A g-C3N4/WO3 photoanode with exceptional ability for photoelectrochemical water splitting†
Haibo
Li
,
Fengyi
Zhao
,
Jincheng
Zhang
,
Lei
Luo
,
Xujing
Xiao
,
Yongchao
Huang
,
Hongbing
Ji
* and
Yexiang
Tong
*
MOE of the Key Laboratory of Bioinorganic and Synthetic Chemistry, KLGHEI of Environment and Energy Chemistry, The Key Lab of Low-carbon Chem & Energy Conservation of Guangdong Province, School of Chemistry and Chemical Engineering, Sun Yat-Sen University, 135 Xingang West Road, Guangzhou 510275, P. R. China. E-mail: chedhx@mail.sysu.edu.cn; jihb@mail.sysu.edu.cn
First published on 12th August 2016
Abstract
Herein, we report a photoanode of g-C3N4/WO3 heterojunctions with exceptional ability and stability for photoelectrochemical (PEC) water splitting which achieved a high photocurrent density of 1.92 mA cm−2 at +1.23 V versus (vs.) RHE which is about 2 times higher than that of the pristine WO3 photoanode (0.71 mA cm−2).
With the increasingly serious environmental pollution and the exhaustion of fossil energy resources, developing clean and reproducible energy to satisfy the growing global demand for energy has become increasingly inevitable and imperative.1–4 Under such a circumstance, hydrogen is believed to be the most promising clean energy and photoelectrochemical (PEC) water splitting is one of the most important pathways to produce hydrogen from water.5–7 Among the numerous photoanode materials, n-type semiconductors, WO3 has been widely studied due to its high chemical stability, environmental friendliness, low cost and natural abundance.8–10 However, WO3 suffers from low solar light absorption due to its large bandgap (Eg 2.5–2.8 eV) and relatively fast electron–hole recombination, which limit its photoconversion efficiency.11,12
To overcome these drawbacks, several strategies have been attempted, which include: (i) impurity element doping,13–15 (ii) surface passivation16,17 and (iii) semiconductor heterojunctions.18–20 What is noteworthy is that the semiconductor-based heterostructure construction, which can tremendously improve the PEC performance by enhancing the absorption of solar radiation and improving the separation of photo-generated carriers, has attracted widespread attention in recent years. For example, several reports have demonstrated that WO3/BiVO4 heterojunction films can enhance the charge separation thus improving the overall photocurrent conversion efficiency,21–23 and Sivula et al. reported that depositing Fe2O3 on WO3 can obviously improve the PEC performance due to an increase in the absorbed photon conversion efficiency.24 Hence, developing WO3 with other semiconductors as a photoanode for PEC water splitting is highly desirable.
The graphite-like carbon nitride (g-C3N4) is a novel and appealing metal-free photocatalyst with various outstanding physicochemical properties, such as excellent electrical conductivity, high chemical and thermal stability, nontoxicity, suitable band-edge position and well-matched band-structure.25,26 In addition, owing to the suitable band-gap (Eg = 2.7 eV), g-C3N4 shows a good response to solar radiation whose wavelength can reach 460 nm. By virtue of the above-mentioned properties, g-C3N4 has recently received considerable attention and has been widely used as a photocatalyst, especially exhibiting brilliant photocatalytic performance for PEC water splitting using solar radiation.27,28 Without doubt, g-C3N4 is an ideal semiconductor material which can be used to improve the PEC properties of WO3. What is more satisfactory is that combining with WO3 to form the heterostructure construction can also effectively overcome the drawbacks of g-C3N4 like the high combination rate of photogenerated charge, low specific surface area and absence of active sites.29 For example, Zhan et al. synthesized g-C3N4/WO3 heterojunction plate array films with enhanced PEC performance via a combination of hydrothermal and dipping–annealing methods, which achieved a maximum photocurrent density of 2.10 mA cm−2 at +2.0 V (vs. RHE), and was almost 2-fold higher than that of the pure WO3 film (0.78 mA cm−2) under illumination.30 And Hou et al. reported the design and fabrication of hybrid three-dimensionally branched WO3 nanosheet array and C3N4 nanosheet (3DB WO3-NA/C3N4-NS) heterojunctions with higher photocurrent density for efficient PEC water oxidation, which can be ascribed to both the enhanced light harvesting from the “window effect”, and the efficient charge separation at the WO3-NA/C3N4-NS heterojunction.8
In this study, we reported the synthesis and characterization of the heterojunction of g-C3N4 nanosheet/WO3 nanorod composites to enhance the PEC water splitting ability of the WO3 photoanode.31 The photocurrent density of the g-C3N4-NS/WO3-NR photoanode achieved 1.92 mA cm−2 at +1.23 V versus (vs.) RHE, which is much higher than that of the pristine WO3 photoanode (0.71 mA cm−2 at +1.23 V vs. RHE). The photocurrent of the g-C3N4-NS/WO3-NR photoanode only decreases to 1.85 mA cm−2 within 400 min of illumination, indicating that the g-C3N4-NS/WO3-NR photoanode is very stable during a long irradiation time at 1.23 V vs. RHE. The incident photon-to-electron conversion efficiency (IPCE) value of the g-C3N4-NS/WO3-NR photoanode reaches 57.79% at 330 nm and 8.38% at 430 nm, which is much higher than that of the pristine WO3-NR photoanode (43.76% at 330 nm and 2.26% at 430 nm). This work suggests that introducing g-C3N4 can effectively improve the PEC performance of WO3 PEC photoanodes.
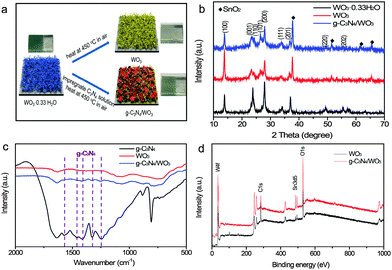
The g-C3N4-NS/WO3 photoanode was prepared on FTO substrates via a two-step process, as schematically illustrated in Fig. 1 (Experimental section). These g-C3N4/WO3 composites were characterized by X-ray diffraction (XRD). Fig. 1b shows the XRD patterns of the prepared samples in comparison to g-C3N4/WO3 and WO3. All the diffraction peaks can be readily indexed to WO3. The XRD pattern of g-C3N4/WO3 exhibits typical peaks corresponding to C3N4 and WO3, indicating the formation of g-C3N4/WO3 composites. Furthermore, the IR-FT studies also clearly show that the peaks in the range of 1250 cm−1 to 1600 cm−1 are detected for g-C3N4/WO3, which belong to g-C3N4 (Fig. 1c). In order to investigate the chemical composition of products, X-ray photoelectron spectroscopy (XPS) analysis was performed. The XPS survey spectra recorded for the WO3 NR and g-C3N4-NS/WO3-NR samples are shown in Fig. 2d. Besides the Sn and Si signals originating from FTO and the C signals originating from adventitious carbon, only W, O and N are detected on the sample surface, confirming that the samples were pure. All the results above showed that g-C3N4/WO3 composites were successfully fabricated on FTO.
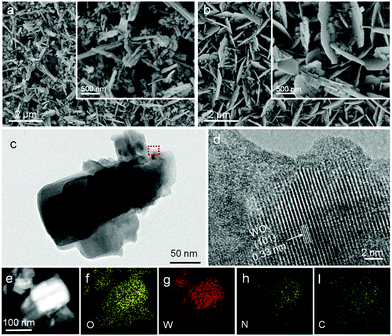
The morphology and crystalline properties of the as-prepared samples were also investigated by SEM and TEM. The morphology of bulk g-C3N4 powder was investigated by SEM, which appeared to have amorphous solid blocks or aggregated particles containing many smaller crystals (Fig. S2, ESI†). Fig. 2a is the SEM of WO3 NRs consisting of a 2 µm length structure. After being impregnated in g-C3N4 solution, some changes in morphology and many nanosheets were observed on the surface of WO3 NRs (Fig. 2b). As shown in the low magnification TEM images (Fig. 2c), the size of g-C3N4/WO3 is about 2 µm, which is very close to that of WO3-NRs and g-C3N4-NSs. Fig. 2d shows the high-resolution TEM image of g-C3N4/WO3. Some clear lattice fringes of WO3 nanocrystals are detected; by measuring these lattice fringes, the resolved interplanar distance is 0.33 nm, corresponding to the (101) plane of WO3. Note that the g-C3N4-NSs are non-crystalline, which were on the surface of WO3. In Fig. 2e–i, the signals of O, W, N and C elements are clearly observed, respectively, implying that g-C3N4-NSs covered the WO3-NR surface uniformly and closely. These results suggest that the g-C3N4/WO3 sample manifests itself as a composite of WO3 and g-C3N4 nanostructures.
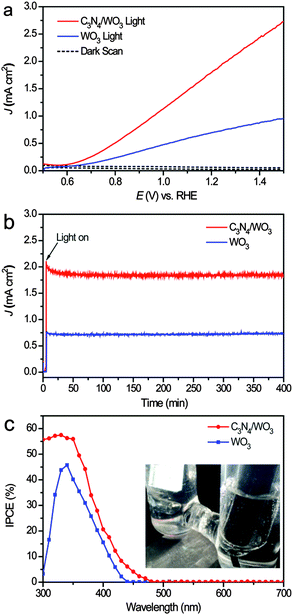
To study the effect of g-C3N4-NSs on WO3-NRs for PEC water splitting, PEC measurements were performed on the pristine WO3-NR photoanode and the g-C3N4-NS/WO3-NR photoanode in a three-electrode electrochemical cell. The advantages of the g-C3N4-NSs grown on the WO3 photoanode were demonstrated by linear sweep voltammogram (LSV) curves in 0.1 M KH2PO4 solution (pH = 7) at a scan rate of 25 mV s−1 under AM 1.5 G illumination (Fig. 3a). The photocurrent density of the g-C3N4-NS/WO3-NR photoanode achieved 1.92 mA cm−2 at +1.23 V vs. RHE, which is much higher than that of the pristine WO3 photoanode (0.71 mA cm−2 at +1.23 V vs. RHE). And the effect of C3N4 loading amounts on the PEC performance was determined by impregnating WO3·0.33H2O into g-C3N4-NS dispersions for different time periods (0.25, 0.5, 1.0, 1.5, 2.0 h) before annealing, and their PEC performances are shown in Fig. S3 (ESI†). As the impregnation time was prolonged, the amount of g-C3N4 loading on WO3 increased, which had a great effect on the PEC performance. When the impregnation time extended from 0 h to 1 h, the photocurrent density of g-C3N4/WO3 increased from 0.71 mA cm−2 to 1.92 mA cm−2 at +1.23 V vs. RHE due to an increase in the amount of g-C3N4/WO3 heterojunctions with increasing g-C3N4 loading amounts. However, the excessive g-C3N4 loading on the surface of WO3 will hinder it from absorbing visible light, and thus the photocurrent density decreased after impregnating into g-C3N4-NS dispersions for more than 1 h.
Furthermore, the stability of the g-C3N4-NS/WO3-NR photoanode was also evaluated and remarked. The current–time (i–t) curves for the pristine WO3-NR and g-C3N4-NS/WO3-NR photoanodes are collected at 1.23 V vs. RHE and shown in Fig. 3b. The photocurrent of the g-C3N4-NS/WO3-NR photoanode only decreases to 1.85 mA cm−2 within 400 min of illumination, indicating that the g-C3N4-NS/WO3-NR photoanode is very stable during the long irradiation time at 1.23 V vs. RHE. This result clearly shows that the g-C3N4-NSs will greatly enhance the ability of WO3-NRs for photoelectrochemical (PEC) water splitting by forming a g-C3N4-NS/WO3-NR heterojunction.
IPCE measurements were further performed to investigate the PEC performances of the pristine WO3-NR photoanode and the g-C3N4-NSs/WO3-NRs photoanode and measured at +1.23 V vs. RHE in 0.1 M KH2PO4 solution (pH = 7). Fig. 3c shows the IPCE spectra of the pristine WO3-NRs photoanode and the g-C3N4-NS/WO3-NR photoanode, which are in accord with their current density–voltage (J–V) characteristics. The IPCE value of the g-C3N4-NS/WO3-NR photoanode reaches 57.79% at 330 nm, which is much higher than that of the pristine WO3-NR photoanode (43.76% at 330 nm). For visible light (>420 nm), the IPCE value of the g-C3N4-NS/WO3-NR photoanode reaches 8.38% at 430 nm, which is much higher than that of the pristine WO3-NR photoanode (2.26% at 430 nm). These results confirmed that the g-C3N4-NSs can improve the photoactive range of the WO3-NRs and enhance the separation of electron–hole pairs. This brilliant photocatalytic activity and stability prove the g-C3N4/WO3 photoanode to be a promising new kind of photoanode material for solar water splitting.
The evolution of O2 was measured by gas chromatography. After adjustment and calculation, the Faradaic efficiency of the g-C3N4/WO3 photoanode was determined to be 81.8% under 4500 s light irradiation (calculated O2 evolution 2.85 µmol, measured O2 evolution 2.33 µmol, measured H2 evolution![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) measured O2 evolution = 4.50 µmol
measured O2 evolution = 4.50 µmol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.33 µmol = 1.93
2.33 µmol = 1.93![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, Fig. S4, ESI†), which indicates that the amount of O2 evolved is slightly less than expected. This number is lower than expected (100%), and it may be as a result of a gas leak during the experiment. On the other hand, previous studies on WO3 electrodes reported that oxidation of water to O2 was not the only photo-oxidation reaction that occurred under light irradiation. The incomplete oxidation of water to peroxide species can easily occur.32,33 In addition, photo-oxidation of anions in the solution present as supporting electrolytes can also compete with the photooxidation of water.33 Compared with previously reported data, the efficiency of a similar photoanode of 3DB WO3-NA/C3N4-NS//CoOx was calculated to be 82.8%, which is close to that of the g-C3N4/WO3 photoanode.8 Moreover, the Faradaic efficiency of Ag-Bi/WO334 and Au/WO335 photoanodes can reach 91.3% and 94.0%, respectively.
1, Fig. S4, ESI†), which indicates that the amount of O2 evolved is slightly less than expected. This number is lower than expected (100%), and it may be as a result of a gas leak during the experiment. On the other hand, previous studies on WO3 electrodes reported that oxidation of water to O2 was not the only photo-oxidation reaction that occurred under light irradiation. The incomplete oxidation of water to peroxide species can easily occur.32,33 In addition, photo-oxidation of anions in the solution present as supporting electrolytes can also compete with the photooxidation of water.33 Compared with previously reported data, the efficiency of a similar photoanode of 3DB WO3-NA/C3N4-NS//CoOx was calculated to be 82.8%, which is close to that of the g-C3N4/WO3 photoanode.8 Moreover, the Faradaic efficiency of Ag-Bi/WO334 and Au/WO335 photoanodes can reach 91.3% and 94.0%, respectively.
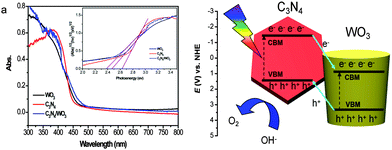
It is known that both the light absorption of the semiconductors and the separation efficiency of photoexcited electron–hole pairs have an important influence on the PEC properties. Diffuse reflectance UV-visible spectra were collected for WO3, C3N4, and g-C3N4-NS/WO3-NR samples to understand the influence of C3N4 on the light harvesting capability. As shown in Fig. 4a, the band edges of all samples were almost similar. These results reveal that the C3N4 coating has no influence on the light absorption ability of WO3, which means that such an enhancement of PEC performance should not be ascribed to the light absorption ability. Their band gap energy (Eg) was evaluated using the following equation:36
| α(hν) = A(hν − Eg)n/2 |
| EVB = X − Ee + 0.5Eg |
In summary, we demonstrated that the g-C3N4-NS/WO3-NR composites exhibited significantly enhanced PEC activity under visible light irradiation. The photocurrent density of the g-C3N4-NS/WO3-NR sample achieved 1.92 mA cm−2 at 1.23 V vs. RHE, which is about 2 times higher than that of pure WO3-NRs (0.71 mA cm−2 at +1.23 V vs. RHE). The IPCE value of the g-C3N4-NS/WO3-NR photoanode reaches 57.79% at 330 nm and 8.38% at 430 nm, which is much higher than that of the pristine WO3-NR photoanode (43.76% at 330 nm and 2.26% at 430 nm). The enhanced activity can be attributed to the separation of photo-generated carriers. Furthermore, the g-C3N4-NS/WO3-NR composites are very stable during a long irradiation time. The photocurrent of the g-C3N4/WO3 photoanode only decreases to 1.85 mA cm−2 within 400 min of illumination at 1.23 V vs. RHE. These findings could open up new opportunities for the design of high-performance anodes.
This work was preliminarily supported by the Natural Science Foundation of China (21425627 and 21476271), the Science and Technology Plan Project (2013B090600036) and Natural Science Foundation (2014A030308012 and 2014KTSCX004) of Guangdong Province, China.
Notes and references
- X. Chang, T. Wang, P. Zhang, J. Zhang, A. Li and J. Gong, J. Am. Chem. Soc., 2015, 137, 8356 CrossRef CAS PubMed.
- Y. Huang, B. Long, H. Li, M. S. Balogun, Z. Rui, Y. Tong and H. Ji, Adv. Mater. Interfaces, 2015, 2, 1500249 CrossRef.
- Y. Huang, H. Li, M.-S. Balogun, W. Liu, Y. Tong, X. Lu and H. Ji, ACS Appl. Mater. Interfaces, 2014, 6, 22920 CAS.
- K.-H. Ye, J.-Y. Wang, N. Li, Z.-Q. Liu, S.-H. Guo, Y.-P. Guo and Y.-Z. Su, Inorg. Chem. Commun., 2014, 45, 116 CrossRef CAS.
- W. Bi, C. Ye, C. Xiao, W. Tong, X. Zhang, W. Shao and Y. Xie, Small, 2014, 10, 2820 CrossRef CAS PubMed.
- F. Lei, Y. Sun, K. Liu, S. Gao, L. Liang, B. Pan and Y. Xie, J. Am. Chem. Soc., 2014, 136, 6826 CrossRef CAS PubMed.
- Y. Zhao, F. Zhao, X. Wang, C. Xu, Z. Zhang, G. Shi and L. Qu, Angew. Chem., Int. Ed., 2014, 53, 13934 CrossRef CAS PubMed.
- Y. Hou, F. Zuo, A. P. Dagg, J. Liu and P. Feng, Adv. Mater., 2014, 26, 5043 CrossRef CAS PubMed.
- B. M. Klepser and B. M. Bartlett, J. Am. Chem. Soc., 2014, 136, 1694 CrossRef CAS PubMed.
- A. J. Rettie, K. C. Klavetter, J.-F. Lin, A. Dolocan, H. Celio, A. Ishiekwene, H. L. Bolton, K. N. Pearson, N. T. Hahn and C. B. Mullins, Chem. Mater., 2014, 26, 1670 CrossRef CAS.
- J. Guo, Y. Li, S. Zhu, Z. Chen, Q. Liu, D. Zhang, W.-J. Moon and D.-M. Song, RSC Adv., 2012, 2, 1356 RSC.
- I. Aslam, C. Cao, M. Tanveer, M. H. Farooq, W. S. Khan, M. Tahir, F. Idrees and S. Khalid, RSC Adv., 2015, 5, 6019 RSC.
- D. W. Hwang, J. Kim, T. J. Park and J. S. Lee, Catal. Lett., 2002, 80, 53 CrossRef CAS.
- Y. Liu, J. Li, W. Li, Y. Yang, Y. Li and Q. Chen, J. Phys. Chem. C, 2015, 119, 14834 CAS.
- C. Liu, H. Tang, J. Li, W. Li, Y. Yang, Y. Li and Q. Chen, RSC Adv., 2015, 5, 35506 RSC.
- T. Singh, R. Müller, J. Singh and S. Mathur, Appl. Surf. Sci., 2015, 347, 448 CrossRef CAS.
- Y. O. Kim, S.-H. Yu, K.-S. Ahn, S. K. Lee and S. H. Kang, J. Electroanal. Chem., 2015, 752, 25 CrossRef CAS.
- C. Ng, A. Iwase, Y. H. Ng and R. Amal, J. Phys. Chem. Lett., 2012, 3, 913 CrossRef CAS PubMed.
- F. Zhan, J. Li, W. Li, Y. Liu, R. Xie, Y. Yang, Y. Li and Q. Chen, Int. J. Hydrogen Energy, 2015, 40, 6512 CrossRef CAS.
- X. Cui, Y. Wang, G. Jiang, Z. Zhao, C. Xu, Y. Wei, A. Duan, J. Liu and J. Gao, RSC Adv., 2014, 4, 15689 RSC.
- J. Su, L. Guo, N. Bao and C. A. Grimes, Nano Lett., 2011, 11, 1928 CrossRef CAS PubMed.
- R. Saito, Y. Miseki and K. Sayama, Chem. Commun., 2012, 48, 3833 RSC.
- Y. Pihosh, I. Turkevych, K. Mawatari, T. Asai, T. Hisatomi, J. Uemura, M. Tosa, K. Shimamura, J. Kubota and K. Domen, Small, 2014, 10, 3692 CrossRef CAS PubMed.
- K. Sivula, F. L. Formal and M. Grätzel, Chem. Mater., 2009, 21, 2862 CrossRef CAS.
- X. Zhang, T. Peng, L. Yu, R. Li, Q. Li and Z. Li, ACS Catal., 2014, 5, 504 CrossRef.
- F. He, G. Chen, Y. Yu, S. Hao, Y. Zhou and Y. Zheng, ACS Appl. Mater. Interfaces, 2014, 6, 7171 CAS.
- Y. Shiraishi, S. Kanazawa, Y. Sugano, D. Tsukamoto, H. Sakamoto, S. Ichikawa and T. Hirai, ACS Catal., 2014, 4, 774 CrossRef CAS.
- Y. He, J. Cai, L. Zhang, X. Wang, H. Lin, B. Teng, L. Zhao, W. Weng, H. Wan and M. Fan, Ind. Eng. Chem. Res., 2014, 53, 5905 CrossRef CAS.
- Y. Chen, W. Huang, D. He, Y. Situ and H. Huang, ACS Appl. Mater. Interfaces, 2014, 6, 14405 CAS.
- F. Zhan, R. Xie, W. Li, J. Li, Y. Yang, Y. Li and Q. Chen, RSC Adv., 2015, 5, 69753 RSC.
- Y. Liu, S. Xie, C. Liu, J. Li, X. Lu and Y. Tong, J. Power Sources, 2014, 269, 98 CrossRef CAS.
- Q. Mi, A. Zhanaidarova, B. S. Brunschwig, H. B. Gray and N. S. Lewis, Energy Environ. Sci., 2012, 5, 5694 CAS.
- J. A. Seabold and K.-S. Choi, Chem. Mater., 2011, 23, 1105 CrossRef CAS.
- Q. Zhao, Z. Yu, W. Yuan and J. Li, Int. J. Hydrogen Energy, 2012, 37, 13249 CrossRef CAS.
- D. Hu, P. Diao, D. Xu and Q. Wu, Nano Res., 2016, 9, 1735 CrossRef CAS.
- I. M. Szilágyi, B. Fórizs, O. Rosseler, Á. Szegedi, P. Németh, P. Király, G. Tárkányi, B. Vajna, K. Varga-Josepovits and K. László, J. Catal., 2012, 294, 119 CrossRef.
- B. Weng, J. Wu, N. Zhang and Y.-J. Xu, Langmuir, 2014, 30, 5574 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6qm00009f |
| This journal is © the Partner Organisations 2017 |