Thermo-sensitive poly(VCL-4VP-NVP) ionic microgels: synthesis, cytotoxicity, hemocompatibility, and sustained release of anti-inflammatory drugs†
Xianjing
Zhou
a,
Qing
Yang
a,
Jianyuan
Li
a,
Jingjing
Nie
b,
Guping
Tang
b and
Binyang
Du
*a
aMOE Key Laboratory of Macromolecular Synthesis and Functionalization, Department of Polymer Science & Engineering, Zhejiang University, Hangzhou 310027, China. E-mail: duby@zju.edu.cn
bDepartment of Chemistry, Zhejiang University, Hangzhou 310027, China
First published on 15th August 2016
Abstract
Thermosensitive poly(VCL-4VP-NVP) ionic microgels were prepared by in situ quaternization crosslinking reaction during surfactant free emulsion polymerization (SFEP) with N-vinylcaprolactam (VCL) as the main monomer, 4-vinylpyridine (4VP) as the quaternizable co-monomer, N-vinyl-2-pyrrolidone (NVP) as the second co-monomer and 1,6-dibromohexane (6Br) as the quaternization crosslinker. The obtained ionic microgels were spherical in shape with a narrow size distribution and exhibited thermo-sensitive behavior. These ionic microgels showed low cytotoxicity at concentrations lower than 25 µg mL−1, excellent hemocompatibility at concentrations up to 1000 µg mL−1, and could be up taken into the cytoplasm regime of HEK-293 cells without entering the nucleus. It was found that these ionic microgels were suitable for the loading and sustained release of a nonsteroidal anti-inflammatory drug, diclofenac sodium (DS). The drug loading content (DLC) of DS in the microgels could reach ca. 12% with an encapsulation efficiency (EE) of up to 68%. Furthermore, 60% loaded DS could be sustainably released at 37 °C from the drug-loaded microgels within 400 min following a first-order exponential kinetics.
Introduction
Non-steroidal anti-inflammatory drugs (NSAIDs) are usually used for the treatment of acute or chronic pain and inflammation conditions.1 The group of NSAIDs includes aspirin, ibuprofen, ketoprofen, salicylic acid (SA), diclofenac, etc. In particular, diclofenac sodium (DS) is a potent NSAID, and widely used in the long-term treatment of inflammation and painful conditions of rheumatic and non-rheumatic origin. However, DS exhibits a half-life in the body of 1.9 h and 65% of the DS is excreted in urine.2 Thus, it is important and necessary to develop new and efficient drug-delivery carriers for the sustained release of DS after oral administration or injection. Research efforts have been carried out to design and fabricate novel polymeric materials for the loading and controlled-release of DS. Schneider et al. fabricated cross-linked polyelectrolyte multilayer films of chitosan/hyaluronan (CHI/HA) and found that about 80% DS could be released from the film into PBS solution within 10 h. However, a first burst release of ca. 60% was observed within about 0.5 h.3 Cassano et al. covalently linked diclofenac moieties to cellulose cotton fibers, which exhibited anti-inflammatory activity and inhibited the formation of foreign body granulomas over 28 days.4 Geever et al. synthesized thermo-sensitive poly(N-isopropylacrylamide-co-N-vinylpyrrolidinone), P(NIPAm-co-NVP), hydrogels crosslinked by poly(ethylene glycol) dimethacrylate and DS-loaded hydrogels could completely release all incorporated drug after ca. 12 h at 30 °C and 37 °C.5 There are also investigations on the sustained release of other NSAIDs like ibuprofen and salicylic acid.6–8 For example, Chandorkar et al. reported the synthesis of a stable cross-linked polyester with SA incorporated in the polymeric backbone, which could be released with a sustained release rate of 3.5% in 4 months.7 Note that SA has a plasma half-life ranging from 2 to 30 h, depending on its concentration.9Microgels are three-dimensional cross-linked polymeric colloidal particles with sizes of 1–1000 nm. By designing and choosing suitable monomers and co-monomers, the resultant microgels could be sensitive to various external stimuli, such as temperature, pH, ionic strength or others.10–26 The cross-linked networks are suitable to entrap the drugs within the microgels and the colloidal characteristic could allow the injection of drug-loaded microgels into the targeted regions where the release of drugs is required. Furthermore, the nanometer size of drug-carriers might also increase their circulation time after intravenous (i.v.) administration.21 Therefore, the potential applications of microgels as drug delivery systems have attracted much attention.16–18,20,24,27–30 For example, Ghugare et al. prepared thermo-sensitive poly(vinyl alcohol)/poly(methacrylate-co-N-isopropylacrylamide) microgels for the sustained release of doxorubicin (DOX).17 Yang et al. reported biocompatible PNVP microgels, which might be useful for the loading and release of a hydrophilic model drug, isoniazid.27 Gu et al. developed pH-responsive and uniform injectable microgels, which consist of a chitosan matrix, enzyme nanocapsules, and recombinant human insulin, for controlled release of insulin.18
In the present work, thermo-sensitive poly(VCL-4VP-NVP) ionic microgels were designed and synthesized for the loading and sustained release of DS. Poly(VCL-4VP-NVP) ionic microgels were prepared by using N-vinylcaprolactam (VCL) as the main monomer, 4-vinylpyridine (4VP) as the quaternizable co-monomer, NVP as the second co-monomer and 1,6-dibromohexane (6Br) as the quaternization crosslinker. PVCL is a water soluble, biocompatible, and thermo-sensitive polymer with a lower critical solution temperature (LCST) of ca. 31–38 °C in aqueous solution.23,24 The PVCL-based microgels had been explored as drug delivery systems for DOX,28,29 calcein,20 nadolol, propranolol and tacrine.30 PNVP is also a water-soluble and biocompatible polymer. Due to its biocompatibility and safety, PNVP has been extensively used in various biomedical applications.31,32 NVP was used here to tune the hydrophilicity and improve the biocompatibility of the resultant poly(VCL-4VP-NVP) ionic microgels. The quaternized 4VP moieties made poly(VCL-4VP-NVP) ionic microgels with positive charges, which are suitable for the delivery of an anionic hydrophilic drug. The morphology, size and microstructure of the obtained poly(VCL-4VP-NVP) ionic microgels were characterized by transmission electron microscopy (TEM), static and dynamic light scattering (SLS and DLS), and electrophoretic light scattering (ELS). The cytotoxicity and hemocompatibility of the obtained ionic microgels as well as their encapsulation and sustained release of DS were investigated and discussed.
Experimental section
Materials
VCL (98%), 2,2′-azobis(2-methylpropionamidine)dihydrochloride (AIBA, 97%), heparin sodium (99%), dimethyl sulfoxide (DMSO) (AR), trypsin (Mw = 23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300), 4′,6-diamidino-2-phenylindole (DAPI), and methyl thiazolyltetrazolium (MTT) were purchased from Aldrich-Sigma. NVP (99%) and 4VP (95%) were purchased from Acros Organics. 6Br was obtained from the Tokyo Chemical Industry Co. Ltd. DS (99%) was obtained from the Zhengzhou Huayao Biotechnology Ltd. (3-Aminopropyl)triethoxysilane (APTES), and fluorescein O-methacrylate 97 (FMA) were purchased from J&K Chemical Ltd. Dulbecco's modified Eagle's medium (DMEM) was obtained from GIBCO Invitrogen Corp. (Greenland NY, USA). Fetal calf serum (FCS) was obtained from Zhejiang Tianhang Biotechnology Co. Ltd. Triton-X (98%) was purchased from the Shanghai Biotechnology Co. Ltd. All of the chemicals were used as received. The phosphate-buffered saline solutions (PBS 0.01 M, pH 7.4 and 6.8) were prepared according to the standard protocols and Milli-Q ultrapure water was used in the cytotoxicity and cell experiments.
300), 4′,6-diamidino-2-phenylindole (DAPI), and methyl thiazolyltetrazolium (MTT) were purchased from Aldrich-Sigma. NVP (99%) and 4VP (95%) were purchased from Acros Organics. 6Br was obtained from the Tokyo Chemical Industry Co. Ltd. DS (99%) was obtained from the Zhengzhou Huayao Biotechnology Ltd. (3-Aminopropyl)triethoxysilane (APTES), and fluorescein O-methacrylate 97 (FMA) were purchased from J&K Chemical Ltd. Dulbecco's modified Eagle's medium (DMEM) was obtained from GIBCO Invitrogen Corp. (Greenland NY, USA). Fetal calf serum (FCS) was obtained from Zhejiang Tianhang Biotechnology Co. Ltd. Triton-X (98%) was purchased from the Shanghai Biotechnology Co. Ltd. All of the chemicals were used as received. The phosphate-buffered saline solutions (PBS 0.01 M, pH 7.4 and 6.8) were prepared according to the standard protocols and Milli-Q ultrapure water was used in the cytotoxicity and cell experiments.
Preparation of poly(VCL-4VP-NVP) ionic microgels
Two series of thermo-sensitive ionic microgels with sample codes of V-4VP and V-4VP-N were prepared via simultaneous quaternized crosslinking reaction during surfactant free emulsion copolymerization (SFEP).14 For the V-4VP series of microgels, VCL and 4VP were used as the main monomer and the co-monomer, respectively. 6Br was used as the quaternary crosslinker. Given amounts of VCL, 4VP, and 6Br were added into 48 mL of deionized water at 60 °C under vigorous stirring. Oxygen was eliminated by bubbling nitrogen through the reaction solution for 30 min. Afterwards, 2 mL of AIBA aqueous solution (5 mg mL−1) was added into the solution to initiate polymerization. The reaction solution was then kept at 60 °C for 12 hours. The obtained microgels were purified by extensive dialysis (MWCO = 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) against deionized water for three days to remove the unreacted molecules. The obtained V-4VP series of microgels were coded as V-4VP-2, V-4VP-6, V-4VP-10, and V-4VP-14, respectively (Table 1). For example, for the microgels with the sample code of V-4VP-10, the capital letter V represented the main monomer VCL, the term 4VP represented the co-monomer 4VP, and the numeric value of 10 represented the molar fraction of 4VP in the feedings of 4VP and VCL, i.e. [4VP]/([4VP] + [VCL]) = 10%. Furthermore, the molar ratio of [6Br]
000) against deionized water for three days to remove the unreacted molecules. The obtained V-4VP series of microgels were coded as V-4VP-2, V-4VP-6, V-4VP-10, and V-4VP-14, respectively (Table 1). For example, for the microgels with the sample code of V-4VP-10, the capital letter V represented the main monomer VCL, the term 4VP represented the co-monomer 4VP, and the numeric value of 10 represented the molar fraction of 4VP in the feedings of 4VP and VCL, i.e. [4VP]/([4VP] + [VCL]) = 10%. Furthermore, the molar ratio of [6Br]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [4VP] was always kept at 1
[4VP] was always kept at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.
2.
| Sample code | VCL/mg | 4VP/mg | 6Br/mg | D T /nm | R g/nm | D h25 /nm | R g/Rh | ξ/mV |
|---|---|---|---|---|---|---|---|---|
| a D T is the diameter of microgels by TEM. b D h25 is the hydrodynamic diameter of microgels by DLS at 25 °C. | ||||||||
| V-4VP-2 | 300 | 4.5 | 5.3 | 370 ± 17 | 122 ± 2 | 372 ± 3 | 0.66 | 14 ± 2 |
| V-4VP-6 | 300 | 13.6 | 15.8 | 82 ± 2 | 82 ± 1 | 262 ± 4 | 0.63 | 20 ± 2 |
| V-4VP-10 | 300 | 23 | 26 | 94 ± 6 | 67 ± 1 | 259 ± 3 | 0.52 | 20 ± 1 |
| V-4VP-14 | 300 | 31 | 36.8 | 104 ± 4 | 69 ± 2 | 200 ± 2 | 0.69 | 26 ± 2 |
The V-4VP-N series of microgels were prepared via a similar procedure by additionally adding the second co-monomer NVP. The obtained V-4VP-N series of microgels were coded as V-4VP-10-N7.5 and V-4VP-10-N15, respectively (Table 2). For example, for the microgels with the sample code of V-4VP-10-N7.5, the capital letters V and N represented the main monomer VCL and the second co-monomer NVP, the term 4VP represented the co-monomer 4-VP, the first numeric value of 10 represented the molar fraction of 4VP in the feedings of 4VP, VCL and NVP, i.e. [4VP]/([4VP] + [VCL] + [NVP]) = 10%, and the second numeric value of 7.5 represented the molar fraction of NVP in the feedings of VCL and NVP, i.e. [NVP]/([VCL] + [NVP]) = 7.5%. Similarly, the molar ratio of [6Br]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [4VP] was always kept at 1
[4VP] was always kept at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.
2.
| Sample code | VCL/mg | NVP/mg | 4VP/mg | 6Br/mg | D T/nm | R g/nm | D h25/nm | R g/Rh | ξ/mV |
|---|---|---|---|---|---|---|---|---|---|
| V-4VP-10-N7.5 | 275 | 18 | 23 | 26 | 98 ± 3 | 61 ± 1 | 224 ± 3 | 0.55 | 17 ± 1 |
| V-4VP-10-N15 | 252 | 36 | 23 | 26 | 102 ± 5 | 61 ± 2 | 198 ± 2 | 0.62 | 25 ± 2 |
Preparation of fluorescent poly(VCL-4VP-NVP) ionic microgels
Fluorescent poly(VCL-4VP-NVP) ionic microgels were synthesized via the same procedure for V-4VP-10-N7.5 microgels as described above by additionally adding 1 wt% of the fluorescent co-monomer, FMA.33 The fluorescent poly(VCL-4VP-NVP) ionic microgels were then coded as V-4VP-10-N7.5-FMA.MTT assay
The relative cytotoxicity of the obtained microgels was evaluated by MTT assay against human embryonic kidney 293 (HEK-293) and human cervical carcinoma HeLa cell lines in a 96 well plate with 1 × 104 cells per well according to the same procedure described previously.27 The relative cell viability was calculated as| Relative cell viability (%) = (ODsample − ODwater)/(ODcontrol − ODwater) × 100% | (1) |
Hemocompatibility
Hemolysis experiments were carried out to evaluate the hemocompatibility of the obtained ionic microgels. Fresh collected rat blood in the heparin washed tube was centrifuged (4 min, 1400 rpm). The supernatant containing plasma was discarded and the red blood cells were further washed with PBS solutions and centrifuged (4 min, 1400 rpm) for several times to remove serum proteins. Erythrocyte suspensions with a concentration of 8 × 106 mL−1 were prepared by dispersing red blood cells in PBS solution. 0.5 mL of microgel suspensions with various concentrations, i.e. 1 µg mL−1, 10 µg mL−1, 25 µg mL−1, 50 µg mL−1, 100 µg mL−1, 500 µg mL−1, and 1000 µg mL−1, were added into 0.5 mL of freshly prepared erythrocyte suspensions, respectively, which were then incubated at 37 °C for 1 h. 0.2% Triton X-100 and PBS solutions were used as positive and blank controls, respectively. After incubation, the samples were centrifuged at 2000 rpm for 5 min. The supernatant was separated from the tubes and its absorbance was measured at 570 nm using an enzyme-linked microplate reader (BIO-RAD 680, USA). The average value was calculated from a triplicate assay. The relative optical density (OD) of the supernatant compared to that of the sample treated with 0.2% Triton X-100 was defined as the percentage of hemolysis:| Hemolysis (%) = (ODMicrogel − ODPBS)/(ODTriton-X − ODPBS) × 100% | (2) |
Drug loading and release
Microgels with sample codes of V-4VP-10 and V-4VP-10-N7.5 were chosen to investigate the drug-loading and release behaviors of the NSAID, and DS, respectively. For loading DS, 100 mg of freeze-dried microgels were added into a 20 mL aqueous solution of DS (1 mg mL−1) under vigorous stirring for 3 h. The mixture was then dialyzed (MWCO = 3500) against deionized water for three days to remove the unloaded drug molecules. The deionized water was frequently changed during dialysis. The microgels with loaded drugs were then lyophilized to a constant weight for further characterization. All of the deionized water used for dialysis was collected. The amounts of unloaded drugs were determined by measuring the absorbance of the collected water using a UV-vis spectrophotometer (Cary 300, Varian Australia Pty Ltd) at 272 nm. The standard calibration curves of DS were experimentally obtained to be y = 31.94298 × x − 0.00679 for DS in deionized water, y = 31.93752 × x + 0.00536 for DS in PBS at pH = 6.8, and y = 31.5587 × x + 0.00177 for DS in PBS at pH = 7.4. The drug loading content (DLC) and encapsulation efficiency (EE) of the microgels were then given as | (3) |
 | (4) |
CLSM measurement
The cellular uptake of poly(VCL-4VP-NVP) ionic microgels without and with loaded DS drugs was tested by using HEK-293 cells as model cells and the fluorescent poly(VCL-4VP-NVP) ionic microgels coded as V-4VP-10-N7.5-FMA according to the previously reported procedure.33 The cells after the uptake of microgels were stained and imaged under a confocal laser scanning microscope (CLSM 410, Carl Zeiss, USA) with a 60× objective to visualize the fluorochromes with the following excitation (Ex) and emission (Em) wavelengths: DAPI (Ex: 350 nm, Em: 470 nm), FMA Green (Ex: 486 nm, Em: 528 nm).34Characterization
The hydrodynamic size and size distribution of the obtained ionic microgels were measured by DLS at a scattering angle θ of 90° as a function of temperature by using a 90 zeta plus particle size analyzer (Brookhaven Instruments Corp.). The wavelength of the laser λ was 635 nm. For each temperature, the sample solutions were equilibrated for 10 min. The zeta potentials ξ of the ionic microgels were also measured by ELS using the same particle size analyzer.SLS measurements of the obtained ionic microgels were carried out at 25 °C by using a Brookhaven BI-200SM spectrometer (λ = 637 nm) in the Department of Polymer Science and Engineering, Soochow University. The range of the scattering angle θ used for SLS was from 30° to 130° with a step of 5°.
The morphology of the obtained ionic microgels was observed by using a TEM (JEOL JEM-1230) operated at an acceleration voltage of 60 kV. The TEM samples were prepared by dip-coating with Formvar-coated copper grids into the microgel suspensions. By absorbing the solvent away using a filter paper, the grids were allowed to dry in air at room temperature before TEM observation.
Fourier transform infrared (FTIR) spectra were recorded on a Vector 22 Bruker spectrometer.
Results and discussion
Characterization of poly(VCL-4VP-NVP) ionic microgels
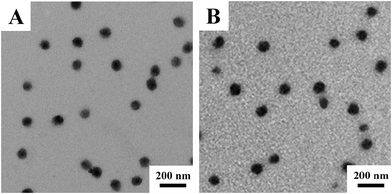
Fig. 1 shows the representative TEM images of the obtained V-4VP series of microgels, i.e. V-4VP-2, V-4VP-6, V-4VP-10, and V-4VP-14 microgels. The V-4VP series of microgels were spherical in shape with narrow size distributions. The average diameters of V-4VP-2, V-4VP-6, V-4VP-10, and V-4VP-14 microgels calculated from the TEM images were 370 ± 17 nm, 82 ± 2 nm, 94 ± 6 nm, and 104 ± 4 nm, respectively (Table 1).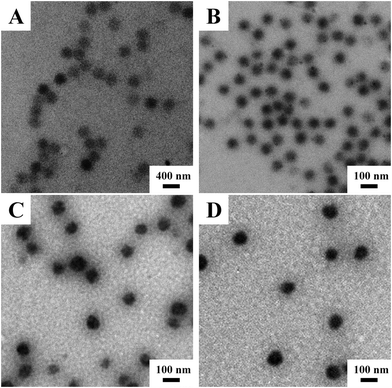 | ||
| Fig. 1 The representative TEM images of (A) V-4VP-2, (B) V-4VP-6, (C) V-4VP-10, and (D) V-4VP-14 microgels. | ||
Fig. 2 shows the hydrodynamic diameters of the V-4VP series of microgels as a function of temperature measured by DLS. As expected, the V-4VP series of microgels exhibited thermo-sensitive behavior and their hydrodynamic diameters decreased with increasing measuring temperatures because the PVCL network strands were thermo-sensitive.19,20,22,23,35–39 Furthermore, increasing the feeding molar ratio of 4VP led to the decrease of microgel sizes. The hydrodynamic diameters of V-4VP-2, V-4VP-6, V-4VP-10, and V-4VP-14 microgels were ca. 372 ± 3 nm, 262 ± 4 nm, 259 ± 3 nm, and 200 ± 2 nm, respectively, at 25 °C. Increasing the feeding molar ratio of 4VP would increase the crosslinking density of the resultant V-4VP series of microgels. Note that the quaternization of the tertiary amine of 4VP with 6Br resulted in the formation of crosslinking networks and thermo-sensitive ionic microgels.14 In our previous report, it was proven that the halogenated compound (6Br) almost completely reacted with the quaternizable co-monomer VIM or 4VP under similar experimental conditions.14 Therefore, the molar ratio of 4VP in the V-4VP series of microgels presented the crosslinking density of the resultant microgel networks. Note that the molar ratio of [6Br]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [4VP] was always kept at 1
[4VP] was always kept at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2. It was thus understandable that the V-4VP microgels with a higher crosslinking density would have smaller sizes. Furthermore, the volume phase transition temperature (VPTT) of the V-4VP series of microgels slightly shifted to the lower temperature and the temperature range of phase transition became broader and broader with increasing crosslinking density.40,41
2. It was thus understandable that the V-4VP microgels with a higher crosslinking density would have smaller sizes. Furthermore, the volume phase transition temperature (VPTT) of the V-4VP series of microgels slightly shifted to the lower temperature and the temperature range of phase transition became broader and broader with increasing crosslinking density.40,41
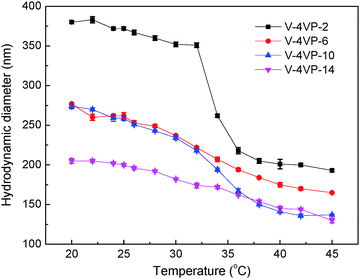 | ||
Fig. 2 The hydrodynamic diameters of the (▪) V-4VP-2, ( ) V-4VP-6, ( ) V-4VP-6, ( ) V-4VP-10, ( ) V-4VP-10, ( ) V-4VP-14 microgels as a function of measuring temperature. ) V-4VP-14 microgels as a function of measuring temperature. | ||
The VPTTs of V-4VP-2, V-4VP-6, V-4VP-10, and V-4VP-14 microgels were determined by differentiating the data of Fig. 2 to be 34, 32, 34, and 30 °C, respectively. The zeta potentials of the V-4VP series of microgels were also measured and are listed in Table 1. The V-4VP series of microgels exhibited positive zeta potentials, which could be attributed to the quaternary nitrogen atom of 4VP and the use of cationic initiator AIBA. The V-4VP-2 microgels with 2% of 4VP had the zeta potential of 14 ± 2 mV and did not exhibit long-term stability in aqueous solution. The V-4VP-14 microgels with the highest crosslinking density had the zeta potential of 26 ± 2 mV but the smallest swell ratio (cf.Fig. 2). Note that swelling ratios of microgels were defined as (Dh25/Dh45)3, where Dh25 and Dh45 represented the hydrodynamic diameters of microgels at 25 °C and 45 °C, respectively. V-4VP-6 and V-4VP-10 had a similar zeta potential of ca. 20 mV and exhibited long-term stability in aqueous solution.
The radius of gyration 〈Rg〉 of the V-4VP series of microgels was also measured at 25 °C by SLS (Fig. S1, ESI† and Table 1). The 〈Rg〉/〈Rh〉 values were calculated to be 0.66, 0.63, 0.52, and 0.69 for V-4VP-2, V-4VP-6, V-4VP-10, and V-4VP-14 microgels, respectively. The value of 〈Rg〉/〈Rh〉 could reflect the crosslinking density distribution of the microgels.13 For uniform hard spheres, 〈Rg〉/〈Rh〉 was 0.778. For PNIPAm microgels with inhomogenous crosslinking network structures, 〈Rg〉/〈Rh〉 usually had the value of 0.55–0.6.42,43 The 〈Rg〉/〈Rh〉 values of the V-4VP series of microgels were similar to those of [poly(N-isopropylacrylamide-co-4-vinylpyridine)/1,4-dibromobutane, PNP4] microgels reported previously, of which the 〈Rg〉/〈Rh〉 values decreased from 0.69 to 0.56 when the quaternization ratios increased from 1/2 to 1 with fixed amounts of co-monomer 4VP.14 Because the VPTTs of the V-4VP series of microgels were lower than the physiological temperature of 37 °C, a hydrophilic monomer NVP was incorporated into the microgels as the second co-monomer to tune the volume phase transition temperature (VPTT) of the resultant microgels close to the physiological temperature of 37 °C. Poly(N-vinylpyrrolidinone) (PNVP) is water-soluble, biocompatible and generally considered safe, and has been approved by the U.S. Food and Drug Administration (FDA) for usage in various biomedical applications.5,44,45
Fig. 3 shows the TEM images of V-4VP-10-N7.5 and V-4VP-10-N15 microgels. Similarly, the V-4VP-N series of microgels were spherical in shape with narrow size distributions. The average diameters of V-4VP-10-N7.5 and V-4VP-10-N15 microgels obtained from the TEM images were 98 ± 3 nm and 102 ± 5 nm, respectively (Table 2). Fig. 4 shows the hydrodynamic diameters of V-4VP-10-N7.5 and V-4VP-10-N15 microgels as a function of temperature measured by DLS. Note that the data for V-4VP-10 microgels were also included in Fig. 4 for comparison. The V-4VP-10-N7.5 and V-4VP-10-N15 microgels remained thermo-sensitive and their hydrodynamic diameters decreased with increasing measuring temperatures. As expected, the incorporation of hydrophilic NVP shifted the VPTTs of the resultant V-4VP-N series of microgels to higher temperatures. The VPTTs of V-4VP-10, V-4VP-10-N7.5 and V-4VP-10-N15 microgels were 34 °C, 38 °C, and 40 °C, respectively. The hydrodynamic diameters Dh25 of V-4VP-10, V-4VP-10-N7.5 and V-4VP-10-N15 microgels were ca. 259 ± 3 nm, 224 ± 3 nm, and 198 ± 2 nm, respectively, at 25 °C. The swelling ratios of V-4VP-10, V-4VP-10-N7.5 and V-4VP-10-N15 microgels calculated from (Dh25/Dh45)3 were ca. 6.75, 3.69, and 2.95, respectively. Interestingly, the incorporation of NVP into the microgel networks decreased the hydrodynamic diameters of the resultant poly(VCL-4VP-NVP) ionic microgels in the swollen states and their swelling ratios. These results were somewhat unexpected. NVP is a hydrophilic monomer and the incorporation of NVP might increase the hydrophilicity of the resultant ionic microgels, which might lead to better swelling properties. Possibly, there was hydrogen bonding among NVP and VCL moieties within the microgel networks, which resulted in the decrease of microgel sizes and the swelling ratio. Another possible reason was that the reaction rate of NVP might be lower than those of VCL and 4VP. As a result, the obtained poly(VCL-4VP-NVP) microgels might have a core–shell like structure with a dense cross-linked core and a loose cross-linked shell, leading to the smaller size in the swollen state. Furthermore, the decrease of VCL might increase the crosslinking density of the core, resulting in the decrease of the swelling ratio. The VPTTs of V-4VP-10-N7.5 and V-4VP-10-N15 microgels were determined to be 38 and 40 °C, respectively, which were slightly higher than the physiological temperature of 37 °C. As expected, the incorporation of hydrophilic NVP shifted the VPTTs of the resultant poly(VCL-4VP-NVP) microgels to higher temperatures and broadened the VPTTs. The radii of gyration 〈Rg〉s of V-4VP-10-N7.5 and V-4VP-10-N15 microgels were measured at 25 °C by SLS to be 61 ± 1 nm and 61 ± 2 nm, respectively (Fig. S2, ESI† and Table 2). The corresponding 〈Rg〉/〈Rh〉 values of V-4VP-10-N7.5 and V-4VP-10-N15 microgels were ca. 0.55 and 0.62, respectively, suggesting that the microgels had inhomogenous crosslinking network structures. The zeta potentials of V-4VP-10-N7.5 and V-4VP-10-N15 microgels were positive (Table 2). V-4VP-10-N7.5 and V-4VP-10-N15 microgels exhibited long-term stability in aqueous solutions.
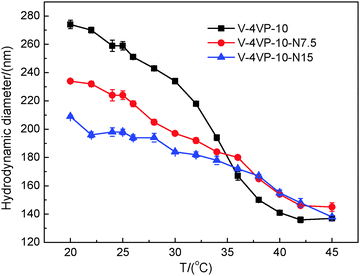 | ||
Fig. 4 The hydrodynamic diameters of the V-4VP-10 (▪), V-4VP-10-N7.5 ( ), V-4VP-10-N15 ( ), V-4VP-10-N15 ( ) microgels as a function of measuring temperature. ) microgels as a function of measuring temperature. | ||
From the above results, the V-4VP-10 and V-4VP-10-N7.5 microgels exhibited long-term stability in aqueous solutions and have VPTTs of 34 °C and 38 °C, respectively, which were lower and higher than the physiological temperature of 37 °C, respectively. Therefore, the V-4VP-10 and V-4VP-10-N7.5 microgels were chosen as the representative microgels for further investigation of their biocompatibility and potential applications as drug-control release materials of NSAIDs.
In vitro cell cytotoxicity of microgels
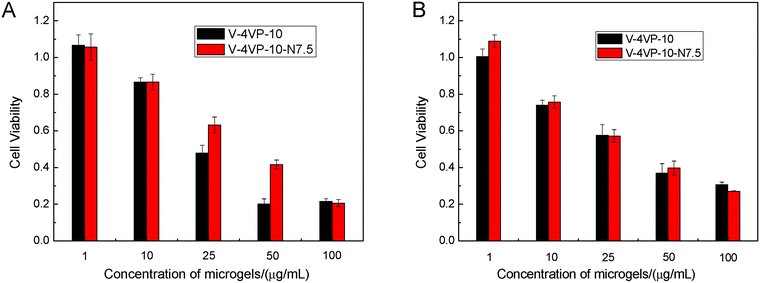
The cytotoxicity of V-4VP-10 and V-4VP-10-N7.5 microgels was first evaluated by an MTT viability assay against HeLa and HEK-293 cell lines. The HeLa cells and HEK-293 cells were used here as the cancer cell and normal cell models, respectively. Fig. 5 shows the cell viability of HeLa and HEK-293 cells after treatment with V-4VP-10 and V-4VP-10-N7.5 microgels for 48 h, respectively. For both HeLa and HEK-293 cells, the cell viability decreased upon increasing the concentration of microgels from 1 to 100 µg mL−1. For the microgels with concentrations up to 10 µg mL−1, the cell viability was close to or higher than 80% for HeLa and HEK-293 cells. For the microgels with a concentration of 25 µg mL−1, the cell viability was close to 60%. By further increasing the concentration of microgels, the cell viability decreased to less than 50%. These MTT results indicated that more than 60% of the normal and cancer cells were viable and metabolically active for the V-4VP-10 or V-4VP-10-N7.5 microgels with concentrations less than 25 µg mL−1 within 48 h. However, these microgels with higher concentration above 25 µg mL−1 become cytotoxic. Furthermore, due to the incorporation of NVP, V-4VP-10-N7.5 microgels showed better cytocompatibility for HeLa cells compared to V-4VP-10 microgels (Fig. 5A). The V-4VP-10 or V-4VP-10-N7.5 microgels contained quaternary nitrogen atoms and carried positive charges, which might result in the strong interaction between the microgels and the negative cell membranes so that the microgels exhibited cytotoxicity towards the cells. Therefore, the concentrations of V-4VP-10 and V-4VP-10-N7.5 microgels should be kept lower than 25 µg mL−1.Hemocompatibility of microgels
To be used as potential drug-control release materials, the microgels might also come in contact with blood. Therefore, the hemocompatibilities of the V-4VP-10 and V-4VP-10-N7.5 microgels were studied by hemolysis assays towards freshly collected rat blood. The release of hemoglobin was used to quantify the membrane-damaging properties of the microgels. The release of hemoglobin from erythrocytes after treatment with 0.2% Triton X-100 and PBS solutions was used as positive and blank controls, which gave 100% and 0% values, respectively.46 Erythrocytes were incubated with V-4VP-10 and V-4VP-10-N7.5 microgels at 37 °C for 1 h. The concentrations of microgels were varied ranging from 1 to 1000 µg mL−1. Fig. 6 shows the hemolysis results of rat red blood cells interacting with the two microgels. No hemolytic effects were observed for the microgels with concentrations up to 1000 µg mL−1, indicating no detectable disturbance and damage of the red blood cell membranes in the presence of the microgels. The hemolysis results indicate that the V-4VP-10 and V-4VP-10-N7.5 microgels were hemocompatible, suggesting that such microgels could be potentially used as drug-control release materials in the blood contacting environment.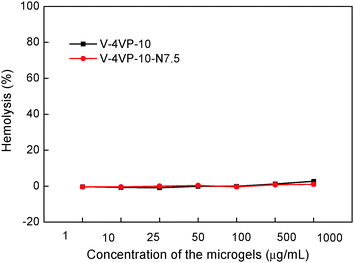 | ||
| Fig. 6 Rat red blood cell hemolysis of the V-4VP-10 and V-4VP-10-N7.5 microgels as a function of concentration. | ||
Drug-loading and release behaviors of microgels
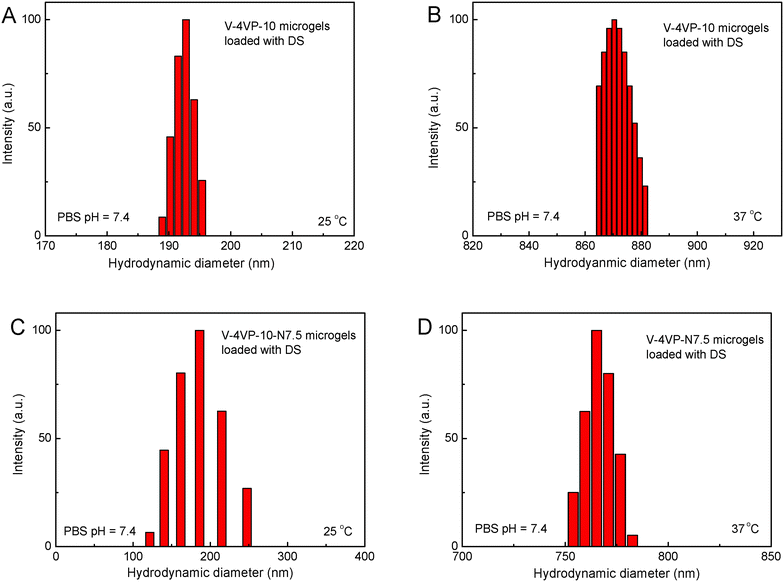
The drug-loading and sustained release behaviors of the V-4VP-10 and V-4VP-10-N7.5 microgels for DS were investigated, respectively, as described in the experimental section. The EEs of V-4VP-10 and V-4VP-10-N7.5 microgels for DS were ca. 68% and 59.3%, respectively. The DLCs of DS in the V-4VP-10 and V-4VP-10-N7.5 microgels were similar and ca. 12.4% and 11.9%, respectively. These results indicated that the incorporation of NVP had no clear effects on the loading of DS in the microgels. The dissolution of DS in PBS solutions released the sodium ions and resulted in drugs with carboxylic anions, which could then be encapsulated into the V-4VP-10 or V-4VP-10-N7.5 ionic microgels via the anion exchange reaction with the bromide counter anions within the microgels.14 The EE of DS was mainly determined by the content of quaternized co-monomer 4VP of the microgels. The V-4VP-10 and V-4VP-10-N7.5 ionic microgels had the same feeding amounts of co-monomer 4VP and quaternization crosslinker 6Br so that the EEs of DS were similar for both of the microgels. Fig. 7 shows the hydrodynamic diameters of V-4VP-10 and V-4VP-10-N7.5 ionic microgels with loaded DS in PBS (pH 7.4) solutions at 25 °C and 37 °C, respectively, as measured by DLS. It can be seen that the hydrodynamic diameters of V-4VP-10 and V-4VP-10-N7.5 microgels in the swollen state at 25 °C decreased after loading with DS (Fig. 7A and C). The size distributions of the two microgels were narrow after loading with DS. This was understandable because the loading of diclofenac with a large molecular structure and the aromatic rings will decrease the hydrophilicity of the microgels. The extent of the size decrease of V-4VP-10-N7.5 microgels was less than that of V-4VP-10 microgels, suggesting that the incorporation of NVP indeed improved the hydrophilicity of the resultant poly(VCL-4VP-NVP) ionic microgels. At a physiological temperature of 37 °C, slight aggregation of V-4VP-10 and V-4VP-10-N7.5 microgels was observed after loading with DS. The sizes of aggregates of DS-loaded V-4VP-10 and V-4VP-10-N7.5 microgels of 37 °C were ca. 870 nm and 770 nm, respectively, which might correspond to the aggregation of 4–6 microgels. As mentioned above, the loading of DS decreased the hydrophilicity of the microgels even in swollen states at 25 °C. At 37 °C, the PVCL network segments also became hydrophobic,23,24 and the stability of the microgels in collapsed states was decreased, leading to the aggregation of adjacent microgels. Fortunately, such aggregates were stable in PBS solutions at 37 °C and the size distributions of aggregates were also narrow, as shown in Fig. 7B and D.The in vitro drug release of DS from the V-4VP-10 and V-4VP-10-N7.5 microgels was then investigated by placing the drug-loaded microgels into the dialysis bags and dialyzing against the PBS (pH 7.4 or 6.8) at a physiological temperature of 37 °C and at room temperature 25 °C, respectively. Note that the pH ranges between 7.3 and 7.45 in the blood and most organs and the pH can drop to a range of 6.4–6.8 in the tumor extracellular region.47–49 Therefore, pH 7.4 and pH 6.8 were selected here to simulate the pH values of blood and the tumor extracellular region, respectively. The cumulative release curves of DS from the drug-loaded V-4VP-10 and V-4VP-10-N7.5 microgels are shown in Fig. 8, respectively. A sustained release was clearly observed within 400 min (Fig. 8). The final drug release percentages could reach ca. 60% for releasing times up to 400 min although a burst release of ca. 20% was observed at the initial time. These results indicated that the interaction between DS and the microgels was weak. DS has a large molecular structure and the aromatic rings of diclofenac are twisted in relation to each other because of the presence of two chlorine atoms in the ortho positions.50 Although there were electrostatic interactions between DS and the microgels, the twisted aromatic rings and the rotation of the linkage between the nitrogen atom and the aromatic rings might weaken the interactions between DS molecules and the microgel matrix. Schneider et al. fabricated cross-linked polyelectrolyte multilayer films of CHI/HA and found that about 80% DS could be released from the film into the PBS solution within 10 h with a first burst release of ca. 60% in the initial 0.5 h.3 The release kinetics of DS from the V-4VP-10 and V-4VP-10-N7.5 microgels in PBS solutions could be well described by the first-order exponential equation given as
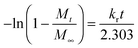 | (5) |
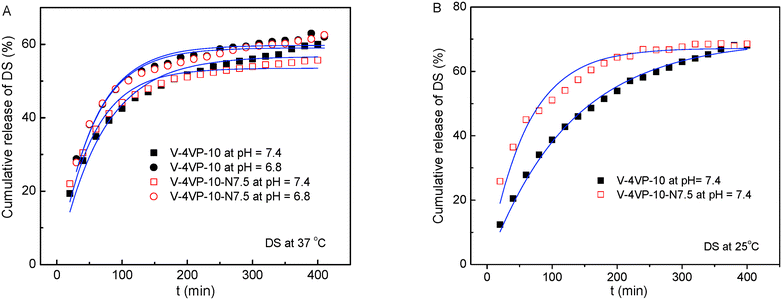 | ||
| Fig. 8 Cumulative release of DS from the drug-loaded V-4VP-10 and V-4VP-10-N7.5 microgels at (A) 37 °C and (B) 25 °C. The blue lines are fits of eqn (5). | ||
| Temperature (°C) | pH | V-4VP-10 | V-4VP-10-N7.5 | ||||
|---|---|---|---|---|---|---|---|
| k r (min−1) | M ∞ (%) | R 2 | k r (min−1) | M ∞ (%) | R 2 | ||
| 37 | 7.4 | 3.4 × 10−2 | 56.8 | 0.957 | 4.4 × 10−2 | 53.5 | 0.960 |
| 6.8 | 4.2 × 10−2 | 59.8 | 0.955 | 4.3 × 10−2 | 59.0 | 0.951 | |
| 25 | 7.4 | 1.8 × 10−2 | 69.8 | 0.994 | 3.8 × 10−2 | 67.2 | 0.952 |
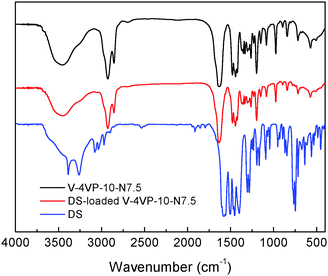
The possible interactions between DS and the microgels were investigated by FTIR. Fig. 9 shows the FTIR spectra of the V-4VP-10-N7.5 microgels, drug (DS) and drug-loaded V-4VP-10-N7.5 microgels. The C–H stretching vibration region (3010–2835 cm−1), the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibration region (1650–1560 cm−1), and strong vinyl double bond peaks in the range 800–1200 cm−1 reflect nearly all the group motions of VCL, 4VP and NVP segments within the microgels.53 The disappearance of the adsorption band at 1600 cm−1 indicated the quaternization reaction of the pyridine ring of 4VP.54 However, it was found that the FTIR spectra of the V-4VP-10-N7.5 microgels and drug-loaded V-4VP-10-N7.5 microgels are almost indistinguishable. Possibly, the electrostatic interaction between DS and the microgels did not change the drug–polymer structures at the molecular level. Furthermore, the characteristic peaks of DS were also indistinguishable from the spectra of drug-loaded microgels, which might be probably due to the low concentrations of drugs within the microgels and the masked effect of the broad bands of drug-loaded microgels. Similar phenomena were observed for DS-loaded V-4VP-10 microgels (Fig. S3, ESI†).
O stretching vibration region (1650–1560 cm−1), and strong vinyl double bond peaks in the range 800–1200 cm−1 reflect nearly all the group motions of VCL, 4VP and NVP segments within the microgels.53 The disappearance of the adsorption band at 1600 cm−1 indicated the quaternization reaction of the pyridine ring of 4VP.54 However, it was found that the FTIR spectra of the V-4VP-10-N7.5 microgels and drug-loaded V-4VP-10-N7.5 microgels are almost indistinguishable. Possibly, the electrostatic interaction between DS and the microgels did not change the drug–polymer structures at the molecular level. Furthermore, the characteristic peaks of DS were also indistinguishable from the spectra of drug-loaded microgels, which might be probably due to the low concentrations of drugs within the microgels and the masked effect of the broad bands of drug-loaded microgels. Similar phenomena were observed for DS-loaded V-4VP-10 microgels (Fig. S3, ESI†).
Cellular uptake of microgels
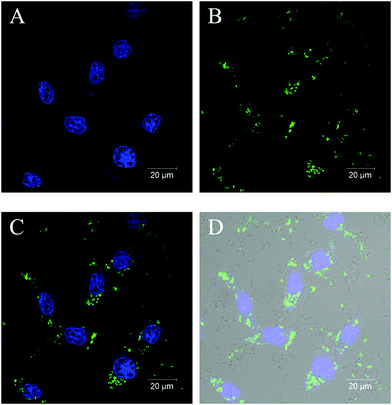
For functioning as a drug delivery and controlled-release vehicle in a living body, whether poly(VCL-4VP-NVP) ionic microgels could be taken up by living cells is crucial. Therefore, the cellular uptake of poly(VCL-4VP-NVP) ionic microgels was investigated. The fluorescent labeled poly(VCL-4VP-NVP) ionic microgels were then synthesized by additionally incorporating 1 wt% of the fluorescent co-monomer FMA and coded as V-4VP-10-N7.5-FMA, as described in the Experimental section. After fluorescein labeling, the V-4VP-10-N7.5-FMA microgels maintained the spherical shape and had hydrodynamic diameters of approximately 206 nm (Fig. S4, ESI†). The fluorescent V-4VP-10-N7.5-FMA microgels were then freeze-dried and redispersed in DMEM. The uptake of V-4VP-10-N7.5-FMA microgels by the HEK-293 cells as the model system was then investigated. Fig. 10 shows the CLSM images of stained HEK-293 cells with the V-4VP-10-N7.5-FMA microgels taken up. It can be seen that the V-4VP-10-N7.5-FMA microgels taken up distributed around the nucleus of HEK-293 cells. Note that the DAPI-stained nucleus of HEK-293 cells appeared to be fluorescent blue (Fig. 10A) and the FMA-labeled V-4VP-10-N7.5-FMA microgels appeared to be fluorescent green (Fig. 10B). Fig. 10C shows the overlapping fluorescence image of Fig. 10A and B. Clearly, the green V-4VP-10-N7.5-FMA microgels locate in the cytoplasm regimes of the HEK-293 cells. By further overlapping with the normal optical image of HEK-293 cells, the outline of the HEK-293 cells was clearly observed (Fig. 10D). The CLSM results clearly indicated that the V-4VP-10-N7.5-FMA microgels could be taken up and internalized by the HEK-293 cells and the V-4VP-10-N7.5-FMA microgels taken up were distributed into the cytoplasm regime of HEK-293 cells without entering the nucleus. The uptake of V-4VP-10-N7.5-FMA microgels loaded with DS drugs by HEK-293 cells also showed similar results (Fig. S5, ESI†). Note that the DS drugs do not show fluorescence signals.Conclusions
Thermo-sensitive poly(VCL-4VP-NVP) ionic microgels with sizes of several hundred nanometers and a narrow size distribution and quaternized 4VP moieties were successfully obtained by in situ quaternization crosslinking reaction during surfactant free emulsion polymerization. The poly(VCL-4VP-NVP) ionic microgels showed low cytotoxicity at a concentration lower than 25 µg mL−1, were hemocompatible at a concentration up to 1000 µg mL−1, and could be taken up into the cytoplasm regime of HEK-293 cells without entering the nucleus. These poly(VCL-4VP-NVP) ionic microgels were suitable for the loading and sustained release of a nonsteroidal anti-inflammatory drug, DS. The drug loading content of DS in the microgels could reach ca. 12% with an encapsulation efficiency up to 68%. 60% loaded DS could be sustainably released from the drug-loaded microgels within 400 min.Competing financial interest
The authors declare no competing financial interest.Acknowledgements
The authors thank Prof. Yingfeng Tu at Soochow University for his help in laser light scattering experiments. The authors thank the National Natural Science Foundation of China (No. 21274129 and 21322406), the Fundamental Research Funds for the Central Universities (2014XZZX003-21), the third level of 2013 Zhejiang Province 151 Talent Project for financial support.Notes and references
- M. J. Thun, S. J. Henley and C. Patrono, J. Natl. Cancer Inst., 2002, 94, 252–266 CrossRef CAS PubMed.
- K. A. Landry and T. H. Boyer, Water Res., 2013, 47, 6432–6444 CrossRef CAS PubMed.
- A. Schneider, C. Vodouhe, L. Richert, G. Francius, E. Le Guen, P. Schaaf, J. Voegel, B. Frisch and C. Picart, Biomacromolecules, 2007, 8, 139–145 CrossRef CAS PubMed.
- R. Cassano, S. Trombino, T. Ferrarelli, E. Barone, V. Arena, C. Mancuso and N. Picci, Biomacromolecules, 2010, 11, 1716–1720 CrossRef CAS PubMed.
- L. M. Geever, C. C. Cooney, J. G. Lyons, J. E. Kennedy, M. J. D. Nugent, S. Devery and C. L. Higginbotham, Eur. J. Pharm. Biopharm., 2008, 69, 1147–1159 CrossRef CAS PubMed.
- U. Hasegawa, A. J. van der Vlies, C. Wandrey and J. A. Hubbell, Biomacromolecules, 2013, 14, 3314–3320 CrossRef CAS PubMed.
- Y. Chandorkar, R. K. Bhagat, G. Madras and B. Basu, Biomacromolecules, 2014, 15, 863–875 CrossRef CAS PubMed.
- Y. Chandorkar, N. Bhaskar, G. Madras and B. Basu, Biomacromolecules, 2015, 16, 636–649 CrossRef CAS PubMed.
- R. Amann and B. A. Peskar, Eur. J. Pharmacol., 2002, 447, 1–9 CrossRef CAS PubMed.
- H. Liu, Z. Wei, M. Hu, Y. Deng, Z. Tong and C. Wang, RSC Adv., 2014, 4, 29344–29351 RSC.
- S. Seiffert, ChemPhysChem, 2013, 14, 295–304 CrossRef CAS PubMed.
- G. R. Hendrickson, M. H. Smith, A. B. South and L. A. Lyon, Adv. Funct. Mater., 2010, 20, 1697–1712 CrossRef CAS.
- D. Kuckling, C. D. Vo, H. J. P. Adler, A. Volkel and H. Colfen, Macromolecules, 2006, 39, 1585–1591 CrossRef CAS.
- X. Zhou, Y. Zhou, J. Nie, Z. Ji, J. Xu, X. Zhang and B. Du, ACS Appl. Mater. Interfaces, 2014, 6, 4498–4513 CAS.
- Z. Cao, T. Y. Chen, X. L. Guo, X. J. Zhou, J. J. Nie, J. T. Xu, Z. Q. Fan and B. Y. Du, Chin. J. Polym. Sci., 2011, 29, 439–449 CrossRef CAS.
- M. Dadsetan, K. E. Taylor, C. Yong, Z. Bajzer, L. Lu and M. J. Yaszemski, Acta Biomater., 2013, 9, 5438–5446 CrossRef CAS PubMed.
- S. V. Ghugare, P. Mozetic and G. Paradossi, Biomacromolecules, 2009, 10, 1589–1596 CrossRef CAS PubMed.
- Z. Gu, T. T. Dang, M. Ma, B. C. Tang, H. Cheng, S. Jiang, Y. Dong, Y. Zhang and D. G. Anderson, ACS Nano, 2013, 7, 6758–6766 CrossRef CAS PubMed.
- A. Imaz and J. Forcada, J. Polym. Sci., Part A: Polym. Chem., 2008, 46, 2510–2524 CrossRef CAS.
- A. Imaz and J. Forcada, J. Polym. Sci., Part A: Polym. Chem., 2010, 48, 1173–1181 CrossRef CAS.
- D. Le Garrec, S. Gori, L. Luo, D. Lessard, D. C. Smith, M. A. Yessine, M. Ranger and J. C. Leroux, J. Controlled Release, 2004, 99, 83–101 CrossRef CAS PubMed.
- A. Pich, A. Tessier, V. Boyko, Y. Lu and H. J. P. Adler, Macromolecules, 2006, 39, 7701–7707 CrossRef CAS.
- F. Schneider, A. Balaceanu, A. Feoktystov, V. Pipich, Y. Wu, J. Allgaier, W. Pyckhout Hintzen, A. Pich and G. J. Schneider, Langmuir, 2014, 30, 15317–15326 CrossRef CAS PubMed.
- H. Zhang, S. Mardyani, W. C. W. Chan and E. Kumacheva, Biomacromolecules, 2006, 7, 1568–1572 CrossRef CAS PubMed.
- X. Zhou, J. Nie, Q. Wang and B. Du, Macromolecules, 2015, 48, 3130–3139 CrossRef CAS.
- Z. B. Li, Y. H. Xiang, X. J. Zhou, J. J. Nie, M. Peng and B. Y. Du, Chin. J. Polym. Sci., 2015, 33, 1516–1526 CrossRef CAS.
- Q. Yang, K. Wang, J. Nie, B. Du and G. Tang, Biomacromolecules, 2014, 15, 2285–2293 CrossRef CAS PubMed.
- Y. Wang, J. Nie, B. Chang, Y. Sun and W. Yang, Biomacromolecules, 2013, 14, 3034–3046 CrossRef CAS PubMed.
- Y. Wang, J. Zheng, Y. Tian and W. Yang, J. Mater. Chem. B, 2015, 3, 5824–5832 RSC.
- H. Vihola, A. Laukkanen, J. Hirvonen and H. Tenhu, Eur. J. Pharm. Sci., 2002, 16, 69–74 CrossRef CAS PubMed.
- R. M. J. Ings, M. Breen, K. Devereux, A. J. Gray, F. E. Edwards, C. Lucas, M. Briggs, B. V. Robinson and D. B. Campbell, Cancer Chemother. Pharmacol., 1990, 27, 106–110 CrossRef CAS PubMed.
- Z. F. Liu and S. Rimmer, J. Controlled Release, 2002, 81, 91–99 CrossRef CAS PubMed.
- Q. Yang, K. Wang, J. Nie, B. Du and G. Tang, Biomacromolecules, 2014, 15, 2285–2293 CrossRef CAS PubMed.
- G. Gollavelli and Y. C. Ling, Biomaterials, 2012, 33, 2532–2545 CrossRef CAS PubMed.
- G. Agrawal, J. Wang, B. Bruester, X. Zhu, M. Moeller and A. Pich, Soft Matter, 2013, 9, 5380–5390 RSC.
- A. Balaceanu, V. Mayorga, W. Lin, M. P. Schuerings, D. E. Demco, A. Boeker, M. A. Winnik and A. Pich, Colloid Polym. Sci., 2013, 291, 21–31 CAS.
- A. Imaz and J. Forcada, Eur. Polym. J., 2009, 45, 3164–3175 CrossRef CAS.
- S. F. Peng and C. Wu, Macromolecules, 2001, 34, 6795–6801 CrossRef CAS.
- S. F. Peng and C. Wu, Macromolecules, 2001, 34, 568–571 CrossRef CAS.
- C. D. Vo, D. Kuckling, H. J. P. Adler and M. Schohoff, Colloid Polym. Sci., 2002, 280, 400–409 CAS.
- Z. Cao, B. Du, T. Chen, J. Nie, J. Xu and Z. Fan, Langmuir, 2008, 24, 12771–12778 CrossRef CAS PubMed.
- M. Bradley and B. Vincent, Langmuir, 2005, 21, 8630–8634 CrossRef CAS PubMed.
- H. Senff and W. Richtering, Colloid Polym. Sci., 2000, 278, 830–840 CAS.
- P. Francois, P. Vaudaux, N. Nurdin, H. J. Mathieu, P. Descouts and D. P. Lew, Biomaterials, 1996, 17, 667–678 CrossRef CAS PubMed.
- L. E. Smith, S. Rimmer and S. MacNeil, Biomaterials, 2006, 27, 2806–2812 CrossRef CAS PubMed.
- D. Fischer, Y. X. Li, B. Ahlemeyer, J. Krieglstein and T. Kissel, Biomaterials, 2003, 24, 1121–1131 CrossRef CAS PubMed.
- R. van Sluis, Z. M. Bhujwalla, N. Raghunand, P. Ballesteros, J. Alvarez, S. Cerdan, J. P. Galons and R. J. Gillies, Magn. Reson. Med., 1999, 41, 743–750 CrossRef CAS PubMed.
- R. Ladj, A. Bitar, M. M. Eissa, H. Fessi, Y. Mugnier, R. Le Dantec and A. Elaissari, Int. J. Pharm., 2013, 458, 230–241 CrossRef CAS PubMed.
- X. Wu, Z. Wang, D. Zhu, S. Zong, L. Yang, Y. Zhong and Y. Cui, ACS Appl. Mater. Interfaces, 2013, 5, 10895–10903 CAS.
- L. B. Lopes, M. V. Scarpa, G. V. J. Silva, D. C. Rodrigues, C. V. Santilli and A. G. Oliveira, Colloids Surf., B, 2004, 39, 151–158 CrossRef CAS PubMed.
- S. Dash, P. N. Murthy, L. Nath and P. Chowdhury, Acta Pol. Pharm., 2010, 67, 217–223 CAS.
- C. Algieri, E. Drioli and L. Donato, J. Appl. Polym. Sci., 2013, 128, 754–760 CrossRef CAS.
- L. Hou and P. Wu, RSC Adv., 2014, 4, 39231–39241 RSC.
- N. Sahiner and A. O. Yasar, J. Colloid Interface Sci., 2013, 402, 327–333 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: SLS data of the microgels, FTIR spectra of the V-4VP-10 microgels and DS-loaded V-4VP-10 microgels, TEM images, DLS data and CLSM images of fluorescent V-4VP-10-N7.5-FMA microgels. See DOI: 10.1039/c6qm00046k |
| This journal is © the Partner Organisations 2017 |