, still water-dispersible) graphene nanosheets into paper-shaped and ordered macrostructures, mainly through flow-directed assembly via filtration. Recently, molecular templates, Langmuir–Blodgett assembly, two-phase interfacial transfer and direct CVD have also been employed to obtain graphene-based or GO-based membranes on selected substrates, and these membranes or papers have been evaluated in water purification, sensing, and energy devices. Fig. 1 shows the results from a search of the publications using the keywords “graphene paper or graphene membrane or graphene film” or “graphene oxide paper or graphene oxide membrane or graphene oxide film” from 2007 to 2015 using the Web of Science database. It is clear that the fabrication and application of graphene have attracted increasing interest in recent years. A review of the preparation of 2D and 3D graphene materials and their application as biosensors, membranes, and hybrid catalysts has been summarized in previous reports.18–21 We believe it is timely to focus on 2D graphene paper-like materials according to the recent research. The overall goal of this review is to provide a critical overview of our current understanding of freestanding graphene papers and their applications. We will discuss how to fabricate, characterize, and functionalize freestanding graphene papers. We will then focus on the applications of graphene papers in sensing, biomimetic, water purification, flexible substrates, and energy devices. Fig. 2 outlines the interest and focus of the present review.
2. How to construct graphene papers
A variety of methods for the preparation of graphene papers/films/membranes have been attempted, with the most commonly used procedures including solution phase assembly, electrochemical deposition, and CVD methods. The details of these commonly used methods are discussed below.2.1 Solution-phase assembly
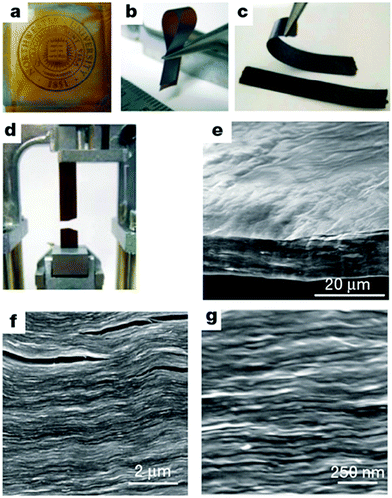 | ||
| Fig. 3 Morphology and structure of GO paper. (a–d) Digital camera images of GO paper. (a) 1 μm thick (the Northwestern University logo is beneath the paper); (b) folded, 5 μm-thick semi-transparent film; (c) folded, 25 μm-thick strip; (d) strip after fracture from tensile loading. (e–g) Low-, middle- and high-resolution SEM side-view images of a 10 μm-thick sample. Reproduced with permission from ref. 22. | ||
2.2 Electrophoretic deposition
Electrophoretic deposition (EPD) has shown to be a useful technique to deposit graphene on conductive substrates.47 Owing to the relatively short processing period and requirement of only simple and cost-effective equipment to produce uniform deposits with high microstructural homogeneity and adequate control of the deposit thickness, EPD offers the possibility of scaling up to large, application relevant dimensions. For instance, Ruoff and co-workers used the deposition of films composed of overlapped and stacked platelets of GO to prepare graphene papers by EPD processes (Fig. 4). The GO platelets migrated toward the positive electrode with oxidation occurring at the anode of the electrolysis cell. The possible electrochemical reactions involved include:| RCOO− → RCOO˙ + e− (oxidation of carboxylate) | (1) |
| RCOO → R˙ + CO2 (oxidative decarboxylation) | (2) |
| 2R → RR (dimerization of radicals) | (3) |
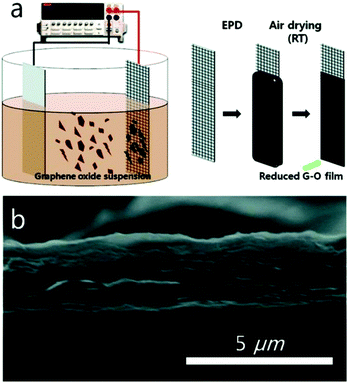 | ||
| Fig. 4 (a) Schematic diagram of the EPD process and (b) cross-sectional SEM image of an EPD–GO film. Reproduced with permission from ref. 48. | ||
The X-ray photoelectron spectroscopy (XPS) analysis shows that, in addition to the deposition, the oxygen containing groups, such as hydroxyl and epoxide on the basal planes, carboxylate and carbonyl functional groups at the edges and other fractions, can also partially be removed during the EPD process. This results in the electrical conductivity of the as-prepared graphene papers being enhanced as high as 1.43 × 104 S m−1. The advantages of this method including the reduction of GO with no need of additional reducing agents and the procedure executed at room temperature has the potential for the high-yield, large-area, low-cost, and environmentally-friendly production of films composed of rGO platelets. This approach should be useful in a variety of applications requiring a facile coating of complex surfaces and shapes.48
These advantages have been further exploited by many application-driven research studies. For example, Hasan et al. prepared a transferable and tunable microstructured GO film through the EPD process. By selecting the appropriate solution pH and deposition voltage, films composed of negatively charged GO sheets can be produced with either a smooth “rug” microstructure on the anode or a porous “brick” microstructure on the cathode.49 A nanostructured porous NiO/RGO hybrid film can also form on an indium tin oxide (ITO) substrate by EPD.50 GO undergoes post-conversion via reduction of GO and is deposited on nickel foam, ITO, stainless steel (SS), and platinum (Pt) to prepare composite films.51,52 Additionally, graphene nanosheets can be directly deposited on the Ni substrate through a similar process.53
2.3 Chemical vapour deposition
To meet the requirement of large-scale, near-perfect graphene films on various substrates, the CVD technique is a direct and promising method which is attractive for electronic devices owing to their high flexibility, transparency, and electrical conductivity.8,54 Commonly, transition metals, such as Cu,55,56 Ni,57–59 and Co,60 and non-metal materials, such as SiO2,61 BN,62 and Si3N4,63 have been used as substrates for the CVD growth of graphene films. However, the non-metal substrates have shown an undesirable limitation of slow growth rate and discontinuous size. A recent advance in the direct fabrication of large-scale graphene films on a Ge substrate has overcome this problem, owing to the high carrier mobility and process compatibility of the semiconductor and the semimetal-characteristics of Ge.642.4 Other methods
In addition, a variety of new assembly strategies have increasingly been developed for the construction of graphene films with hierarchical structures and high performances, inspired by the micro-/nano-assembled structures of materials found in nature. Cao et al. developed a simple, smart, and scalable method to simultaneously assemble and reduce GO nanosheets into large-size chemically-converted graphene films with high conductivity and mechanical stability, triggered by the unsteady microenvironment from the reduction–oxidation reactions between GO and the active-metal substrate.65 Honeycomb structural graphene films, which were prepared using a template method, display excellent properties, such as large porosity, high conductivity, and robust chemical and mechanical stability. These films exhibit promising potential usage in various applications, including serving as biological scaffolds, catalysts, and sensors.66,67 A bubble structural graphene film was also fabricated using monodispersed poly(methyl methacrylate) (PMMA) latex spheres as the sacrificial templates.40 Highly conductive, free-standing, and flexible porous carbon thin films can be prepared through chemical activation of rGO paper,68,69 as reported by Ruoff's group. Moreover, a novel strategy for the preparation of GO papers was developed by mechanically pressing a graphene aerogel that was obtained by freeze-drying a GO solution. This kind of graphene paper shows a unique structure with folded graphene sheets, and the d-space is reduced to 0.77 nm, which is narrower than the graphene paper prepared using vacuum filtration methods (typically 0.83–1.0 nm).702.5 Post-treatment of graphene oxide paper
Generally, when GO paper is prepared using solution phase assembly, its conductivity is very poor owing to GO containing many oxygen groups. Such GO paper is hardly suitable for many applications related to electrochemical sensors and energy storage. Thus, the GO paper is normally subjected to post-treatment to gain the necessary conductivity. This can be achieved by chemical reduction, high-temperature annealing and electrochemical reduction. Hydrogen iodine (HI) has been used as an effective reductant to reduce GO paper for enhanced electrical conductivity. Annealing is another effective method used to remove oxygen-containing groups from GO paper to improve its conductivity. The d-space of graphene paper is approximately 1 nm, which is appropriate for the mass transportation of most ions. However, this needs to be further improved in some cases. To do so, hydrazine vapour was used as a reducing agent to create a porous structure between the interlayer graphene papers. In addition, a unique process for the preparation of highly conductive and oxygen-free graphene thin paper with a carbon/oxygen ratio reduced to 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 can be employed by means of Ar+ ion irradiation of the GO papers (Fig. 5). The composition of graphene paper in terms of the carbon/oxygen ratio and the types of individual oxygen-containing groups is monitored throughout the process. Angle-resolved high resolution X-ray photoelectron spectroscopy (XPS) was used to investigate the depth profile of carbon and oxygen within the rGO paper. A C/O ratio of over 100 on the surface and 40 in the bulk material was observed. To provide insight into the processes of oxygen removal from GO paper through low energy Ar+ ion bombardment, the gases released during irradiation are analysed using mass spectroscopy. It is found that the Ar+ ion beam can be applied as a technique for the fabrication of highly reduced graphene papers with high conductivity. Such highly conductive graphene papers have great potential for applications in the construction of microelectronic and sensing devices.71
1 can be employed by means of Ar+ ion irradiation of the GO papers (Fig. 5). The composition of graphene paper in terms of the carbon/oxygen ratio and the types of individual oxygen-containing groups is monitored throughout the process. Angle-resolved high resolution X-ray photoelectron spectroscopy (XPS) was used to investigate the depth profile of carbon and oxygen within the rGO paper. A C/O ratio of over 100 on the surface and 40 in the bulk material was observed. To provide insight into the processes of oxygen removal from GO paper through low energy Ar+ ion bombardment, the gases released during irradiation are analysed using mass spectroscopy. It is found that the Ar+ ion beam can be applied as a technique for the fabrication of highly reduced graphene papers with high conductivity. Such highly conductive graphene papers have great potential for applications in the construction of microelectronic and sensing devices.71
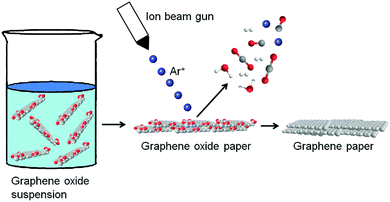 | ||
| Fig. 5 Preparation of graphene papers by Ar+ ion irradiation. Reproduced with permission from ref. 71. | ||
3. How to functionalize graphene papers
The emerging field of free-standing and flexible paper-like materials based on graphene sheets has become the focus of considerable research in recent years because of the scientific and technological significance of these materials. In particular, multifunctional flexible graphene-based films or papers are in high demand in a number of fields.3.1 Graphene–polymer composite films/papers
Polymers, as important components in the nanoworld, have been applied in the areas of sensor and energy conversion and storage. They have also widely been used in the functionalization of graphene paper to improve conductivity, biocompatibility, and mechanical properties of nanostructured composites.72–74 In general, the polymer is stabilized with graphene paper through non-covalent bonding such as electrostatic, hydrogen bonding, and π–π stacking interactions. The layer-by-layer assembly method is a facile, low-cost way to fabricate polymer/graphene hybrid papers with precise control over the structure and thickness on both the micro- and nanoscales, and has the great advantage of being able to fabricate graphene–polymer composite films through the electrostatic interaction of oppositely-charged objects or hydrogen bonding. A variety of polymers have been incorporated into graphene-based films with a uniform multilayer structure and novel functionality, such as polyaniline (PANI),73 poly(sodium 4-styrenesulfonate),74 poly(diallyldimethylammonium),75 poly(p-phenylene vinylene),43 and poly(sodium 4-styrenesulfonate).76The method was used to fabricate GO–PEI films consisting of a poly(ethylene terephthalate) substrate (Fig. 6), and the resulting hybrid films can be used as a gas barrier. Nanostructure and oxygen barrier properties of the GO–PEI film were shown to be highly dependent on the pH of the starting material solutions. For example, using a GO suspension with a pH of 3.5 resulted in the assembly of a film that exhibited very dense and ordered structures and delivered oxygen with very low transmission rates (the lowest rate was <0.05 cm3 m−2 day−1).77
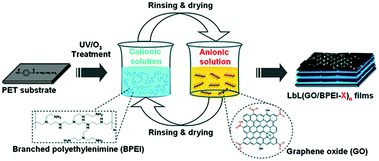 | ||
| Fig. 6 Schematic of the preparation of GO/BPEI films via layer-by-layer self-assembly. Reproduced with permission from ref. 77. | ||
In addition to the layer-by-layer assembly, solution-casting is another popular method to assemble polymer/graphene paper owing to the advantages of easily-obtained, simple apparatus and size-controllability. Recently, PVDF (polyvinylidene fluoride), PVP (polyvinyl pyrrolidone),78,79 PANI,80 poly(benzimidazole),81 and poly(VDF-TrFE)72 modified graphene papers have been synthesized using the solution-casting approach. For example, a PANI nanorod array was electrodeposited on a graphene paper that was prepared using the solution-casting assembly strategy, and the thickness of the PANI layer was controlled via electropolymerization time. In addition, free-standing, flexible, conductive, reduced GO/Nafion (RGON) hybrid films were obtained using solution chemistry that involved self-assembly and directional convective-assembly. These films display high conductivity facilitated electron transfer (ET), low interfacial resistance, and have excellent performances towards electrochemical biosensing of organophosphates (OP).83
Given the limited number of active functional groups on the surface of most pure carbon materials, forming covalent linkages between the polymer matrix and such carbon surfaces (when used as a composite filler) has posed a general challenge. In contrast, GO platelets contain surfaces rich in reactive functional groups, and a number of approaches for introducing covalent bonds between the GO platelets and polymers can be thus achieved. For instance, both the grafting-from and grafting-to approaches have been used for the attachment of a broad range of polymers. Dopamine and PEI, acting as reducing agents, were used to reduce and simultaneously functionalize GO, which further assembled into functional graphene papers.84,85
In addition, other methods have also been attempted, although there are few reports so far. For example, an interesting case is the fabrication of polycaprolactone (PCL)/graphene papers based on a PCL brush on the surface by polymerization of caprolactone from the functional groups on graphene.86
3.2 Graphene–nano-object composite films/papers
Graphene displays extremely useful physicochemical properties, but it also possesses a weakness in the expected functions, such as catalysis, redox performance, or energy storage. From the viewpoint of practical fabrication, it is necessary to decorate graphene with functional groups. Graphene is highly compatible with different solvents, and graphene papers or films offer a large surface area. Additionally, graphene obtained by the reduction of GO may still possess some oxygen groups, which enable the loading or growing of nano-objects.Several methods have been reported to fabricate functionalized nano-objects with graphene paper or films. To date, graphene paper is functionalized with nano-objects mainly through the following procedures:
(1) Some inorganic functional materials cover mainly the surface rather than bind to the interior of the graphene sheet. These nano-objects functionalize graphene hybrid materials mostly obtained by the growth of metal and semiconductor nanostructures on the graphene film by various processes. Such highly transparent conducting films can be obtained by spin-coated,87 physical vapour,88 and pulse electrochemical89 Ag nanowires or nanocrystals on the graphene film to significantly enhance the electrical properties and surface plasmon signal of the graphene film. Pulse laser deposition was applied to deposit V2O5 films on the graphene paper surface to yield flexible energy storage devices.90 MS (NiS and CoS),91 Ni(OH)2 and NiOOH,92–94 TiO,95,96 indium–gallium–zinc-oxide,97 and Au@PtNPs, PtNPs, or PtNP/MnO2 nanowires98–101 are well-designed for deposition on the graphene paper surface through advanced techniques. Furthermore, Duck Hyun Lee presented a well-designed carbon hybrid film that was prepared by the self-assembly of versatile growth carbon nanotubes on graphene films (Fig. 7),54 which displayed good mechanical properties and a high performance in the bending test.
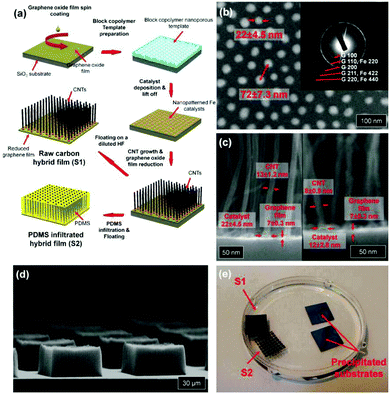 | ||
| Fig. 7 (a) Schematic illustration of the fabrication process for carbon hybrid films. A multilayer graphene oxide film is spin-coated onto a SiO2/Si substrate from an aqueous dispersion. The following block-copolymer lithography creates a nanopatterned iron catalyst array on the graphene oxide film. Highly aligned vertical CNT arrays are grown by catalytic PE-CVD. The underlying graphene oxide film is reduced and becomes electrically conductive during this high temperature PE-CVD growth. The fabricated hybrid film with (S1) or without (S2) PDMS infiltration is detached from the Si substrate by wet-etching the SiO2 layer. (b) SEM image of iron catalyst particles on the graphene film. The inset image shows the electron diffraction pattern. (c) Cross-sectional SEM images of hybrid films. (d) SEM image of a square-patterned CNT array on the graphene film. (e) Photograph of S1 and S2 floating on a water surface. Reproduced with permission from ref. 54. | ||
More interestingly, AuNPs102 and NiO103,104 can be in situ grown on the graphene paper surface to form multifunctional hybrid graphene paper.
(2) Loading NPs into the interiors of graphene sheets (Fig. 8). To insert a nano-object into the interlayer graphene paper, flow-directed assembly is the most efficient way to prepare nano-object (Prussian blue nanoparticles (PBNPs),24 Au@PBNPs,105 Na2/3Fe1/2Mn1/2O2 nanoparticles,106 and SnO2 nanoparticles107) hybrid graphene paper. Flow-directed assembly of a mixture of graphene and Fe3O4 nanoparticles has also been used to prepare graphene paper. A hybrid graphene paper was reported with a high loading of Fe3O4, reaching up to 10 percent of the total composition. The hybrid graphene paper also exhibited excellent electrical conductivity and mechanical strength. The most notable characteristic is its super paramagnetism, which can easily be tuned through modulation of the loading of the Fe3O4 nanoparticles.108 Moreover, graphene/MnO2 paper was fabricated by filtration of the chemically reduced mixture of GO solution with Mn(NO3)2 and KMnO4.109
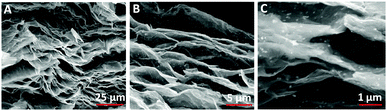 | ||
| Fig. 8 Cross-sectional SEM images of Au@PBNP rGO papers with various magnifications. Reproduced with permission from ref. 105. | ||
A homogeneous mixture of the GO suspension with AuNP self-assembles at the air/liquid interface, resulting in multi-layered GO–Au NP composite films. The reduced GO–Au NP film shows increased electrode kinetics and a cyclic voltammetric response in proportion to the amount of Au NPs, and an enhancement of the anodic peak current was observed compared with that of the reduced GO films.110 Transparent and flexible thin film transistors with a high performance, based on solution processed graphene nanosheet (GNS)–amorphous indium–gallium–zinc-oxide composites, have been developed.111
In addition, Zhang et al. reported a facile, rapid, scalable, and environmentally-friendly method of making rGO/Ni(OH)2 paper by the EPD process.51 NiCo2O4 has been grown on the surfaces of porous N-doped graphene sheets through hydrogen bonding, van der Waals forces, or covalent interactions with the functional groups of graphene, such as –COOH. The as-prepared hierarchically porous graphene paper with NiCo2O4 has shown a remarkable OER catalytic activity.112 N-doped graphene–SnO2 papers have been prepared by inducing the 7,7,8,8-tetracyanoquinodimethane anion as both the nitrogen source and complexing agent to format a sandwich structure, which exhibits a large capacity, high rate capability, and excellent cycling stability.113 In addition, AuNP-embedded porous graphene thin films for the electrochemical sensing of H2O2 were prepared through LBL self-assembly and subsequent annealing technologies.114
Not only can some foreign groups endow graphene paper with special functions, the special structures that can be obtained during the preparation process, such as porous and bubble structures,115 were also used to functionalize graphene paper. Moreover, free-standing elemental-doped (e.g. N)116 graphene film can be prepared directly using CVD.
4. Uses of graphene papers
Graphene papers, with the advantages of low price, high quality, and simple synthesis processes, have the potential to bridge the gap between nanoscale graphene sheets and real macro-scale applications of graphene as summarized in Table 1.| Materials | Preparation method | Assembly principle | Application | Advantage | Ref. |
|---|---|---|---|---|---|
| Graphene paper | Vacuum filtration | Vacuum π–π stack | Robust, used as a host to combine with various functional units | 22 | |
| Graphene paper | Vacuum filtration | Biomimetic | 125–127 | ||
| Graphene paper | LBL assembly | Langmuir–Blodgett or electrostatics | Enhance the conductive and protection film for electrodes | Controllable thickness | 31–33 |
| Graphene paper | Two-interface growth | Brownian motion | Free-standing | 41 | |
| Graphene film | Solution coated | Physical absorption, electrostatics | Electrode | 42–46 | |
| Graphene paper | EPD | Electricity | High conductivity | 48 | |
| Graphene film | EPD | Electricity | Supercapacitor electrode | High specific capacitance | 53 |
| Graphene film | Vacuum filtration | Vacuum π–π stack | Water purification | High pore structure | 117 |
| N-graphene paper with NiCo2O4 | Vacuum filtration | Vacuum π–π stack | OER catalyst | 112 | |
| PBNPs or Au@PBNP-graphene paper | Vacuum filtration | Vacuum π–π stack | Free-standing electrode | Robust, could be used as a host to combine with a functionalized group | 24 and 105 |
| RGD-peptide–graphene paper | Vacuum filtration | Vacuum π–π stack | Living-cell sensor | High sensitivity and good selectivity | 161 |
| Graphene film | CVD | Flexible substrate | Rollable and transparent | 132 | |
| PANI–graphene paper | Solution casting | Supercapacitor electrode | Flexible, high conductivity | 82 | |
| Graphene film | CVD | Dual-gate devices | High conductivity, transparent | 56 | |
| Graphene film | Solution casting | Transparent, robust | 65 | ||
| Graphene film | CVD | Electrodes | High conductivity, transparent | 57 |
4.1 Water purification
Graphene derivative papers or membranes for molecular sieving purification permeation through nanometer pores are important in the design of materials for use in filtration and separation techniques. To achieve high solvent permeability, filtration membranes must be as thin as possible, while retaining their high mechanical strength and solvent resistance. Papers or membranes made from GO are particularly interesting candidates in this subject. In this section, we discuss recently demonstrated graphene derivative papers or membranes which are used for molecular sieving purification.Grossman et al. reported that nanometer-scale pores in single-layer freestanding graphene can effectively filter out NaCl salt from water.117 The graphene membrane's ability to prevent the salt passage depends critically on pore diameter with adequately sized pores allowing for water flow while blocking ions (Fig. 9). Additionally, they investigated the role of chemical functional groups bonded to the edges of graphene pores. The results suggest that commonly occurring hydroxyl groups can roughly double the water flux owing to their hydrophilic character. The water permeability of this graphene membrane is several orders of magnitude higher than that from conventional reverse osmosis membranes.
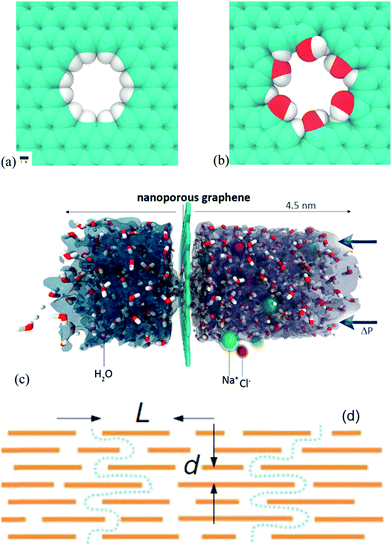 | ||
| Fig. 9 (a) Hydrogenated and (b) hydroxylated graphene pores, and (c) side view of the computational system investigated. Reproduced with permission from ref. 117. (d) Schematic view of the possible permeation through GO laminates. Typical L/d is ∼1000. Reproduced with permission from ref. 118. | ||
Another option for molecular sieving is the low-friction flow of solvent through two-dimensional capillaries formed by closely spaced graphene sheets. Nair et al. observed that submicrometer-thick GO membranes can be completely impermeable to liquids, vapours, and gases, including helium, but allow unimpeded permeation of water.118 They commented on the mechanism involved in the following way: monolayer water proceeds through the capillaries formed in the GO membranes. GO laminates consist of crystallites stacked on top of each other. The groups attached to the graphene sheets lead to a relatively large d-spacing. Importantly, such groups tend to cluster and leave large, percolating regions of graphene sheets not oxidized. Therefore, GO laminates are likely to have empty spaces between the non-oxidized regions of the graphene sheets. Because d for the reduced GO is approximately 4 Å, the width of the empty space δ can be estimated to be approximately 5 Å, which is sufficient to accommodate a monolayer of water (Fig. 9d). The described GO membranes can be used as barrier films in the design of filtration and separation materials and for the selective removal of water.
The nearly frictionless surface of the non-oxidized GO facilitates the extremely fast flow of water molecules. Joshi et al. further reported that ions smaller in size than the GO nanochannels can permeate in the GO membrane at a speed orders of magnitude faster than would occur through simple diffusion.119 Size exclusion appears to be the dominant sieving mechanism, even though other possible methods have not been completely excluded.
Indeed, the GO membrane represents the next generation of ultrathin, high-flux, energy-efficient membranes for precise ionic and molecular sieving in aqueous solution; however, as commented by Mi,120 further research is still needed to thoroughly understand the transport of water and solutes in the GO membrane, especially to fundamentally elucidate other potential separation mechanisms in addition to size exclusion. In addition to the porous graphene monolayer and GO membranes, diamond-like carbon (DLC) is a promising alternative in the permeation of solvents.121 DLC was made just over 40 years ago by Aisenberg and Chabot.122 Thin transparent films made from compounds very similar to diamond were initially synthesized by means of an ion-beam deposition method. Currently, the widely-used technique for DLC deposition is plasma CVD using organic compounds. Karan et al. demonstrated that DLC membranes can be prepared to exhibit extremely high solvent permeability while maintaining considerable mechanical strength.121 They reported the ultrafast permeation of organic solvents through DLC nanosheets, which were free-standing amorphous carbon membranes with a thickness ranging from 10 to 40 nm. Furthermore, the membranes exhibit an excellent separation performance for organic solutes. Interestingly, the solvent flux was mainly affected by the viscosity of the solvent, rather than its molecular size or dipole moment.
4.2 Biomimetic devices
Graphene paper also has potential applications in actuators, electromagnetic-free generators, robots and artificial muscles,42,123–125 as it displays a fast response to external stimuli. Mu et al.125,126 developed a series of graphene monolayer (GM) papers with a gradient rGO/GO structure. In the gradient GM paper, the GO region could readily adsorb/desorb water molecules in response to environmental humidity, temperature, or light, which resulted in the swelling/shrinking of the GO sheets. In contrast, the rGO region was inert to water molecules. Considering this behaviour, together with the excellent photothermal properties of rGO and GO, as well as its high flexibility and mechanical robustness, this graphene paper provides great potential for photoresponsive actuator application. Mu et al. exploited these properties to produce GM paper with reversible, fast (≈0.3 s), powerful (7.5 × 105 N kg−1 force output), and controllable mechanical deformation and recovery, in response to moisture, heat, and light. The response of this water-driven actuator to multiple stimuli allows the fabrication of artificial muscles and electric generators. Furthermore, it was shown that with a programmed dual-gradient (both vertical and lateral) structure, a self-folding all-graphene origami could be developed to demonstrate three capabilities: (i) production of pre-designed shapes, (ii) walking, and (iii) turning a corner (Fig. 10).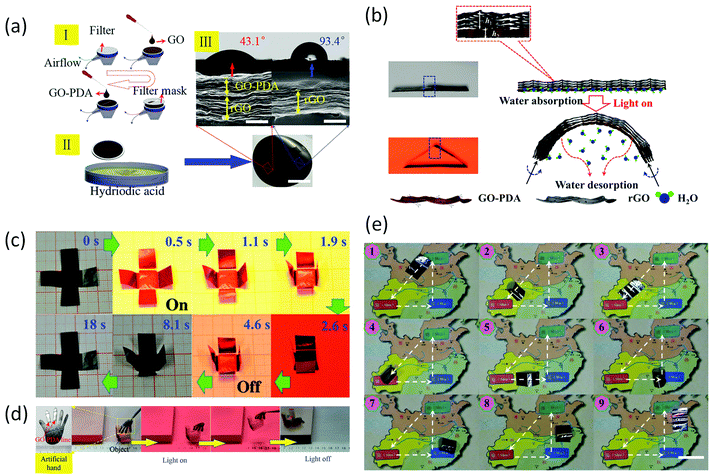 | ||
| Fig. 10 (a) (I) Schematic illustration of the mask-assisted filtration process (scale bar, 2 cm). (II) Cross-sectional SEM images of GO–PDA/rGO and rGO regions following reduction by HI (scale bar, 1 μm). (III) CA measurement of the GO–PDA/rGO surface (43.1°) and rGO surface (93.4°) of dual-gradient GM. (b) Schematic representations of the structures and mechanisms of the graphene paper. With no NIR light irradiation, the GO–PDA/rGO region flattens. A flat free-standing GO–PDA/rGO region starts to bend immediately upon exposure to NIR light irradiation. This bending/unbending mechanism is completely reversible over many cycles. (c) Time profiles of self-folding movements of a cross-shaped piece of paper with and without NIR light irradiation. The sample was placed on the platform and illuminated with NIR light (100 mW cm−2) normal to its surface (light is incident from above). (d) Optical images showing an artificial/robotic hand holding an object driven by light irradiation. (e) Optical images showing the “micro robot” walking and turning on a map driven by light irradiation. Reproduced with permission from ref. 126. | ||
The skin is highly sensitive to pressure and temperature, and is therefore an important feedback source of crucial information. Considering its excellent mechanical, electrical, and thermoelectric properties, graphene papers have attracted increasing attention to mimic the sensing behaviour of natural skin. For example, Hou et al. reported the first graphene paper-based self-healing, mechanically strong and stretchable, pressure-sensing device.127 The device is composed of a piezoelectric polymer layer and a porous graphene layer with a healing substrate. The integrated graphene/polymer hybrid film mimics both the mechanical self-healing and pressure sensitivity behaviour of natural skin without the requirement of an external power supply (Fig. 11). More encouragingly, the ultimate strain and tensile strength of such devices were even two and ten times larger than the corresponding values of human skin, respectively.
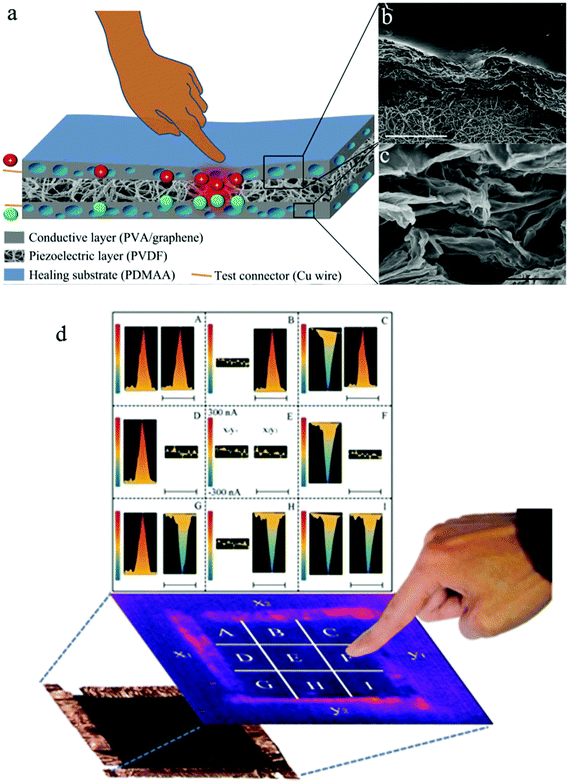 | ||
| Fig. 11 Schematic illustration and images of graphene-paper based touch sensors. (a–c) A self-healing and stretchable PDMAA/PVA/graphene–PVDF sandwiched thin-film sensor. Reproduced with permission from ref. 127. (d) A compressive graphene sensor pad. Reproduced with permission from ref. 28. | ||
More recently, Hou et al. reported a novel paper-like graphene foam that is free-standing, flexible and elastic. It demonstrated temperature sensitivity based on the thermoelectric effects of graphene. Furthermore, this elastic graphene foam exhibited pressure sensing behaviour upon finger pressing. A proof-of-concept graphene pressure sensor pad was manufactured that could locate finger-pressure points and measure the pressure levels. All of the above sensing abilities were demonstrated without the need for an internal/external power supply. This unique passive all-graphene flexible thin-film sensor may open up new opportunities for the development of electronic skin.
4.3 Use as flexible substrates
Graphene films that have recently become available on a large scale exhibit good optical transparency, electrical and thermal conductivities, and high temperature stability and mechanical flexibility. Therefore, graphene film, as a solid substrate conducting platform to support functional groups, has attracted a lot of interest. For the fabrication of light-emitting diodes, high-quality GaN/ZnO co-axial nanorod heterostructures were grown directly on graphene films. The nanostructured LEDs exhibited strong blue emission, even under room-light illumination.97 In another study, ZnO nanowire functionalized graphene films were used as an optical device for constructing a nanostructured LED light (Fig. 12).128 Transparent macroporous graphene thin films (MGTFs) were prepared using an ice crystal-induced phase separation technique. Such MGTFs have shown a wide range of applications as substrates, as they can be used as porous scaffolds with high conductivity for the electrochemical deposition of various semiconductors and rare metal nanoparticles, such as CdSe, ZnO, and Pt, as well as successive deposition of different materials. Notably, the macroporous structured and nanoparticle-decorated MGTFs (e.g. Pt@MGTFs and CdSe@MGTFs) showed an enhanced performance as electrodes for the oxygen reduction reaction (ORR) and photoelectrochemical H2 generation.129 Gyu-Chul Yi's group successfully grew ZnO on a graphene film. They demonstrated the position- and morphology-controlled growth of ZnO nanostructures on graphene layers without using any template mask or metal catalyst.130 The microstructures of the epitaxial GaN thin films grown on graphene layers using ZnO nanowalls were then investigated. The predominant dislocations in the GaN thin films grown on the ZnO-coated graphene layers were mixed-type dislocations. The typical threading dislocation density in the films was found to range from 1.2 × 109 to 2.4 × 109 cm−2, which is comparable to that of GaN on Si substrates and slightly higher than that of GaN on sapphire substrates.131 Fully-flexible transparent 3D structure nanogenerators were fabricated by depositing 1D ZnO nanorods on a 2D graphene film. This type of nanocomposite is suitable for self-powered rollable transparent device applications, such as flexible self-powered touch sensors, wearable artificial skin, fully flexible display mobile devices, and battery supplements for wearable smart or cell phones.132 In another example, the power-generating phenomenon of a single GO film with a preformed oxygen-containing group gradient was reported. The GO film was prepared by a moisture/electric field co-induced gradient process of oxygen-containing groups on a vacuum-filtrating GO film, and this strategy was termed “moisture-electric annealing”. The as-prepared GO film was able to provide a moisture-driven voltage output of approximately 35 mV with a power density of approximately 4.2 mW m−2 by harvesting energy from moisture diffusion with a high energy conversion efficiency of up to 62%. This efficiency clearly surpasses the fluidic-electric generators and is even better than those of the currently popular piezoelectric generators with an optimized structure. More interestingly, an extra-power-free respiratory monitor was also built by using the moisture flow of human breath as a clean and renewable power source.133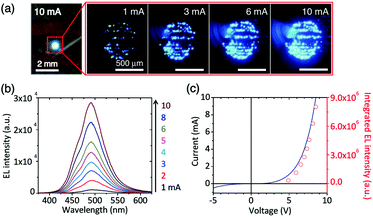 | ||
| Fig. 12 Visible nanostructured LEDs on graphene films. (a) Photograph and optical microscopy images of the light emission from the LED at different applied currents. (b) Power-dependent EL spectra at applied currents of 1–10 mA. (c) I–V characteristic curve of the LED (black solid line) and a plot of the integrated EL intensity (red open circles) as a function of the applied bias voltage. Reproduced with permission from ref. 128. | ||
Graphene paper, as a flexible substrate, has been used not only for the growth of nano-objects, but also as a good supporting material for immobilizing enzymes and microbes to construct membrane-free biofuel cells.134–136
4.4 Applications in sensors
The self-assembled 2D graphene paper/film/membrane, functionalized with nano-objects, polymers, and biomolecules, are promising candidates for sensing applications. In particular, in the field of electrochemical biosensors with high sensitivity, these candidates have found widespread uses in clinical diagnosis, environment monitoring, and for quality control in industrial, food, and agricultural products.Graphene paper as a sensing platform for the detection of H2O2 and glucose (Table 2) has been attractive owing to its high conductivity, bio-compostable, sufficient flexibility, and robust mechanical strength. Besides the graphene film–GOD (glucose oxidase) electrochemical sensor for the detection of glucose, other electrochemical sensors have also been built, for example, the LBL self-assembly of GOD and chitosan nitrogen-doped graphene was used to fabricate a glucose biosensor. This biosensor operates at a low potential of −0.2 V (vs. Ag/AgCl), exhibiting a high sensitivity of 10.5 mA cm−2 M−1, and a detection limit (LOD) of 64 μM.141 A GOD–AuNP–PBNPs–chitosan complex142 or PANI/AuNPs143 as the electron transfer mediator and assistant groups to combine GOD with graphene paper, has been proposed as a method for the determination of glucose concentration in whole blood samples within the physiological concentration range (3.9–7.8 mM). Based on the CVD of a graphene film modified by GOD, field-effect transistors for real-time glucose and glutamate sensing have been reported.144 Moreover, graphene paper has been functionalized by non-enzymatic catalysts using different techniques. PtNP/MnO2 nanowires/graphene paper as a free-standing paper electrode was used for the non-enzymatic detection of H2O2, enabling the monitoring of H2O2 with a linear dynamic range of 2.0 μM to 13.33 mM and a LOD down to 1.0 μM (S/N = 3).98 The sensor response is linear to glucose concentrations in the range from 0.1 mM to 30.0 mM with a detection limit of 0.02 mM (S/N = 3) and a detection sensitivity of 58.54 mA cm−2 mM−1. In addition to those mentioned above, dip-coating gold nanoparticles,145 NiO nanoparticles loaded on graphene paper using the EPD process,93 AgNCs grown controllably directly on graphene paper,89 and the LBL self-assembly of AuNPs-embedded porous graphene thin films114 have been used to build sensing platforms for the detection of glucose or/and H2O2.
| Materials | Catalyst | Analyst | Methods | LOD | Ref. |
|---|---|---|---|---|---|
| AuNP/graphene paper | AuNPs | Glucose | Chronoamperometry | 5 μM | 145 |
| AuNP/PANI/graphene paper–GOD | AuNPs | Glucose | DPV | 143 | |
| PBNP/graphene paper–GOD | GOD | Glucose | Chronoamperometry | 10 μM | 24 |
| Chitosan/graphene–GOD | GOD | Glucose | Chronoamperometry | 64 μM | 141 |
| SiO2@CeO2/graphene paper–GOD | GOD | Glucose | Colorimetric | 0.2 mM | 150 |
| PtNP/MnO2 nanowires/graphene paper | PtNPs | Glucose | Chronoamperometry | 0.02 mM | 99 |
| NiO/graphene paper | NiO | Glucose | Chronoamperometry | 0.1 μM | 93 |
| PBNP/graphene paper–GOD | GOD | Glucose | Chronoamperometry | 8.4 μM | 151 |
| AuNP/graphene–GOD | GOD | Glucose | Chronoamperometry | 4.1 μM | 152 |
| PtNP/graphene film | PtNPs | Glucose | Chronoamperometry | 1 μM | 100 |
| ZrO2/graphene film-GOD | GOD | Glucose | Chronoamperometry | 45.6 μM | 153 |
| GOD–AuNP–PBNP chitosan/graphene | GOD | Glucose | Chronoamperometry | 10 μM | 142 |
| AuNP/PANI/graphene paper–GOD | AuNPs | H2O2 | DPV | 143 | |
| AuNP/graphene paper | AuNPs | H2O2 | Chronoamperometry | 2 μM | 145 |
| PBNP/graphene paper | PBNPs | H2O2 | Chronoamperometry | 5 μM | 24 |
| SiO2@CeO2/graphene paper | H2O2 | Colorimetric | 9 nM | 164 | |
| PtNP/MnO2 nanowires/graphene paper | PtNPs | H2O2 | Chronoamperometry | 1 μM | 98 |
| HRP/graphene film | HRP | H2O2 | CVs | 1.17 mM | 154 |
| PBNP/graphene paper | PBNPs | H2O2 | Chronoamperometry | 1.5 μM | 151 |
| AgNC/graphene film | AgNCs | H2O2 | Chronoamperometry | 3 μM | 89 |
| AuNP-embedded porous graphene thin films | AuNPs | H2O2 | Chronoamperometry | 0.1 μM | 114 |
| PtNP/graphene film | PtNPs | H2O2 | Chronoamperometry | 0.2 μM | 100 |
We have successfully fabricated graphene paper doped with chemically compatible Prussian blue nanoparticles (PBNPs) and Au@PBNPs105 as a nanohybrid electrocatalyst. Prussian blue was loaded on the graphene nanosheet via electrostatic attraction. The hybrid materials were characterized using AFM, SEM, and TEM (Fig. 13 and 14), and indicate that the graphene nanosheet was successfully decorated by PBNPs. However, the surface of the graphene paper was very smooth, and water molecules were also trapped together with the PBNPs on the graphene paper, which could provide a microenvironment for the accommodation of bio-macromolecules. Cyclic voltammograms (CVs) reveal that heterogeneous electron transfer (ET) reactions arising from the confined PBNPs were reversible and ET kinetics were controlled via mixed diffusion and surface confinement. Using the hybrid materials as a platform for the detection of H2O2, the electrocatalytic reduction of H2O2 started at 0.4 V (vs. SCE) and tended to reach a maximum effect at potentials more negative than −0.1 V, and a linear relation between electrocatalytic current density and H2O2 concentration was observed in the range of 1–7 mM, with a detection limit of approximately 5 μM.24
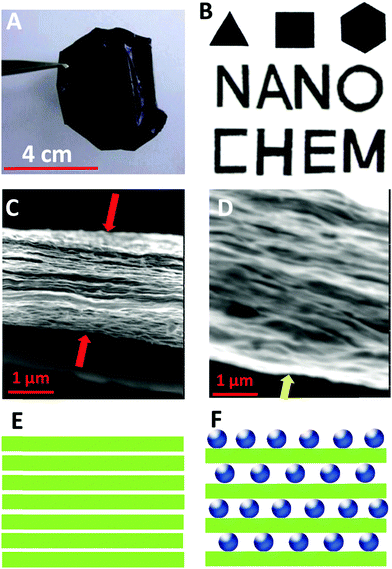 | ||
| Fig. 13 AFM and TEM images of various nanosheets. (A) An AFM image of the rGO nanosheets, (B) an AFM image of the PBNP–rGO hybrid nanosheets with a large scanned area, (C) high resolution topography and(D) three-dimensional AFM images of PBNP–rGO hybrid nanosheets, and (E and F) TEM images of the PBNP–rGO hybrid nanosheets. The AFM substrate used is mica. The scanned areas: (A) 5 μm × 5 μm, (B) 2 μm × 2 μm, (C and D) 0.45 μm × 0.45 μm. Reproduced with permission from ref. 24. | ||
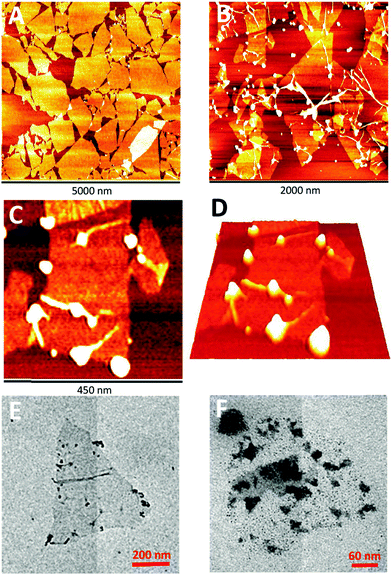 | ||
| Fig. 14 Photographs and SEM images of the graphene papers. (A and B) Photographs of different-shaped patterns and letters of the PBNP–rGO papers. Cross-sectional SEM images of (C) rGO and (D) PBNP–rGO papers. Schematic illustrations of the proposed side-view structures of (E) rGO and (F) PBNP–rGO papers. Reproduced with permission from ref. 24. | ||
Electrochemiluminescence (ECL) sensors are very important. Therefore, ECL sensors have recently been developed based on graphene paper. For example, a novel biosensor was developed using immobilized Ru(bpy)32+ and alcohol dehydrogenase (ADH) on a graphene/bovine serum albumin composite film for the detection of ethanol.137 Xu et al. used a poly(sodium 4-styrenesulfonate) functionalized graphene/Nafion composite film to prepare a paper-based solid-state electrochemiluminescence sensor and used it to detect tripropylamine. A low detection limit (S/N = 3) of 5.0 nM was achieved. The sensor exhibited excellent reproducibility (relative standard deviations of 0.63% for continuous 45 cycles) and long-term stability (∼80% of its initial ECL intensity could be retained over 3 months). Furthermore, highly porous Fe3O4 nanocrystal clusters as well as poly(sodium 4-styrenesulfonate) were used to hybridize graphene paper. These types of graphene papers showed high sensing performances for specific compounds containing tertiary amino groups and DNA with guanine and adenine, when they were used in an ECL sensor.146,147
Impedimetric immunosensors for the detection of bacteria have been a hot topic because of the frequency of food safety accidents. An efficient, low-cost, and robust impedimetric immunosensor for the rapid and sensitive detection of Escherichia coli O157:H7 (E. coli O157:H7) was developed using a gold nanoparticles hybrid free-standing graphene paper electrode. The graphene paper was prepared via the chemical reduction of GO paper obtained from the vacuum filtration method. The gold nanoparticles were grown on the surface of the graphene paper electrode using a one-step electrodeposition technique. Immobilization of anti-Escherichia coli O157:H7 antibodies on the paper electrode were performed using the biotin–streptavidin system. Electrochemical impedance spectroscopy (EIS) was used to detect E. coli O157:H7 captured on the paper electrode with a broad linear range (1.5 × 102–1.5 × 107 cfu mL−1) and a low detection limit (1.5 × 102 cfu mL−1).138
Photoelectrochemical sensing is another key technique as a branch of electrochemical sensing, based on a TiO2–graphene complex nanopaper. An advanced photoelectrochemical biosensing device for the detection of carcinoembryonic antigens has been proposed working at a relatively low applied potential.148
Moreover, a microfluidic biochip was prepared using graphene paper and functionalized with antibodies, enzymes, and aptamers as a biosensor for the multiplexed detection of different metabolites, such as glucose, lactate, xanthine, and cholesterol. The results showed that the graphene biosensor arrays can detect multiple metabolites on a microfluidic paper sensitively, rapidly, and simultaneously.149
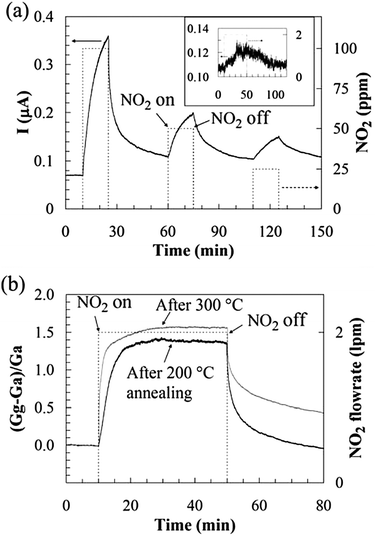 | ||
| Fig. 15 (a) Room-temperature sensing behaviour of GO after being annealed in Ar at 200 °C for 1 h. The inset shows the sensor response to 2 ppm NO2, where the conductance increased by ∼12% with 40 min NO2 exposure. (b) Annealing at 300 °C improved sensitivity and response time but lengthened the recovery time, compared to annealing at 200 °C. Reproduced with permission from ref. 156. | ||
Moreover, based on the reflectance change with varied optical and geometric properties of the sensing layers absorbing the gas molecules, Some et al. constructed a new type of one-headed polymer optical sensor array with hydrophilic GO and hydrophobic rGO for sensing volatile organics.163 The sensing device offered the ability to distinguish between tetrahydrofuran (THF) and dichloromethane, owing to the strong interactions of the oxygenated groups of GO with polar organic THF.164 Furthermore, the GO sensor displayed a high sensitivity under extreme conditions, such as strongly acidic or basic conditions, or a high humidity environment.
In addition to the gas species, GO-based assemblies showed high sensitivity to moisture,42,124 which can be attributed to the planar structure and abundance of oxygen-containing groups on the GO sheets. Park et al. constructed a smart paper actuator composed of adjacent GO and CNT films, based on the sensitivity of GO to humidity.165
Graphene paper based colorimetric sensors display a lot of advantages, such as visualization, low cost simplicity, rapidity, and high sensitivity. A new kind of colorimeter sensors for the detection of hazardous volatile organics, including THF, chloroform, methanol, and dimethylformamide (DMF), were developed based on a graphene–polydiacetylene (PDA) composite film. First, graphene film was immersed in a PCDA solution to immobilize PCDA on the graphene nanosheet through π–π stacking. After UV illumination treatment, the precursor self-assembled and was converted into “blue-phase” PDA. Owing to the merits of the large surface area and high transparency of the graphene film as an efficient support, the colorimetric response from the structural conformation changes of PDA could be visually detected at a relatively high concentration of organics. The well-fitting relationship between the chromatic response and the organic concentration in the range of 0.01–10% quantitatively provides the foundation for organic detection in practical applications.166
Thanks to its good biocompatibility, a free-standing hybrid film sensor would be a promising candidate for biological, medical, and pharmaceutical applications. Heldt et al. fabricated a microdevice sensor for the detection of proteins in solution by measuring the surface electrical resistivity of graphene/cellulose composite paper as a function of protein concentration.167 Different kinds of bioactive molecule (e.g. glucose oxidase, cholesterol oxidase, cholesterol esterase, lactate oxidase, and HRP) functionalized SiO2@CeO2/graphene papers were used for the colorimetric detection of lactate, uric acid, cholesterol and glucose.150 Zuo et al. presented microfluidic systems for the detection of two infectious pathogens—Staphylococcus aureus and Salmonella enterica—using fluorescence marked aptamer-functionalized graphene paper.149 A pH sensor built using upconversion luminescence nanomaterial hybrid graphene paper,168 and high-performance flexible potentiometric sensing devices using free-standing graphene paper as functional ion-selective electrodes have also been reported.169
4.5 Application in energy devices
Increasing research efforts have been focused on developing flexible, lightweight, and even wearable energy systems because of their potential applications in wearable electronic devices such as flexible displays, paper-like mobile phones, and other collapsible functional devices. In this section, we summarize several key types of wearable energy systems, including batteries, supercapacitors, and fuel cells, which are based on graphene papers.| Cathode: LiCoO2 ↔ Li1 − xCoO2 + xLi+ + xe− | (4) |
| Anode: 6C + xLi+ + xe− ↔ LixC6 | (5) |
| Total reaction: 6C + LiCoO2 ↔ Li1 − xCoO2 + LixC6 | (6) |
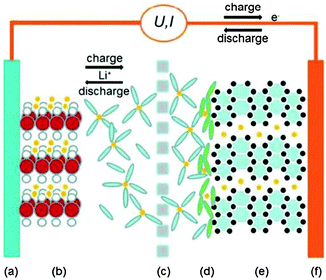 | ||
| Fig. 16 Schematic illustration of a LIB cell: (a) aluminium current collector; (b) cathode; (c) porous separator; (d) solid electrolyte interface layer; (e) graphite anode; and (f) copper current collector. Reproduced with permission from ref. 170. | ||
According to the above reactions, it is easy to notice that the electrode material holds the key for the electrochemical performance of the LIBs. Research efforts have thus been focused on exploring new electrode materials including graphene based paper-like electrodes.
Graphene paper shows an extraordinary cyclic stability as 99% of its capacitance is retained after 5000 cycles at a high current density of 20 A g−1, which is attributed to its unique folding structure which allows more access to the Li-ion insertion active sites and facilitates electrode materials coming into contact with the electrolytes. Pure graphene paper was prepared by vacuum filtration of graphene dispersions and used as the LIB cathode.90,171 The paper exhibited distinguishable electrochemical properties compared with the traditional graphite cathode. For example, a sharp peak at 1.47 V was observed in the first lithiation process in the cyclic voltammograms. A flat discharge plateau was observed at 2.20 V in the first discharge curve, and a corresponding discharge capacity of 582 mA h g−1 (to the cut off voltage of 2.0 V) was obtained.172 However, during the preparation or reduction process of pure GO papers, the free single-layer graphene nanosheets will aggregate and restack owing to the interplanar π–π interactions and van der Waals forces between the graphene layers. In this case, a possible reduction of the surface area of the graphene films and the diffusion of the electrolyte ions occurs, which results in a decrease in the electrochemical performance. Therefore, a number of strategies have been developed to prevent the aggregation of graphene sheets to increase the surface area and promote transport of the electrolyte ions, including elemental doping, designing a special structure, adding spacers, crumpling the graphene sheets, and template-assisted growth.
To increase the power capability of graphene paper, a graphene sheet with carbon vacancy defects (pores) in the plane was developed, which was synthesized by a wet chemistry approach, and applied to Li-ion storage batteries. This kind of material displays much enhanced Li-ion storage capacity and transport properties than without the holey defects, while not adversely affecting the electrical conductivity or ductility.173 Non-annealed graphene paper, prepared by the reduction of prefabricated GO paper with hydrazine hydrate, was employed as the sole component of a binder-free lithium-ion battery anode, circumventing the polymer binders and other additives required for the fabrication of conventional electrodes. The binder-free anode fabricated from this non-annealed paper possessed an excellent cycling ability, while exhibiting a voltage versus capacity profile similar to that of a polymer-bound graphene powder anode. Kinetic barriers may exist for Li ion diffusion through the layered paper structure as decreasing the current rate from 50 to 10 mA g−1 increased the reversible capacity by over 150%.174 Obviously, in such compact and ordered structures, a large current rate causes the loss of the reversible capacity. In order to facilitate Li diffusion between the graphene layers and reduce the side effects caused by a large charge rate, the pores can further be introduced into the graphene layers, with the pore sizes ranging from a nanometer to micrometer scale. Photoflash and lasers were used to reduce the free-standing graphene paper owing to the photothermal reduction of GO yielding an expanded structure with micrometer-scale pores, cracks, and inter sheet voids, as the high rate capable anodes for lithium-ion batteries. This open-pore structure enables access to the underlying sheets of graphene for lithium ions and facilitates efficient intercalation kinetics even at ultrafast charge/discharge rates of >100C. Importantly, photothermally reduced graphene anodes are structurally robust and display an outstanding stability and cycling ability. At charge/discharge rates of ∼40C, photoreduced graphene anodes delivered a steady capacity of ∼156 mA h g−1 (anode) continuously over 1000 charge/discharge cycles, providing a stable power density of ∼10 kW Kg−1 (anode).175 Graphene honeycomb film has been shown to exhibit enhanced LIB performance with a reversible capacity of 1612 mA h g−1 and a reversible retention capability of 71%, compared with conventional graphene films with a reversible capacity of 1076 mA h g−1 and a reversible retention capability of 63%, owing to the high porosity and robust chemical and mechanical stability.67 The folded graphene paper, made by mechanically pressing graphene aerogel, presents first discharge and charge capacities of 1091 and 864 mA h g−1, respectively, with a coulombic efficiency of 79.2%, which is improved to 98% for the second charge–discharge cycle.70
Researchers have also presented a one-pot, post-fabrication amine-modification strategy for converting insulating GO paper into conductive, “alkylated” graphene paper. The amine modification “alkylates” the stacked GO sheets and creates a stabilized structure that can be maintained under vigorous hydrazine reduction conditions. The resulting “alkylated” graphene paper possesses a well-ordered structure and uniform conductivity, in contrast to graphene papers prepared in the absence of structure-stabilizing amines. In this sense, post-fabrication chemical modification can be an attractive strategy for engendering a desirable combination of structures and properties in graphene papers. While the HA-graphene paper does not have the highest conductivity observed to date, it features built-in alkyl functionalities that facilitate novel material processing and diverse applications. For example, it may be desirable to have a biocompatible material with large spacing that can support cell growth and encapsulate enzymes for catalysis. In addition, functionalization may turn graphene paper into a high-capacity, well-ordered medium for ion storage in applications concerning super capacitors and lithium-ion batteries.29
Owing to the sandwiched structure exhibiting sufficient space for π–π interactions and van der Waals forces between the graphene layers, there have been a lot of reports on the use of sandwiched graphene papers to improve LIB performances by solving the limitations of compact graphene paper with respect to Li-ion and electrolyte diffusion, by taking advantage of the graphene network for keeping sandwiched NPs in good electrical contact and in a loosely stacked porous structure for facile Li-ion and electrolyte transportation.23,108
Fe3O4 exhibits a high theoretical capacity, eco-friendliness, and natural abundance, so the sandwiched graphene/Fe3O4 paper is an ideal electrode material. When used as the cathode of a Li-ion battery, it showed a specific capacity of 940 mA h g−1 at 200 mA g−1, much higher than that of pure Fe3O4 (200 mA h g−1) and the traditional graphene/Fe3O4 electrode (400 mA h g−1).176 The most widely explored pseudo-capacitor materials include transition metal oxide and hydroxide nanoparticles and nanosheets, such as SnO2,108 CuO,177 NiO or Ni(OH)2,92–94 MnO2,178 and Co3O4.179
More complicated graphene-based paper structures have been designed for achieving a better performance. The 7,7,8,8-tetracyanoquinodimethane anion was used as a nitrogen source and complexing agent to fabricate structural N-doped graphene–SnO2 sandwich papers. It has been used in lithium-ion batteries, with the specific capacity maintained at values as high as 683 mA h g−1 and 619 mA h g−1 after the current density was increased to 1000 mA g−1 and 2000 mA g−1, respectively. Even at a current density of 5000 mA g−1, this material still delivered a capacity of 504 mA h g−1, which is 1.35 times the theoretical capacity of graphite (372 mA h g−1) (Fig. 17).108 Si nanoparticles were used to reconstitute regions of highly stacked graphene. This type of material exhibits high Li-ion storage capacities and cycling stability. An electrode was prepared with a storage capacity of 42![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 mA h g−1 after 50 cycles and 41
200 mA h g−1 after 50 cycles and 41![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 mA h g−1 after 200 cycles that decreased by 0.5% per cycle.180
500 mA h g−1 after 200 cycles that decreased by 0.5% per cycle.180
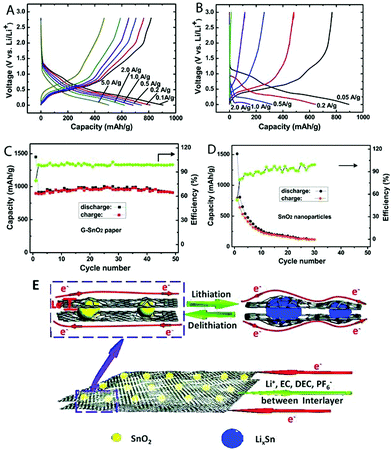 | ||
| Fig. 17 Charge/discharge curves of (A) N-doped G–SnO2 papers and (B) commercial SnO2 nanoparticles (20–50 nm) at various current densities. Cycling performance of N-doped G–SnO2 paper (C) and SnO2 nanoparticles (D) at a current density of 50 mA g−1. (E) Schematic representation showing paths for lithium-ions and electrons (Le) in the N-doped G–SnO2 paper. Reproduced with permission from ref. 108. | ||
A novel anode material of Li-ion batteries was prepared by LBL assembly of graphene–MnO2 to an ultrathin, free-standing nanotube film with a unique nanostructure. The unique film structure does not only enhance Li-ion diffusion to the active sites of the film, but also enhances the electrical conductivity of the large surface area graphene layers. Consequently, the composite film demonstrated not only a reversible capacity of 686 mA h g−1 at a current rate of 100 mA g−1, but also 208 mA h g−1 at a high current rate of 1600 mA g−1. Furthermore, the capacity became stabilized at ∼500 mA h g−1 after cycling at various current rates.178
To increase the Li-ion insertion and extraction rate between layers of graphene paper, a unique 3-D nanostructure was fabricated using hybrid TiO2 pillars as the lithium ion battery anode materials. At a current rate of 2 A g−1, the specific capacity could reach 122 mA h g−1 after 100 charge/discharge cycles. More remarkably, the flexible graphene/TiO2 hybrid paper showed excellent stability when the rates were decreased from 4 A g−1 back to 200 mA g−1, with the retained capacity of 175 mA h g−1.181
Co3O4 nanosheets were assembled with graphene nanosheets through electrostatic interactions, then graphene paper was fabricated via vacuum filtration. When used as a binder-less and free-standing electrode for lithium ion batteries, the hybrid film delivered a high specific capacity (1400 mA h g−1 at 100 mA g−1 based on the total electrode weight), enhanced rate capability, and excellent cyclic stability (1200 mA h g−1 at 200 mA g−1 after 100 cycles).179
In another study, vertically aligned carbon nanotubes (VACNTs) were directly grown on the GP using a thermal CVD method. The as-prepared freestanding VACNT/GP film was employed as an integrated electrode combining both the active component and current collector for LIB, and good high-rate capability and cycling performance were demonstrated. When serving as the counter electrode of a DSSC, the VACNT/GP film displayed superior overall performance to the GP and TCNT/GP films and a slightly lower efficiency when compared with the Pt electrode. Owing to its good electrical conductivity, high thermal and chemical stability, and desirable flexibility, the VACNT/GP film may find application in high performance, flexible energy storage and conversion devices.182
Kim et al. reported the development of flexible graphene/mesoporous carbon films, which show a specific capacitance value of 86.7 F g−1 in a 1 M H2SO4 solution.186 Later, Mitlin et al. reported a sponge-like graphene based supercapacitor, which showed an extremely high power density of 48 kW kg−1.187
In addition to pure graphene, activated graphene materials have proven to be more promising as candidates for SC electrodes. R. S. Ruoff et al. reported that the specific surface area of the graphene prepared by microwave exfoliation of GO was significantly enhanced to 3100 m2 g−1 after activation by KOH. The activation treatment of graphene not only increased its specific surface area but also enabled a superior supercapacitor performance. A rectangle-like CV behaviour was maintained at a scan rate as high as 500 mV s−1 in an organic electrolyte. Graphene paper was prepared by holey graphene (h-graphene) to improve the accessibility of the electrolytes and ions to the graphene surface. The ultracapacitor electrodes based on h-graphene showed a remarkably improved volumetric capacitance with an approximately 700% increase compared to that of regular graphene electrodes.188
Boron, nitrogen, phosphorus, oxygen, and sulphur are the most commonly used elements for doping graphene. This could lead to an increase of the charge carrier concentration, specific surface area, and enhanced capacitance retention. Among these elements, a maximum proton loading can be achieved with boron substitution, further establishing the ability of B doping to enhance the interfacial capacitance.189 However, previous studies indicate that the actual capacitive performance is much lower than the anticipated value estimated from the ultrahigh theoretical surface area, which arises from the aggregation and restacking of graphene nanosheets in graphene papers. Therefore, various graphene-based composites/hybrids, including metal oxide nanoparticle/graphene and polymer chain/graphene papers, have been demonstrated to solve the above problem. Wang's group demonstrated a simple method for preparing flexible, free-standing, three-dimensional porous graphene/MnO2 nanorod and graphene/Ag hybrid thin-film electrodes. These graphene hybrid films accelerate ion and electron transport by providing lower ion-transport resistances and shorter diffusion-distances, thus exhibiting high specific capacitances and power performances, and also having excellent mechanical flexibility.190
Graphene/manganese dioxide (MnO2) composite papers (GMCPs) have been fabricated by a simple three-step route: preparation of the GO/MnO2 composite (GOMC) dispersion, subsequent vacuum filtration of the GOMC dispersion to achieve GO/MnO2 composite paper (GOMCP), and finally, thermal reduction of GOMCP to generate GMCP. The morphology and microstructure of the prepared samples were characterized using field-emission scanning electron microscopy, transmission electron microscopy, Raman spectroscopy, Fourier transformation infrared spectroscopy, thermal gravimetric analysis, and X-ray photoelectron spectroscopy. Moreover, as a binder-free and flexible electrode material for supercapacitors, the electrochemical properties of the prepared GMCP were evaluated using cyclic voltammetry and galvanostatic charge/discharge tests. As a result, the specific capacitance of the GMCP with a MnO2 weight ratio of 24% (GMCP-24) reached 256 F g−1 at a current density of 500 mA g−1, and also showed good cycle stability, indicating a promising potential application as an effective electrode material for supercapacitors.191
Asymmetric SCs can be fabricated by using different graphene composite/hybrid papers as positive and negative electrodes, respectively. Compared with conventional symmetric SCs, asymmetric SCs can make full use of the different potential windows of the two electrodes to broaden the maximum operating voltage of the cell system, which leads to an obvious improvement in the specific capacitance and energy density. The ability to fabricate flexible electrodes that exhibit very large surface areas and unusual or novel physical and electronic properties, and that can be assembled into asymmetric SCs, is therefore necessary for applications.
Polymer/graphene composite papers have also attracted great attention because of their relatively high strength when compared to pure inorganic based electrodes. Moreover, when applying graphene in conductive polymers, the conductivity of the polymers would also be increased. G. Shi et al. reported a flexible SC based on a polyaniline/graphene film.192 The conductivity of polyaniline fibres was found to be increased up to 44% when the composite was made with graphene. This flexible composite SC had a specific capacitance of 210 F g−1 at a current density of 0.3 A g−1.
Supercapacitor performance is significantly affected by the transport rates of electrons and ions, which usually depend on the microstructure and conductive characteristics of the electrode materials. These can be improved by fabricating interconnected electrode materials with a high conductivity and porous structure.21 3-D graphene materials, consisting of micro-, meso-, and even macroporous networks, can provide high surface area, light-weight, and fast ion/electron transport.
The rGO foam supercapacitor is able to bend. For example, Chen et al. reported a “leavening” process to transform compact graphene paper to porous graphene film (Fig. 18).29 They used a filtration assembled graphene paper as a dough and hydrazine vapour as a foaming and reducing agent. Cross-sectional SEM images indicated the formation of an open porous graphene foam network with pore sizes in the range of sub-micrometer to several micrometres. They concluded that the hydrazine vapour induced gaseous species (such as H2O and CO2) were responsible for the formation of the porous structure. They built flexible supercapacitors by using freestanding rGO foams as both current collectors and electrodes. The specific capacitance of the resulting rGO foam was 110 F g−1. It was found that there was only a very slight difference in the CV curves when the distance between the two sides of the supercapacitor was changed from 3 to 2 cm under bending.
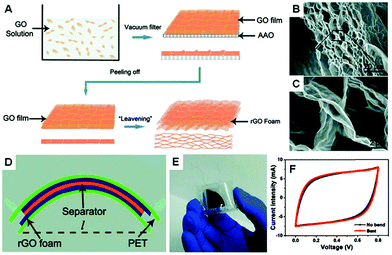 | ||
| Fig. 18 (A) Schematic drawings illustrating the process to prepare rGO foams. (B and C) Cross-sectional SEM images of the rGO foams. (D and E) Schematic diagram and optical image of the flexible rGO foam supercapacitor. (F) CV curves of the rGO foam supercapacitor before bending and while bent. Reproduced with permission from ref. 29. | ||
5. Concluding remarks and perspectives
Scale-up assembly of nanomaterials such as graphene and its derivatives is a promising way to enable the materials to be connected to real-world applications. In comparison with 2D graphene nanosheets, their macroscopic assemblies are much more visible, handable, and programmable, as well-demonstrated in the literature studies outlined above.Graphene papers (or membranes) have features of low-cost, ease of processing and versatile functionality. They can be prepared by solution-phase assembly, EPD and CVD methods, and further functionalized specifically by polymers, nano-objects, enzymes, and proteins to endow them with different desirable functions based on different structures. These composite materials in the form of paper are emerging and proving to meet a number of applications in the fields of sensing, water purification, and energy conversion and storage.
Although graphene papers as a kind of new free-standing and flexible material have shown several remarkable advantages as discussed, their physiochemical properties and diversity of functionality are expected to be continuously improved by ongoing research. Both mechanical strength and conductivity are the key parameters for graphene papers to meet future flexible electronics. Another challenging issue could be their biocompatibility if graphene papers are used in biomedical devices. Materials chemistry should play a vital role in offering the solutions to these challenges. Thus, it could be anticipated that the future research efforts would focus on the further improvement of their mechanical strength and conductivity, controllable engineering of the structure and spacer between interlayers, and local positioning functionalization.
The continuous efforts to improve the performances of graphene papers will lead the way to real-life products, even as potential substitutes for many long-standing functional materials such as silicon, metal oxides, conducting polymers and even biomaterials. Towards such a purpose, cost-efficiency and large-scale production are two essential factors that have yet to be fully proven. However, if we take the industrial success of carbon fibres as an example, our confidence should not lag behind the expectation that graphene paper based materials can meet these milestones in the near future.
Acknowledgements
This work was supported by DFF-FTP (the Danish Research Council for Technology and Product Science, Project No. 12-127447). C. H. is grateful for financial support by the Ørsted-Marie-Curie Cofunded postdoc fellowship, the Shanghai ChenGuang Program (15CG33), the Shanghai Natural Science Foundation (16ZR1401500), the Shanghai Sailing Program (16YF1400400), and the China Postdoctoral Science Foundation. M. Z. acknowledges the CSC PhD scholarship (No. 201306170047). H. W. thanks financial support by the NSFC (No. 51572046, 51503035), SNSF (15ZR1401200), Eastern Scholar, PSARL (16XD1400100), and PITDU (No. 111-2-04).Notes and references
- K. S. Novoselov, A. K. Geim, S. V. Morozov, D. Jiang, Y. Zhang, S. V. Dubonos, I. V. Grigorieva and A. A. Firsov, Science, 2004, 306, 666–669 CrossRef CAS PubMed
.
- K. S. Novoselov, D. Jiang, F. Schedin, T. J. Booth, V. V. Khotkevich, S. V. Morozov and A. K. Geim, Proc. Natl. Acad. Sci. U. S. A., 2005, 102, 10451–10453 CrossRef CAS PubMed
.
- K. S. Novoselov, A. K. Geim, S. V. Morozov, D. Jiang, M. I. Katsnelson, I. V. Grigorieva, S. V. Dubonos and A. A. Firsov, Nature, 2005, 438, 197–200 CrossRef CAS PubMed
.
- Y. Hernandez, V. Nicolosi, M. Lotya, F. M. Blighe, Z. Sun, S. De, I. T. McGovern, B. Holland, M. Byrne, Y. K. Gun'ko, J. J. Boland, P. Niraj, G. Duesberg, S. Krishnamurthy, R. Goodhue, J. Hutchison, V. Scardaci, A. C. Ferrari and J. N. Coleman, Nat. Nanotechnol., 2008, 3, 563–568 CrossRef CAS PubMed
.
- C. Valles, C. Drummond, H. Saadaoui, C. A. Furtado, M. He, O. Roubeau, L. Ortolani, M. Monthioux and A. Penicaud, J. Am. Chem. Soc., 2008, 130, 15802–15804 CrossRef CAS PubMed
.
- A. B. Bourlinos, V. Georgakilas, R. Zboril, T. A. Steriotis and A. K. Stubos, Small, 2009, 5, 1841–1845 CrossRef CAS PubMed
.
- Y. F. Hao, M. S. Bharathi, L. Wang, Y. Y. Liu, H. Chen, S. Nie, X. H. Wang, H. Chou, C. Tan, B. Fallahazad, H. Ramanarayan, C. W. Magnuson, E. Tutuc, B. I. Yakobson, K. F. McCarty, Y. W. Zhang, P. Kim, J. Hone, L. Colombo and R. S. Ruoff, Science, 2013, 342, 720–723 CrossRef CAS PubMed
.
- X. S. Li, W. W. Cai, J. H. An, S. Kim, J. Nah, D. X. Yang, R. Piner, A. Velamakanni, I. Jung, E. Tutuc, S. K. Banerjee, L. Colombo and R. S. Ruoff, Science, 2009, 324, 1312–1314 CrossRef CAS PubMed
.
- Z. Z. Sun, Z. Yan, J. Yao, E. Beitler, Y. Zhu and J. M. Tour, Nature, 2010, 468, 549–552 CrossRef CAS PubMed
.
- G. Eda, G. Fanchini and M. Chhowalla, Nat. Nanotechnol., 2008, 3, 270–274 CrossRef CAS PubMed
.
- S. Stankovich, D. A. Dikin, G. H. B. Dommett, K. M. Kohlhaas, E. J. Zimney, E. A. Stach, R. D. Piner, S. T. Nguyen and R. S. Ruoff, Nature, 2006, 442, 282–286 CrossRef CAS PubMed
.
- X. Fan, W. Peng, Y. Li, X. Li, S. Wang, G. Zhang and F. Zhang, Adv. Mater., 2008, 20, 4490–4493 CrossRef CAS
.
- K. V. Emtsev, A. Bostwick, K. Horn, J. Jobst, G. L. Kellogg, L. Ley, J. L. McChesney, T. Ohta, S. A. Reshanov, J. Roehrl, E. Rotenberg, A. K. Schmid, D. Waldmann, H. B. Weber and T. Seyller, Nat. Mater., 2009, 8, 203–207 CrossRef CAS PubMed
.
- L. Y. Jiao, L. Zhang, X. R. Wang, G. Diankov and H. J. Dai, Nature, 2009, 458, 877–880 CrossRef CAS PubMed
.
- D. V. Kosynkin, A. L. Higginbotham, A. Sinitskii, J. R. Lomeda, A. Dimiev, B. K. Price and J. M. Tour, Nature, 2009, 458, 872–875 CrossRef CAS PubMed
.
- A. Rouhanipour, M. Roy, X. L. Feng, H. J. Rader and K. Mullen, Angew. Chem., Int. Ed., 2009, 48, 4602–4604 CrossRef CAS PubMed
.
- L. J. Zhi and K. Mullen, J. Mater. Chem., 2008, 18, 1472–1484 RSC
.
- G. Liu, W. Jin and N. Xu, Chem. Soc. Rev., 2015, 44, 5016–5030 RSC
.
- H. Gao and H. Duan, Biosens. Bioelectron., 2014, 65, 404–419 CrossRef PubMed
.
- X. Zhao, P. Zhang, Y. Chen, Z. Su and G. Wei, Nanoscale, 2015, 7, 5080–5093 RSC
.
- Y. Shao, M. F. El-Kady, L. J. Wang, Q. Zhang, Y. Li, H. Wang, M. F. Mousavi and R. B. Kaner, Chem. Soc. Rev., 2015, 44, 3639–3665 RSC
.
- D. A. Dikin, S. Stankovich, E. J. Zimney, R. D. Piner, G. H. B. Dommett, G. Evmenenko, S. T. Nguyen and R. S. Ruoff, Nature, 2007, 448, 457–460 CrossRef CAS PubMed
.
- G. Wang, X. Sun, F. Lu, H. Sun, M. Yu, W. Jiang, C. Liu and J. Lian, Small, 2012, 8, 452–459 CrossRef CAS PubMed
.
- N. Zhu, S. Han, S. Gan, J. Ulstrup and Q. Chi, Adv. Funct. Mater., 2013, 23, 5297–5306 CrossRef CAS
.
- H. Chen, M. B. Mueller, K. J. Gilmore, G. G. Wallace and D. Li, Adv. Mater., 2008, 20, 3557–3561 CrossRef CAS
.
- O. C. Compton, D. A. Dikin, K. W. Putz, L. C. Brinson and S. T. Nguyen, Adv. Mater., 2010, 22, 892–896 CrossRef CAS PubMed
.
- X. Y. Lin, X. Shen, Q. B. Zheng, N. Yousefi, L. Ye, Y. W. Mai and J. K. Kim, ACS Nano, 2012, 6, 10708–10719 CrossRef CAS PubMed
.
- C. Hou, H. Wang, Q. Zhang, Y. Li and M. Zhu, Adv. Mater., 2014, 26, 5018–5024 CrossRef CAS PubMed
.
- Z. Niu, J. Chen, H. H. Hng, J. Ma and X. Chen, Adv. Mater., 2012, 24, 4144–4150 CrossRef CAS PubMed
.
- Q. Shi, C. Hou, H. Wang, Q. Zhang and Y. Li, J. Mater. Chem. A, 2015, 3, 9882–9889 CAS
.
- G. K. Ramesha and S. Sampath, J. Phys. Chem. C, 2009, 113, 7985–7989 CAS
.
- M. A. Raj and S. A. John, J. Phys. Chem. C, 2013, 117, 4326–4335 CAS
.
- S. L. Yang, B. F. Xu, J. Q. Zhang, X. D. Huang, J. Ye and C. Z. Yu, J. Phys. Chem. C, 2010, 114, 4389–4393 CAS
.
- L. J. Cote, F. Kim and J. Huang, J. Am. Chem. Soc., 2009, 131, 1043–1049 CrossRef CAS PubMed
.
- F. Kim, L. J. Cote and J. Huang, Adv. Mater., 2010, 22, 1954–1958 CrossRef CAS PubMed
.
- D. D. Kulkarni, I. Choi, S. Singamaneni and V. V. Tsukruk, ACS Nano, 2010, 4, 4667–4676 CrossRef CAS PubMed
.
- Q. Zheng, W. H. Ip, X. Lin, N. Yousefi, K. K. Yeung, Z. Li and J. K. Kim, ACS Nano, 2011, 5, 6039–6051 CrossRef CAS PubMed
.
- X. Lin, J. Jia, N. Yousefi, X. Shen and J.-K. Kim, J. Mater. Chem. C, 2013, 1, 6869–6877 RSC
.
- C. Chen, Q.-H. Yang, Y. Yang, W. Lv, Y. Wen, P.-X. Hou, M. Wang and H.-M. Cheng, Adv. Mater., 2009, 21, 3007–3011 CrossRef CAS
.
- C. M. Chen, Q. Zhang, C. H. Huang, X. C. Zhao, B. S. Zhang, Q. Q. Kong, M. Z. Wang, Y. G. Yang, R. Cai and D. S. Su, Chem. Commun., 2012, 48, 7149–7151 RSC
.
- S. J. Woltornist, A. J. Oyer, J. M. Carrillo, A. V. Dobrynin and D. H. Adamson, ACS Nano, 2013, 7, 7062–7066 CrossRef CAS PubMed
.
- S. Borini, R. White, D. Wei, M. Astley, S. Haque, E. Spigone, N. Harris, J. Kivioja and T. Ryhanen, ACS Nano, 2013, 7, 11166–11173 CrossRef CAS PubMed
.
- B.-H. Wee and J.-D. Hong, Adv. Funct. Mater., 2013, 23, 4657–4666 CrossRef CAS
.
- X. Li, G. Zhang, X. Bai, X. Sun, X. Wang, E. Wang and H. Dai, Nat. Nanotechnol., 2008, 3, 538–542 CrossRef CAS PubMed
.
- T. Li, J. R. Hauptmann, Z. M. Wei, S. Petersen, N. Bovet, T. Vosch, J. Nygard, W. P. Hu, Y. Q. Liu, T. Bjornholm, K. Norgaard and B. W. Laursen, Adv. Mater., 2012, 24, 1333–1339 CrossRef CAS PubMed
.
- S. Seo, M. Min, J. Lee, T. Lee, S. Y. Choi and H. Lee, Angew. Chem., Int. Ed., 2012, 51, 108–112 CrossRef CAS PubMed
.
- A. Chavez-Valdez, M. S. Shaffer and A. R. Boccaccini, J. Phys. Chem. B, 2013, 117, 1502–1515 CrossRef CAS PubMed
.
- S. J. An, Y. W. Zhu, S. H. Lee, M. D. Stoller, T. Emilsson, S. Park, A. Velamakanni, J. H. An and R. S. Ruoff, J. Phys. Chem. Lett., 2010, 1, 1259–1263 CrossRef CAS
.
- S. A. Hasan, J. L. Rigueur, R. R. Harl, A. J. Krejci, I. Gonzalo-Juan, B. R. Rogers and J. H. Dickerson, ACS Nano, 2010, 4, 7367–7372 CrossRef CAS PubMed
.
- G. F. Cai, J. P. Tu, J. Zhang, Y. J. Mai, Y. Lu, C. D. Gu and X. L. Wang, Nanoscale, 2012, 4, 5724–5730 RSC
.
- H. Zhang, X. Zhang, D. Zhang, X. Sun, H. Lin, C. Wang and Y. Ma, J. Phys. Chem. B, 2013, 117, 1616–1627 CrossRef CAS PubMed
.
- S. Liu, J. Ou, J. Wang, X. Liu and S. Yang, J. Appl. Electrochem., 2011, 41, 881–884 CrossRef CAS
.
- Y. Chen, X. Zhang, P. Yu and Y. Ma, J. Power Sources, 2010, 195, 3031–3035 CrossRef CAS
.
- D. H. Lee, J. E. Kim, T. H. Han, J. W. Hwang, S. Jeon, S. Y. Choi, S. H. Hong, W. J. Lee, R. S. Ruoff and S. O. Kim, Adv. Mater., 2010, 22, 1247–1252 CrossRef CAS PubMed
.
- B. Aleman, W. Regan, S. Aloni, V. Altoe, N. Alem, C. Girit, B. S. Geng, L. Maserati, M. Crommie, F. Wang and A. Zettl, ACS Nano, 2010, 4, 4762–4768 CrossRef CAS PubMed
.
- S. Lee, K. Lee and Z. H. Zhong, Nano Lett., 2010, 10, 4702–4707 CrossRef CAS PubMed
.
- K. S. Kim, Y. Zhao, H. Jang, S. Y. Lee, J. M. Kim, K. S. Kim, J. H. Ahn, P. Kim, J. Y. Choi and B. H. Hong, Nature, 2009, 457, 706–710 CrossRef CAS PubMed
.
- A. Reina, X. Jia, J. Ho, D. Nezich, H. Son, V. Bulovic, M. S. Dresselhaus and J. Kong, Nano Lett., 2009, 9, 30–35 CrossRef CAS PubMed
.
- M. Losurdo, M. M. Giangregorio, P. Capezzuto and G. Bruno, Phys. Chem. Chem. Phys., 2011, 13, 20836–20843 RSC
.
- M. E. Ramon, A. Gupta, C. Corbet, D. A. Ferrer, H. C. P. Movva, G. Carpenter, L. Colombo, G. Bourianoff, M. Doczy, D. Akinwande, E. Tutuc and S. K. Banerjee, ACS Nano, 2011, 5, 7198–7204 CrossRef CAS PubMed
.
- H. Bi, S. R. Sun, F. Q. Huang, X. M. Xie and M. H. Jiang, J. Mater. Chem., 2012, 22, 411–416 RSC
.
- Z. Liu, L. Song, S. Z. Zhao, J. Q. Huang, L. L. Ma, J. N. Zhang, J. Lou and P. M. Ajayan, Nano Lett., 2011, 11, 2032–2037 CrossRef CAS PubMed
.
- J. Y. Chen, Y. L. Guo, Y. G. Wen, L. P. Huang, Y. Z. Xue, D. C. Geng, B. Wu, B. R. Luo, G. Yu and Y. Q. Liu, Adv. Mater., 2013, 25, 992–997 CrossRef CAS PubMed
.
- G. Wang, M. Zhang, Y. Zhu, G. Q. Ding, D. Jiang, Q. L. Guo, S. Liu, X. M. Xie, P. K. Chu, Z. F. Di and X. Wang, Sci. Rep., 2013, 3, 2465 Search PubMed
.
- X. B. Cao, D. P. Qi, S. Y. Yin, J. Bu, F. J. Li, C. F. Goh, S. Zhang and X. D. Chen, Adv. Mater., 2013, 25, 2957–2962 CrossRef CAS PubMed
.
- S. Y. Yin, Y. Goldovsky, M. Herzberg, L. Liu, H. Sun, Y. Y. Zhang, F. B. Meng, X. B. Cao, D. D. Sun, H. Y. Chen, A. Kushmaro and X. D. Chen, Adv. Funct. Mater., 2013, 23, 2972–2978 CrossRef CAS
.
- S. Y. Yin, Y. Y. Zhang, J. H. Kong, C. J. Zou, C. M. Li, X. H. Lu, J. Ma, F. Y. C. Boey and X. D. Chen, ACS Nano, 2011, 5, 3831–3838 CrossRef CAS PubMed
.
- L. L. Zhang, X. Zhao, M. D. Stoller, Y. Zhu, H. Ji, S. Murali, Y. Wu, S. Perales, B. Clevenger and R. S. Ruoff, Nano Lett., 2012, 12, 1806–1812 CrossRef CAS PubMed
.
- Y. Zhu, S. Murali, M. D. Stoller, K. J. Ganesh, W. Cai, P. J. Ferreira, A. Pirkle, R. M. Wallace, K. A. Cychosz, M. Thommes, D. Su, E. A. Stach and R. S. Ruoff, Science, 2011, 332, 1537–1541 CrossRef CAS PubMed
.
- F. Liu, S. Song, D. Xue and H. Zhang, Adv. Mater., 2012, 24, 1089–1094 CrossRef CAS PubMed
.
- P. Simek, Z. Sofer, O. Jankovsky, D. Sedmidubsky and M. Pumera, Adv. Funct. Mater., 2014, 24, 4878–4885 CrossRef CAS
.
- S. H. Bae, O. Kahya, B. K. Sharma, J. Kwon, H. J. Cho, B. Ozyilmaz and J. H. Ahn, ACS Nano, 2013, 7, 3130–3138 CrossRef CAS PubMed
.
- J. Shang, Y. Zhang, L. Yu, X. Luan, B. Shen, Z. Zhang, F. Lv and P. K. Chu, J. Mater. Chem. A, 2013, 1, 884–890 CAS
.
- N. Ruecha, R. Rangkupan, N. Rodthongkum and O. Chailapakul, Biosens. Bioelectron., 2014, 52, 13–19 CrossRef CAS PubMed
.
- A. K. Sarker and J. D. Hong, Langmuir, 2012, 28, 12637–12646 CrossRef CAS PubMed
.
- S. Liu, X. Liu, Z. Li, S. Yang and J. Wang, New J. Chem., 2011, 35, 369–374 RSC
.
- D. K. Huang, B. Y. Zhang, Y. B. Zhang, F. Zhan, X. B. Xu, Y. Shen and M. K. Wang, J. Mater. Chem. A, 2013, 1, 1415–1420 CAS
.
- W. Qi, Z. Xue, W. Yuan and H. Wang, J. Mater. Chem. B, 2014, 2, 325 RSC
.
- J. T. Chen, Y. J. Fu, Q. F. An, S. C. Lo, S. H. Huang, W. S. Hung, C. C. Hu, K. R. Lee and J. Y. Lai, Nanoscale, 2013, 5, 9081–9088 RSC
.
- X. Yan, J. Chen, J. Yang, Q. Xue and P. Miele, ACS Appl. Mater. Interfaces, 2010, 2, 2521–2529 CAS
.
- Y. Wang, J. Yu, L. Chen, Z. Hu, Z. Shi and J. Zhu, RSC Adv., 2013, 3, 20353 RSC
.
- H.-P. Cong, X.-C. Ren, P. Wang and S.-H. Yu, Energy Environ. Sci., 2013, 6, 1185–1191 CAS
.
- B. G. Choi, H. Park, T. J. Park, M. H. Yang, J. S. Kim, S. Y. Jang, N. S. Heo, S. Y. Lee, J. Kong and W. H. Hong, ACS Nano, 2010, 4, 2910–2918 CrossRef CAS PubMed
.
- X. L. Hu, R. R. Qi, J. Zhu, J. Q. Lu, Y. Luo, J. Y. Jin and P. K. Jiang, J. Appl. Polym. Sci., 2014, 131, 39754 Search PubMed
.
- H. Liu, T. Kuila, N. H. Kim, B.-C. Ku and J. H. Lee, J. Mater. Chem. A, 2013, 1, 3739–3746 CAS
.
- T. D. Dao, J.-E. Hong, K.-S. Ryu and H. M. Jeong, Chem. Eng. J., 2014, 250, 257–266 CrossRef CAS
.
- H. O. Choi, D. W. Kim, S. J. Kim, S. B. Yang and H. T. Jung, Adv. Mater., 2014, 26, 4575–4581 CrossRef CAS PubMed
.
- P. Mulpur, R. Podila, K. Lingam, S. K. Vemula, S. S. Ramamurthy, V. Kamisetti and A. M. Rao, J. Phys. Chem. C, 2013, 117, 17205–17210 CAS
.
- L. J. Zhong, S. Y. Gan, X. G. Fu, F. H. Li, D. X. Han, L. P. Guo and L. Niu, Electrochim. Acta, 2013, 89, 222–228 CrossRef CAS
.
- H. Gwon, H.-S. Kim, K. U. Lee, D.-H. Seo, Y. C. Park, Y.-S. Lee, B. T. Ahn and K. Kang, Energy Environ. Sci., 2011, 4, 1277–1283 CAS
.
- H. Bi, W. Zhao, S. Sun, H. Cui, T. Lin, F. Huang, X. Xie and M. Jiang, Carbon, 2013, 61, 116–123 CrossRef CAS
.
- G. F. Cai, J. P. Tu, J. Zhang, Y. J. Mai, Y. Lu, C. D. Gu and X. L. Wang, Nanoscale, 2012, 4, 5724–5730 RSC
.
- Y. Zhang, X. P. Xiao, Y. J. Sun, Y. Shi, H. C. Dai, P. J. Ni, J. T. Hu, Z. Li, Y. H. Song and L. Wang, Electroanalysis, 2013, 25, 959–966 CrossRef CAS
.
- B. Zhan, C. Liu, H. Chen, H. Shi, L. Wang, P. Chen, W. Huang and X. Dong, Nanoscale, 2014, 6, 7424–7429 RSC
.
- H.-B. Yao, L.-H. Wu, C.-H. Cui, H.-Y. Fang and S.-H. Yu, J. Mater. Chem., 2010, 20, 5190 RSC
.
- X. Sun, M. Xie, J. J. Travis, G. Wang, H. Sun, J. Lian and S. M. George, J. Phys. Chem. C, 2013, 117, 22497–22508 CAS
.
- C. H. Lee, Y. J. Kim, Y. J. Hong, S. R. Jeon, S. Bae, B. H. Hong and G. C. Yi, Adv. Mater., 2011, 23, 4614–4619 CrossRef CAS PubMed
.
- F. Xiao, Y. Li, X. Zan, K. Liao, R. Xu and H. Duan, Adv. Funct. Mater., 2012, 22, 2487–2494 CrossRef CAS
.
- F. Xiao, Y. Q. Li, H. C. Gao, S. B. Ge and H. W. Duan, Biosens. Bioelectron., 2013, 41, 417–423 CrossRef CAS PubMed
.
- B. Liang, L. Fang, Y. C. Hu, G. Yang, Q. Zhu and X. S. Ye, Nanoscale, 2014, 6, 4264–4274 RSC
.
- X. Zan, Z. Fang, J. Wu, F. Xiao, F. Huo and H. Duan, Biosens. Bioelectron., 2013, 49, 71–78 CrossRef CAS PubMed
.
- Y. K. Kim, H. K. Na and D. H. Min, Langmuir, 2010, 26, 13065–13070 CrossRef CAS PubMed
.
- S. Chen, J. Duan, J. Ran, M. Jaroniec and S. Z. Qiao, Energy Environ. Sci., 2013, 6, 3693–3699 CAS
.
- S. Chen, J. Zhu, L. Qiu, D. Li and X. Wang, Chem. – Eur. J., 2013, 19, 7631–7636 CrossRef CAS PubMed
.
- M. Zhang, A. Halder, C. Hou, J. Ulstrup and Q. Chi, Bioelectrochemistry, 2016, 109, 87–94 CrossRef CAS PubMed
.
- H. L. Zhu, K. T. Lee, G. T. Hitz, X. G. Han, Y. Y. Li, J. Y. Wan, S. Lacey, A. V. Cresce, K. Xu, E. Wachsman and L. B. Hu, ACS Appl. Mater. Interfaces, 2014, 6, 4242–4247 CAS
.
- J. F. Liang, Y. Zhao, L. Guo and L. D. Li, ACS Appl. Mater. Interfaces, 2012, 4, 5742–5748 CAS
.
- J. Liang, Y. Xu, D. Sui, L. Zhang, Y. Huang, Y. Ma, F. Li and Y. Chen, J. Phys. Chem. C, 2010, 114, 17465–17471 CAS
.
- A. Sumboja, C. Y. Foo, X. Wang and P. S. Lee, Adv. Mater., 2013, 25, 2809–2815 CrossRef CAS PubMed
.
- F. Liu, Y. Piao, K. S. Choi and T. S. Seo, Carbon, 2012, 50, 123–133 CrossRef CAS
.
- M.-K. Dai, J.-T. Lian, T.-Y. Lin and Y.-F. Chen, J. Mater. Chem. C, 2013, 1, 5064–5071 RSC
.
- S. Chen and S. Z. Qiao, ACS Nano, 2013, 7, 10190–10196 CrossRef CAS PubMed
.
- X. Wang, X. Cao, L. Bourgeois, H. Guan, S. Chen, Y. Zhong, D.-M. Tang, H. Li, T. Zhai, L. Li, Y. Bando and D. Golberg, Adv. Funct. Mater., 2012, 22, 2682–2690 CrossRef CAS
.
- Q. Xi, X. Chen, D. G. Evans and W. Yang, Langmuir, 2012, 28, 9885–9892 CrossRef CAS PubMed
.
- L. Z. Sheng, Y. Liang, L. L. Jiang, Q. Wang, T. Wei, L. T. Qu and Z. J. Fan, Adv. Funct. Mater., 2015, 25, 6545–6551 CrossRef CAS
.
- G. R. S. Iyer, J. Wang, G. Wells, M. P. Bradley and F. Borondics, Nanoscale, 2015, 7, 2289–2294 RSC
.
- D. Cohen-Tanugi and J. C. Grossman, Nano Lett., 2012, 12, 3602–3608 CrossRef CAS PubMed
.
- R. R. Nair, H. A. Wu, P. N. Jayaram, I. V. Grigorieva and A. K. Geim, Science, 2012, 335, 442–444 CrossRef CAS PubMed
.
- R. K. Joshi, P. Carbone, F. C. Wang, V. G. Kravets, Y. Su, I. V. Grigorieva, H. A. Wu, A. K. Geim and R. R. Nair, Science, 2014, 343, 752–754 CrossRef CAS PubMed
.
- B. X. Mi, Science, 2014, 343, 740–742 CrossRef CAS PubMed
.
- S. Karan, S. Samitsu, X. Peng, K. Kurashima and I. Ichinose, Science, 2012, 335, 444–447 CrossRef CAS PubMed
.
- S. Aisenberg and R. Chabot, J. Appl. Phys., 1971, 42, 2953–2958 CrossRef CAS
.
- H. H. Cheng, J. Liu, Y. Zhao, C. G. Hu, Z. P. Zhang, N. Chen, L. Jiang and L. T. Qu, Angew. Chem., Int. Ed., 2013, 52, 10482–10486 CrossRef CAS PubMed
.
- D. W. Boukhvalov, M. I. Katsnelson and Y. W. Son, Nano Lett., 2013, 13, 3930–3935 CrossRef CAS PubMed
.
- J. Mu, C. Hou, B. Zhu, H. Wang, Y. Li and Q. Zhang, Sci. Rep., 2015, 5, 9503 CrossRef CAS PubMed
.
- J. Mu, C. Hou, H. Wang, Y. Li, Q. Zhang and M. Zhu, Sci. Adv., 2015, 1, e1500533 Search PubMed
.
- C. Hou, T. Huang, H. Wang, H. Yu, Q. Zhang and Y. Li, Sci. Rep., 2013, 3, 3138 Search PubMed
.
- K. ul Hasan, M. O. Sandberg, O. Nur and M. Willander, Adv. Opt. Mater., 2014, 2, 326–330 CrossRef CAS
.
- J. Sun, M. A. Memon, W. Bai, L. Xiao, B. Zhang, Y. Jin, Y. Huang and J. Geng, Adv. Funct. Mater., 2015, 25, 4334–4343 CrossRef CAS
.
- Y.-J. Kim, H. Yoo, C.-H. Lee, J. B. Park, H. Baek, M. Kim and G.-C. Yi, Adv. Mater., 2012, 24, 5565–5569 CrossRef CAS PubMed
.
- H. Yoo, K. Chung, Y. S. Choi, C. S. Kang, K. H. Oh, M. Kim and G.-C. Yi, Adv. Mater., 2012, 24, 515–518 CrossRef CAS PubMed
.
- D. Choi, M.-Y. Choi, W. M. Choi, H.-J. Shin, H.-K. Park, J.-S. Seo, J. Park, S.-M. Yoon, S. J. Chae, Y. H. Lee, S.-W. Kim, J.-Y. Choi, S. Y. Lee and J. M. Kim, Adv. Mater., 2010, 22, 2187–2192 CrossRef CAS PubMed
.
- F. Zhao, H. Cheng, Z. Zhang, L. Jiang and L. Qu, Adv. Mater., 2015, 27, 4351–4357 CrossRef CAS PubMed
.
- Y. Song, C. Chen and C. Wang, Nanoscale, 2015, 7, 7084–7090 RSC
.
- C. Liu, S. Alwarappan, Z. Chen, X. Kong and C. Z. Li, Biosens. Bioelectron., 2010, 25, 1829–1833 CrossRef CAS PubMed
.
- A. S. Campbell, Y. J. Jeong, S. M. Geier, R. R. Koepsel, A. J. Russell and M. F. Islam, ACS Appl. Mater. Interfaces, 2015, 7, 4056–4065 CAS
.
- W. H. Gao, Y. S. Chen, J. Xi, S. Y. Lin, Y. W. Chen, Y. J. Lin and Z. G. Chen, Biosens. Bioelectron., 2013, 41, 776–782 CrossRef CAS PubMed
.
- Y. Wang, J. Ping, Z. Ye, J. Wu and Y. Ying, Biosens. Bioelectron., 2013, 49, 492–498 CrossRef CAS PubMed
.
- Z. Zheng, Y. Du, Z. Wang, Q. Feng and C. Wang, Analyst, 2013, 138, 693–701 RSC
.
- F. Liu, K. S. Choi, T. J. Park, S. Y. Lee and T. S. Seo, BioChip J., 2011, 5, 123–128 CrossRef CAS
.
- M. M. Barsan, M. David, M. Florescu, L. Tugulea and C. M. A. Brett, Bioelectrochemistry, 2014, 99, 46–52 CrossRef CAS PubMed
.
- X. Zhong, R. Yuan and Y. Q. Chai, Sens. Actuators, B, 2012, 162, 334–340 CrossRef CAS
.
- F.-Y. Kong, S.-X. Gu, W.-W. Li, T.-T. Chen, Q. Xu and W. Wang, Biosens. Bioelectron., 2014, 56, 77–82 CrossRef CAS PubMed
.
- Y. X. Huang, X. C. Dong, Y. M. Shi, C. M. Li, L. J. Li and P. Chen, Nanoscale, 2010, 2, 1485–1488 RSC
.
- F. Xiao, J. B. Song, H. C. Gao, X. L. Zan, R. Xu and H. W. Duan, ACS Nano, 2012, 6, 100–110 CrossRef CAS PubMed
.
- Y. Xu, B. Lou, Z. Lv, Z. Zhou, L. Zhang and E. Wang, Anal. Chim. Acta, 2013, 763, 20–27 CrossRef CAS PubMed
.
- Y. Xu, Z. Lv, Y. Xia, Y. Han, B. Lou and E. Wang, Anal. Bioanal. Chem., 2013, 405, 3549–3558 CrossRef CAS PubMed
.
- Y. Zhang, L. Ge, S. Ge, M. Yan, J. Yan, D. Zang, J. Lu, J. Yu and X. Song, Electrochim. Acta, 2013, 112, 620–628 CrossRef CAS
.
- P. Zuo, X. Li, D. C. Dominguez and B.-C. Ye, Lab Chip, 2013, 13, 3921–3928 RSC
.
- L. Deng, C. G. Chen, C. Z. Zhu, S. J. Dong and H. M. Lu, Biosens. Bioelectron., 2014, 52, 324–329 CrossRef CAS PubMed
.
- X. Y. Bai, G. H. Chen and K. K. Shiu, Electrochim. Acta, 2013, 89, 454–460 CrossRef CAS
.
- X. D. Cao, Y. K. Ye, Y. Li, X. Xu, J. C. Yu and S. Q. Liu, J. Electroanal. Chem., 2013, 697, 10–14 CrossRef CAS
.
- C. J. Cai, M. W. Xu, S. J. Bao, C. Lei and D. Z. Jia, RSC Adv., 2012, 2, 8172–8178 RSC
.
- Q. A. Zhang, Y. Qiao, L. Zhang, S. Y. Wu, H. Zhou, J. W. Xu and X. M. Song, Electroanalysis, 2011, 23, 900–906 CrossRef CAS
.
- O. Leenaerts, B. Partoens and F. Peeters, Phys. Rev. B: Condens. Matter Mater. Phys., 2008, 77, 125416 CrossRef
.
- G. Lu, L. E. Ocola and J. Chen, Appl. Phys. Lett., 2009, 94, 083111 CrossRef
.
- S. V. Samsonau, S. D. Shvarkov, F. Meinerzhagen, A. D. Wieck and A. M. Zaitsev, Sens. Actuators, B, 2013, 182, 66–70 CrossRef CAS
.
- J. Hassinen, J. Kauppila, J. Leiro, A. Maattanen, P. Ihalainen, J. Peltonen and J. Lukkari, Anal. Bioanal. Chem., 2013, 405, 3611–3617 CrossRef CAS PubMed
.
- F. Yavari, Z. Chen, A. V. Thomas, W. Ren, H. M. Cheng and N. Koratkar, Sci. Rep., 2011, 1, 166 Search PubMed
.
- M. Q. Xu, J. F. Wu and G. C. Zhao, Sensors, 2013, 13, 7492–7504 CrossRef CAS PubMed
.
- C. X. Guo, S. R. Ng, S. Y. Khoo, X. T. Zheng, P. Chen and C. M. Li, ACS Nano, 2012, 6, 6944–6951 CrossRef CAS PubMed
.
- Z. Jiang, J. Li, H. Aslan, Q. Li, Y. Li, M. Chen, Y. Huang, J. P. Froning, M. Otyepka, R. Zboril, F. Besenbacher and M. Dong, J. Mater. Chem. A, 2014, 2, 6714–6717 CAS
.
- S. Some, Y. Xu, Y. Kim, Y. Yoon, H. Qin, A. Kulkarni, T. Kim and H. Lee, Sci. Rep., 2013, 3, 1868 Search PubMed
.
- O. C. Compton, S. W. Cranford, K. W. Putz, Z. An, L. C. Brinson, M. J. Buehler and S. T. Nguyen, ACS Nano, 2012, 6, 2008–2019 CrossRef CAS PubMed
.
- S. Park, J. An, J. W. Suk and R. S. Ruoff, Small, 2010, 6, 210–212 CrossRef CAS PubMed
.
- X. Wang, X. Sun, P. A. Hu, J. Zhang, L. Wang, W. Feng, S. Lei, B. Yang and W. Cao, Adv. Funct. Mater., 2013, 23, 6044–6050 CrossRef CAS
.
- C. L. Heldt, A. K. Sieloff, J. P. Merillat, A. R. Minerick, J. A. King, W. F. Perger, H. Fukushima and J. Narendra, Sens. Actuators, B, 2013, 181, 92–98 CrossRef CAS
.
- L. A. Yan, Y. N. Chang, W. Y. Yin, X. D. Liu, D. B. Xiao, G. M. Xing, L. N. Zhao, Z. J. Gu and Y. L. Zhao, Phys. Chem. Chem. Phys., 2014, 16, 1576–1582 RSC
.
- J. Ping, Y. Wang, K. Fan, W. Tang, J. Wu and Y. Ying, J. Mater. Chem. B, 2013, 1, 4781–4791 RSC
.
- C. Xu, B. Xu, Y. Gu, Z. Xiong, J. Sun and X. S. Zhao, Energy Environ. Sci., 2013, 6, 1388–1414 CAS
.
- O. C. Compton, B. Jain, D. A. Dikin, A. Abouimrane, K. Amine and S. T. Nguyen, ACS Nano, 2011, 5, 4380–4391 CrossRef CAS PubMed
.
- C. Wang, D. Li, C. O. Too and G. G. Wallace, Chem. Mater., 2009, 21, 2604–2606 CrossRef CAS
.
- X. Zhao, C. M. Hayner, M. C. Kung and H. H. Kung, ACS Nano, 2011, 5, 8739–8749 CrossRef CAS PubMed
.
- A. Abouimrane, O. C. Compton, K. Amine and S. T. Nguyen, J. Phys. Chem. C, 2010, 114, 12800–12804 CAS
.
- R. Mukherjee, A. V. Thomas, A. Krishnamurthy and N. Koratkar, ACS Nano, 2012, 6, 7867–7878 CrossRef CAS PubMed
.
- R. H. Wang, C. H. Xu, J. Sun, L. Gao and C. C. Lin, J. Mater. Chem. A, 2013, 1, 1794–1800 CAS
.
- Y. Liu, W. Wang, L. Gu, Y. W. Wang, Y. L. Ying, Y. Y. Mao, L. W. Sun and X. S. Peng, ACS Appl. Mater. Interfaces, 2013, 5, 9850–9855 CAS
.
- A. P. Yu, H. W. Park, A. Davies, D. C. Higgins, Z. W. Chen and X. C. Xiao, J. Phys. Chem. Lett., 2011, 2, 1855–1860 CrossRef CAS
.
- R. H. Wang, C. H. Xu, J. Sun, Y. Q. Liu, L. Gao and C. C. Lin, Nanoscale, 2013, 5, 6960–6967 RSC
.
- J. K. Lee, K. B. Smith, C. M. Hayner and H. H. Kung, Chem. Commun., 2010, 46, 2025–2027 RSC
.
- T. Hu, X. Sun, H. T. Sun, M. P. Yu, F. Y. Lu, C. S. Liu and J. Lian, Carbon, 2013, 51, 322–326 CrossRef CAS
.
- S. Li, Y. Luo, W. Lv, W. Yu, S. Wu, P. Hou, Q. Yang, Q. Meng, C. Liu and H.-M. Cheng, Adv. Energy Mater., 2011, 1, 486–490 CrossRef CAS
.
- L. L. Zhang and X. S. Zhao, Chem. Soc. Rev., 2009, 38, 2520–2531 RSC
.
- M. Winter and R. J. Brodd, Chem. Rev., 2004, 104, 4245–4269 CrossRef CAS PubMed
.
- P. Simon and Y. Gogotsi, Nat. Mater., 2008, 7, 845–854 CrossRef CAS PubMed
.
- S. H. Lee, H. W. Kim, J. O. Hwang, W. J. Lee, J. Kwon, C. W. Bielawski, R. S. Ruoff and S. O. Kim, Angew. Chem., Int. Ed., 2010, 49, 10084–10088 CrossRef CAS PubMed
.
- Z. Xu, Z. Li, C. M. B. Holt, X. Tan, H. Wang, B. S. Amirkhiz, T. Stephenson and D. Mitlin, J. Phys. Chem. Lett., 2012, 3, 2928–2933 CrossRef CAS PubMed
.
- X. Han, M. R. Funk, F. Shen, Y. C. Chen, Y. Li, C. J. Campbell, J. Dai, X. Yang, J. W. Kim, Y. Liao, J. W. Connell, V. Barone, Z. Chen, Y. Lin and L. Hu, ACS Nano, 2014, 8, 8255–8265 CrossRef CAS PubMed
.
- T. Liao, C. Sun, Z. Sun, A. Du, D. Hulicova-Jurcakova and S. C. Smith, J. Mater. Chem., 2012, 22, 13751–13755 RSC
.
- Y. L. Shao, H. Z. Wang, Q. H. Zhang and Y. G. Li, J. Mater. Chem. C, 2013, 1, 1245–1251 RSC
.
- Z. Li, Y. Mi, X. Liu, S. Liu, S. Yang and J. Wang, J. Mater. Chem., 2011, 21, 14706–14711 RSC
.
- Q. Wu, Y. Xu, Z. Yao, A. Liu and G. Shi, ACS Nano, 2010, 4, 1963–1970 CrossRef CAS PubMed
.
Footnote |
| † These authors contributed equally to this work. |
| This journal is © the Partner Organisations 2017 |