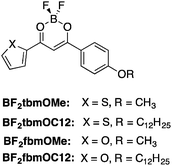
Stimuli responsive furan and thiophene substituted difluoroboron β-diketonate materials†
William A.
Morris
,
Tristan
Butler
,
Milena
Kolpaczynska
and
Cassandra L.
Fraser
*
Department of Chemistry, University of Virginia, Charlottesville, Virginia 22904, USA. E-mail: fraser@virginia.edu
First published on 29th July 2016
Abstract
Difluoroboron β-diketonate (BF2bdk) compounds exhibit solid-state switchable luminescence under excitation by UV light. This property is usually manifested as a blue-shift in emission when dye films are thermally annealed followed by a red-shift in response to mechanical shear (i.e. mechanochromic luminescence). Here we report thiophene and furan heterocycle-substituted dyes bearing short methoxy and long dodecyloxy chain substituents. Optical properties of the dyes were investigated in solution, pristine powders, and films on paper and glass. The structural and thermal properties of the dyes were also investigated by powder X-ray diffraction (XRD) and differential scanning calorimetry (DSC), respectively. The methoxy-substituted dyes exhibited neither thermal nor mechano-responsive behavior, however, addition of a longer C12 alkoxyl chain substituent resulted in stimuli-responsive behavior. The furan and thiophene dodecyloxy-substituted dyes both exhibit high-contrast reversible luminescence switching between crystalline, blue-shifted and amorphous red-shifted emissive states. For the furan dye, gentle heating produced a green emissive form while melting followed by rapid cooling produced an orange emissive form. The thiophene dye, on the other hand, exhibited blue-shifted emission when annealed below its melting temperature and red-shifted emission when smeared (mechanochromic luminescence). These transformations for both dyes were found to be completely reversible, making them potential candidates for applications requiring reusable, functional materials.
Introduction
Tetracoordinate boron complexes are receiving much attention for their attractive optical properties and high stability compared to tri-coordinate boron species.1,2 Difluoroboron β-diketonate (BF2bdk) complexes are of particular interest due to their impressive emission properties and the facile synthesis of β-diketonates via Claisen condensation from a variety of commercially available ketones and esters.1,3–7 Desirable properties of BF2bdks include high extinction coefficients,8–10 high quantum yields,8–10 two-photon absorption cross-sections,8,11 a range of emission colors in solution and the solid state,10,12 solvatochromism,4 and the ability to impart significant intramolecular charge transfer (ICT) character in unsymmetrical analogues.13 Previously we reported the unique mechanochromic luminescent (ML) properties6,7,12,14,15 of BF2bdk dyes as well as their ability to act as dual-emissive ratiometric oxygen sensors in polymer media.3,16–19 Mechanoluminescence in these dyes involves switching between a short-wavelength (blue-shifted) ordered emissive state and long-wavelength (red-shifted) amorphous emissive state. The former is accessible via thermal annealing and the latter, by applying shear force to the annealed material.14 Exciton migration to lower energy emitting H-aggregates in the amorphous state is believed to be responsible for the red-shift in fluorescence observed upon smearing.20 Substituents attached to the bdk ligand, such as halides7,21 or alkoxyl chains of varying length,6 are also known to affect ML properties. Aryl substituted BF2bdks with donor–acceptor motifs typically exhibit high-contrast, reversible ML.12,14,22Heteroaromatic systems with impressive optical properties are abundant. As reported by Rasmussen and coworkers, oligothiophene materials have high quantum yields as well as significant solid-state emission.23,24 End-capping DTP oligomers with thienyl groups produced red-shifted absorption and emission maxima in comparison to phenyl end-caps.23 Yam et al. have synthesized a functional material capable of memory storage utilizing a ternary logic state by combining benzothiadiazole and BF2bdk moieties. This material derives its unique capabilities from charge transfer between the benzothiadiazole and BF2bdk moieties.25 More recently, Kato and coworkers have demonstrated liquid crystalline materials based on oligothiophenes exhibiting tunable, reversible shear-induced ML.26 Oxygen heteroaromatic materials also show responsive behavior. Greco et al. have reported reversible temperature dependent emission in solution of furan-containing nucleosides.27 Naeem et al. have synthesized stimuli-responsive anthracene–benzoxazole (ABO) and anthracene–thiazole (ABT) compounds in which the anthracene acts as an electron donor and the benzoxazole or thiazole moieties act as electron acceptors. The ABO dye exhibited stable ML while the ABT dye recovered mechanically-induced emission changes almost instantly. The differences in behavior were ascribed to the heteroatoms (i.e. oxygen vs. sulfur).28
Kim and coworkers have recently reported diketopyrrolopyrrole (DPP) derivatives showing mechanical and thermally responsive emissive behavior. They found that the aforementioned properties were also heavily influenced by the length of alkyl chain substituents. In particular, a DPP derivative bearing a C8H17 chain (DPP8) could access a stable, supercooled liquid state with characteristic low quantum yield red emission. Once in this state, the material was stable despite subsequent thermal treatment. However, a change to the crystalline, high quantum yield, blue-shifted emissive state could be brought about via mechanical shear force creating sufficiently large nucleation sites. They attributed this to an extremely small ΔG between the red emitting, supercooled liquid and the yellow emitting crystalline solid. The C8H17 substituent afforded this unique functionality while other shorter and longer chains did not. This further demonstrates that the length of an alkyl chain substituent can have substantial effects on thermal properties.29
In this study, four compounds were synthesized in order to probe the consequences of replacing one of the aryl moieties in BF2bdks dyes with either a thiophene or furan heterocycle (Fig. 1). Based on previous work, we reasoned that thiophene and furan heterocycles might bring about more red-shifted emissions with high quantum yields.23 In fact, a recent study by Kolpaczynska et al. revealed that thiophene-substituted BF2bdks displayed red-shifted room temperature phosphorescence (RTP) in a polylactic acid (PLA) matrix.19 To probe alkyl chain length effects, methoxy and dodecyloxy substituents were attached to the 4-positions of the phenyl rings. The optical properties of the dyes were studied in CH2Cl2 solution as well as in the solid state as both films on weighing paper and thinner films on glass substrates. Density functional theory (DFT) calculations were performed to model the HOMO and LUMO molecular orbital (MO) diagrams and the absorption spectra in CH2Cl2 solution. Specifically, BF2tbmOMe and BF2fbmOMe dyes as well as BF2tbmOC3 were explored in order to simulate the effects of chain lengths beyond CH3 on solution optical properties. Pristine powders were studied using differential scanning calorimetry (DSC) to detect thermally induced phase transitions. Powder X-ray diffraction (XRD) analytical techniques were used on both pristine powders and films on glass to gauge the crystallinity of various states. Thermally responsive properties were also investigated.
Experimental methods
Materials
Solvents THF and CH2Cl2 were dried over 3 Å molecular sieves activated at 300 °C as previously described.30 Reactions were monitored using silica TLC plates. Compounds purchased from Sigma-Aldrich were reagent grade and used without further purification. Methyl 4-dodecyloxybenzoate31 and thiophene-substituted dyes19 were synthesized by a previously reported method. Additional synthetic details are provided in the ESI.†Methods
1H NMR (600 MHz) spectra were recorded in dilute CDCl3 solvent using a Varian VRMS/600 instrument. Spectra were referenced to the signals for residual protio-CDCl3 at 7.26 ppm and coupling constants were reported in Hz. Mass spectra were recorded using a Micromass Q-TOF Ultima spectrometer using electrospray ionization (ESI) MS techniques. UV-vis spectra were collected on a Hewlett-Packard 8452A diode-array spectrophotometer. Steady-state fluorescence emission spectra were obtained on a Horiba Fluorolog-3 Model FL3-22 spectrofluorometer (double-grating excitation and double-grating emission monochromator). Time-correlated single-photon counting (TCSPC) fluorescence lifetime measurements were performed with a NanoLED-370 (λex = 369 nm) excitation source and a DataStation Hub as the SPC controller. Lifetime data were analyzed with DataStation v2.4 software from Horiba Jobin Yvon. Fluorescence quantum yields, ΦF, in CH2Cl2 were calculated versus a dilute anthracene solution in ethanol as a standard using a previously described method32 and the following values: ΦF anthracene in ethanol = 0.27,33n20D ethanol = 1.36, n20D CH2Cl2 = 1.424. Optically dilute CH2Cl2 solutions of all samples were prepared in 1 cm path length quartz cuvettes with absorbances <0.1 (a.u.). Powder XRD patterns were obtained using a Panalytical X'Pert Pro MPD diffractometer operating at 40 kV and 40 mA using Cu Kα radiation. DSC was performed on the pristine powders using a TA Instruments DSC 2920 Modulated DSC and data were analyzed using the Universal Analysis software V 2.3 from TA Instruments. Thermograms were recorded using the standard mode and temperature ramp rates of 5 °C min−1 or 10 °C min−1. A conditioning cycle followed by a second heating/cooling cycle were measured for each sample.Films on weighing paper were created by smearing a small amount of dye onto 5 × 5 cm2 pieces of weighing paper with nitrile examination gloves. The samples were weighed to ensure a dye mass of ∼1–3.5 mg spread out over the entire 5 × 5 cm2 area. A Laurel Technologies WS-650S spin-coater was used to make the spin-cast films. The films were fabricated by preparing 10−2 M solutions of the dyes and applying ∼5 drops of these solutions to circular microscope cover glass slides 25 mm in diameter rotating at 3000 rpm. The films were dried in vacuo for 15 min before further processing. Thin films for XRD analysis of BF2fbmOC12 were fabricated by adding 10 drops of a saturated toluene solution to 18 × 18 mm2 square microscope cover glass slides and evaporating the solvent in air, yielding films in the green phase. Orange films were produced by heating green BF2fbmOC12 films above the melting point with a heat gun (i.e. holding the heat source ∼3 cm from the film for ∼1 s) followed by rapid cooling in air at room temperature. Drop-cast films of BF2tbmOC12 were prepared by applying ∼20 drops of a 10−2 M solution to circular microscope cover glass slides 25 mm in diameter. The films were allowed to dry under ambient conditions and then further dried in vacuo for 15 min prior to performing measurements.
Results and discussion
Optical properties in solution
All thiophene and furan compounds showed very similar absorptions and emissions in CH2Cl2 solution with absorbance maxima (λabs) in the range of 413–418 nm and peak emissions (λem) in the range of 437–445 nm (Table 1). Little variation in λabs, λem, and fluorescence lifetime (τF) was observed despite the difference in alkyl chain length and heteroatom substitution (Table 1 and Fig. S1, ESI†). This is typical for this class of dyes.6| Dye | λ abs (nm) | ε (M−1 cm−1) | λ em (nm) | τ F (ns) | Φ F |
|---|---|---|---|---|---|
| a Absorption maxima. b Extinction coefficients calculated at the absorption maxima. c Fluorescence emission maxima excited at 369 nm (except BF2tbmOMe excited at 350 nm). d Fluorescence lifetime excited with a 369 nm light-emitting diode (LED) monitored at the emission maximum. All fluorescence lifetimes are fitted to single-exponential decay. e Relative quantum yield, with anthracene in EtOH as a standard. | |||||
| BF2tbmOMe | 417 | 56![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
445 | 2.06 | 0.38 |
| BF2tbmOC12 | 418 | 62![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
441 | 2.08 | 0.71 |
| BF2fbmOMe | 413 | 28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
437 | 1.93 | 0.35 |
| BF2fbmOC12 | 415 | 65![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
439 | 1.92 | 0.48 |
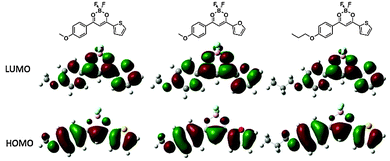
Density functional theory (DFT) was used to model the molecular orbital (MO) diagrams and the absorption spectra in CH2Cl2 solution of BF2tbmOMe and BF2fbmOMe as well as a compound bearing a C3H7 chain (BF2tbmOC3) to simulate alkyl chain lengths beyond three carbons (Fig. 2, Tables S3 and S4, ESI†). The computed absorption spectra reveal the strongest transitions to be from the highest occupied molecular orbital (HOMO) to the lowest unoccupied molecular orbital (LUMO) for all three dyes. Furthermore, these transitions are predominantly π–π* in character, as expected from the experimental data. In the past, the solution properties of BF2bdks have proved to be relatively insensitive to alkyl chain length,6 consistent with the fact that amplitude in the HOMOs and LUMOs does not extend past the first carbon in the alkyl chain. With the exception of BF2tbmOC12 (ΦF = 0.71), all other derivatives showed modest fluorescent quantum yields (ΦF) ranging from ΦF = 0.35 for BF2fbmOMe, to ΦF = 0.48 for BF2fbmOC12.
A trend can be established based on alkyl chain length, as C12H25 derivatives possess higher ΦF relative to their methoxy-substituted counterparts. An increase in ΦF when going from methoxy to dodecyloxy chains has been previously observed for BF2dibenzoylmethane (BF2dbm) derivatives.6
Pristine powders
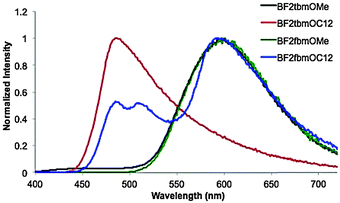
The emissions of dye pristine powders were measured under ambient conditions (Table S1, ESI† and Fig. 3). The OMe derivatives BF2tbmOMe and BF2fbmOMe show very similar broad red-shifted emission; whereas, the C12H25 derivatives show quite different emission spectra. A single short wavelength emission profile was observed for BF2tbmOC12, while BF2fbmOC12 showed a weak, structured blue-shifted peak similar to the thiophene-substituted counterpart and a much more intense, broad, red-shifted peak. Excitation spectra were monitored at the λmax for the blue-shifted and red-shifted transitions. Both excitation spectra were nearly identical from 300–480 nm, suggesting that the two peaks arise from the same ground-state species (Fig. S2, ESI†). This may indicate that the orange emission is due to excimer formation, or energy transfer to other lower energy emitting species in the excited state.34These results indicate that the identity of the heteroatom (i.e. S or O) has very little effect on the emission wavelength of pristine powders and that the dominant factor in determining the emission color is the alkyl chain length. The blue-shifted emission observed in C12H25 powders has been previously ascribed to the steric hindrance of the C12H25 chain limiting dye–dye interactions that red shift emission.6 In methoxy-substituted analogues, dye–dye interactions such as π-stacking and longer range effects that would red shift emission are more probable since the β-diketonate cores are closer together, undiluted by packed alkyl chain domains.6,17 This result is analogous to a study performed by Nguyen et al. wherein BF2dbm derivatives with long alkoxyl chains exhibited more blue-shifted solid-state emissions compared to shorter chain derivatives.6
Thermally responsive emission
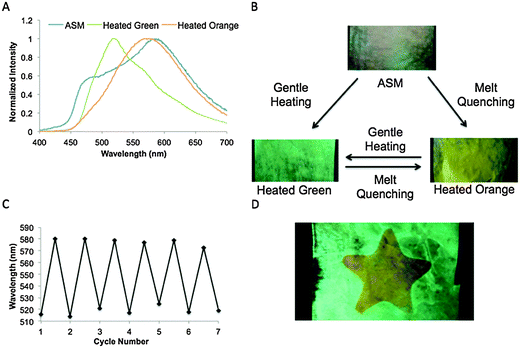
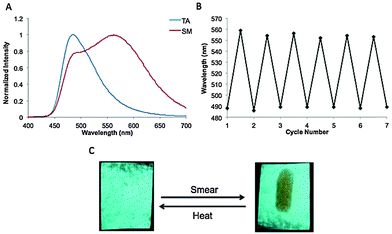
In the process of testing these dyes for ML properties, it was discovered that the long chain furan dye BF2fbmOC12 exhibited highly controllable and reversible changes in emission in response to different methods of heating while the thiophene dye BF2tbmOC12 did not show this same behavior. The emission spectrum of the melted BF2fbmOC12 powder was also obtained. The melted phase of BF2fbmOC12 was accessed by heating the pristine powder above the melting point until melted (∼5 seconds) then rapidly removing the heat source and cooling in an ice bath. The resultant melt-quenched material appeared orange to the eye and the emission spectrum showed only a single broad peak at 581 nm corresponding to the red-shifted peak in the spectrum of the dye as a pristine powder (Fig. 3).Although, this property could be observed for powders, the effect was much more accessible and reversible with films of the material. When the dye was initially smeared into a film on weighing paper and subjected to UV illumination, it was evident that the film represented a heterogeneous mixture of emissive states, one being blue-shifted (λem = ∼470 nm), and the other being red-shifted (λem = 587 nm) and this was corroborated by an emission spectrum of the as-smeared (ASM) dye. If the film was gently heated for five seconds (e.g. with a heat gun from approximately one foot away), a homogenous film with green emission (λem = 519 nm) could be obtained. Heating for one minute in an oven at 125 °C (just below the melting point of 127 °C) produced the same results. The orange emissive state, on the other hand, was accessible via a different method of thermal processing, namely melt quenching. Intense heat sufficient to melt the film was applied (e.g. with a heat gun by holding the heat source approximately one inch from the film). When the heat source was removed rapidly and the film cooled quickly in the air, a homogenous, orange emitting film (λem = 575 nm) was obtained. This state is not achieved if the dye is allowed to cool slowly on a heat conductive surface, such as a laboratory bench top. Instead, under these circumstances, the warm surface provides the gentle heating necessary to access the green emissive state. The orange emissive state was also obtained by submersion of the hot film in liquid N2 which provided sufficiently rapid cooling. Regardless of how the orange state was achieved, the transformation between orange and green emissive states was found to be completely reversible. By cycling back and forth between gentle heating and melt quenching in air, the dye could be carried through seven cycles with no deterioration in responsiveness.
Given that a furan functional group is present in this dye, the heat-induced transition could be due to a Diels–Alder cycloaddition.35 To test this possibility, material from both the orange and green forms were re-dissolved and subjected to UV-vis (Fig. S3, ESI†) and 1H NMR spectral analysis. These analyses suggested that the green and orange-emitting chemical species are identical in terms of chemical composition, inconsistent with formation of a Diels–Alder adduct in one and not the other. Therefore, data are consistent with a thermally induced physical change, not a chemical change.
Like other BF2bdk dye materials, the relatively blue-shifted BF2fbmOC12 dye film may represent a thermodynamically stable, ordered emissive state while the more red-shifted film represents a kinetically accessible, metastable, amorphous emissive state. In addition, lifetimes were recorded for films in both orange and green states (Table S2, ESI†). The orange form had a significantly longer τpw0 (7.61 ns) when compared to the green form (τpw0 = 0.80 ns). This behavior is very similar to other BF2bdk materials exhibiting ML,14,15,20,31 only this time the amorphous, metastable emissive state is accessible via heat instead of mechanical perturbation.
Through experimentation, we were able to discover an immediate potential use of this dye for thermal printing.36 By heating a metal object, applying it to the annealed green dye film, removing it and then quickly cooling by submersion in liquid N2, clear images could be printed (Fig. 4). Since this transformation is reversible, the process can be repeated many times on a single film. Alternative substrates for thermal printing are desirable because many papers currently used contain bisphenol A (BPA), which is potentially hazardous to human health.37 In terms of industrial applications, this material could be used in any process that requires heating to high temperature followed by rapid cooling. Such an example of a potential application could be in the frozen food industry, where many products need to be cooked and then frozen quickly to avoid the formation of large ice crystals, which can damage the product.38 If the dye film were encapsulated in a clear, heat-conductive container attached to the product, it could be used as a quick, easy indicator of quality control.
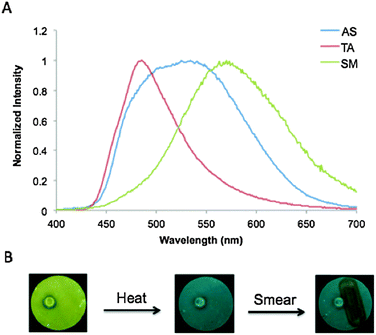
Interestingly, the BF2fbmOC12 dye did not exhibit the same thermally responsive, reversible changes in emission properties as a spin-cast film from CH2Cl2 solution on glass. The AS film was found to have red emission (λem = 597 nm) with a long τpw0 under UV illumination (Fig. 5 and Table S2, ESI†). Whether the film was gently heated with a heat gun or annealed in an oven, only a red, long-lifetime emission remained and the green emissive state could not be obtained. Therefore, it appears that spin casting locks this dye into a red-shifted emissive state. This is likely a processing or thickness effect that, at this time, we are unable to explain. However, it was possible to fabricate thermally responsive films of the BF2fbmOC12 dye on glass by drop-casting from a saturated toluene solution. Slow evaporation yielded a glass film of BF2fbmOC12 in the green phase (λem = 512 nm) and the orange phase (λem = 551 nm) was produced through the melting and room temperature cooling of the green thin films using a heat gun in the same way as the films on weighing paper (Fig. 5).
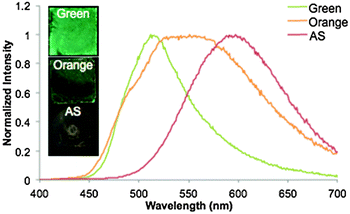 | ||
| Fig. 5 Emission of BF2fbmOC12 as films on glass. Drop-cast films on glass from toluene solutions in both green and orange forms are compared to a spin-cast film from CH2Cl2 solution (AS = as spun). | ||
Curiously, the C12H25 derivative of the furan-substituted dye exhibited stimuli responsive solid-state emission while the methoxy-substituted counterpart did not have this property. A previous study has shown that incorporation of an alkoxyl chain longer than OCH3 into difluoroboron dibenzoylmethane (dbm) fluorophores can grant ML properties.6 Perhaps the ability of the alkoxyl chains to break up closer intermolecular π–π interactions creates a greater variety of available states that may be accessed through different processing methods. In this example and others,39–41 the addition of long alkoxyl chain substituents to solid-state emitting fluorophores is an effective strategy for imparting switchable luminescence behavior. Both Yang et al.41 and Xu et al.40 have both reported organic molecules with mechanically switchable emissive behavior in the solid state brought about by the addition of long aliphatic chains. They proposed, with emission and X-ray diffraction data, that the alkyl chains disrupt close supramolecular interactions such as π–π stacking. They believe this balancing of π–π and aliphatic interactions can create multiple emissive states and thus impart switchable emissive behavior.40
Differential scanning calorimetry
The dyes BF2tbmOC12 and BF2fbmOC12 show thermally responsive emission whereas their methoxy-substituted counterparts do not. The differences between these samples and the nature of the thermally accessible green and orange states of BF2fbmOC12 were investigated using differential scanning calorimetry (DSC). Samples were heated above their melting points and cooled to 0 °C at a constant ramp rate (5 °C min−1 or 10 °C min−1).The thermal properties were reported using thermograms of the second heating/cooling cycle of each compound (Table 2 and Fig. S4, ESI†). The melting points of methoxy-substituted BF2tbmOMe (265.9 °C) and BF2fbmOMe (216.5 °C) were higher compared to their alkoxylated counterparts BF2tbmOC12 (137.0 °C) and BF2fbmOC12 (127.7 °C). The lower melting points observed in C12H25 samples is further evidence that the alkoxyl chains are capable of disrupting stronger intermolecular interactions. In general, thiophene dyes had slightly higher melting points than furan dyes, but the alkoxyl chain length, by far, had the greatest effect on melting temperature.
| T m (°C) | ΔHc (kJ mol−1) | T c (°C) | ΔHc (kJ mol−1) | |
|---|---|---|---|---|
| a All data was taken from the 2nd cycle. b Melting point given in °C as the peak of the major endothermic transition. c Enthalpy of the transition given in kJ mol−1. d Crystallization point given in °C as the peak of the major exothermic transition. | ||||
| BF2tbmOMe | 265.9 | 65.0 | 197.2 | 61.3 |
| BF2tbmOC12 | 137.0 | 505.7 | 111.8 | 515.6 |
| BF2fbmOMe | 216.5 | 142.3 | 163.0 | 101.1 |
| BF2fbmOC12 | 127.7 | 526.2 | 103.4 | 520.9 |
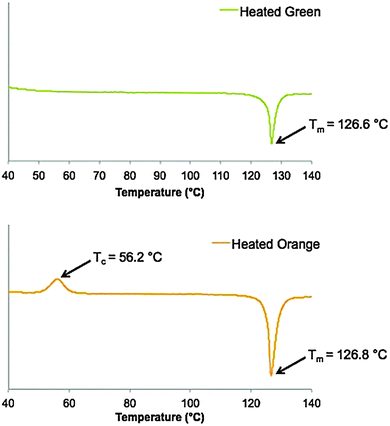
In order to investigate transformations in BF2fbmOC12 brought about by heat, the DSC thermograms of the first heating cycle of both the green and orange powders with a constant ramp rate (10 °C min−1) were compared (Fig. 6). Because BF2tbmOC12 does not respond to heat in the same way as BF2fbmOC12, only the thermal properties of BF2fbmOC12 were studied in depth. When the green form of BF2fbmOC12 was heated only the melting point was observed at 127 °C, but an additional crystallization peak at 56.2 °C was observed when the orange form was heated. Chujo and coworkers have reported similar behavior for powders of difluoroboron β-diiminates in crystalline and amorphous emissive forms.42 This is further evidence that the orange form represents an amorphous, metastable state while the green form represents a thermodynamically stable, crystalline state.
However, one question remains. Why does the furan-substituted derivative exhibit this thermally responsive switchable behavior while the thiophene-substituted dye does not? The answer may be found in comparing π-stacking energies and intermolecular interactions. According to a computational study performed by Huber et al., furan forms a slightly closer and stronger π-stacking interaction with benzene than thiophene due to the smaller size of the oxygen atom compared to sulfur.43 Furthermore, it stands to reason that the stronger hydrogen bonding affinity afforded by the oxygen atom could also contribute to closer interactions between molecules. If the furan-substituted dye molecules interact with each other strongly in the H-aggregates present in the amorphous form,20 a larger energy barrier would have to be overcome to switch from the amorphous to the crystalline emissive form. This could explain why the films and powders of BF2fbmOC12 become trapped in the amorphous phase when their melts are rapidly quenched. The answer could also lie in the favorability of the ordered emissive state over the amorphous state for the BF2tbmOC12 dye. The pristine powder of this dye showed preference for the ordered emissive, blue state and could not be easily converted to the amorphous phase like its furan counterpart. Also the melting point of the BF2tbmOC12 dye pristine powder (always in the blue form) is higher than that of the furan dye, suggesting a greater degree of stability.
Mechanochromic luminescence
Although BF2fbmOC12 showed excellent responsiveness to heating, this dye and both methoxy-substituted dyes did not exhibit the ML properties typical for many BF2bdks.6,7,14 However, BF2tbmOC12 did possess ML properties. While BF2bdks exhibit a degree of mechano-responsiveness as bulk powders, that can be activated by prolonged grinding, this property is greatly amplified when the dye materials are fabricated as films.6,7,14,22,31 Therefore the dye was fabricated into both thin films on glass substrates and thicker films on weighing paper substrates. When the BF2tbmOC12 dye weighing paper film is annealed for ten minutes at 130 °C (below the melting temperature of 137 °C), it exhibits blue emission and the spectrum reveals a relatively narrow peak (485 nm) (Fig. 7). When the thermally annealed (TA) film is smeared with a cotton swab, the emission changes to yellow and the spectrum reveals a much broader red-shifted peak (562 nm). This 77 nm shift in emission after smearing represents a high contrast ML material. Furthermore, the ML property is highly reversible. A process of annealing a film at 130 °C for a minute and then smearing was repeated seven times without any noticeable decline in responsiveness. This excellent reversibility could make this dye useful in many applications in which force responsiveness is required.To test for thickness and substrate effects, spin-cast films of BF2tbmOC12 on glass were also fabricated. The process by which spin-cast films are fabricated allow for much thinner films than can be achieved by smearing dye powders onto paper substrates. In the past, we have observed that thinner spin-cast films on glass display slightly different properties than films on weighing paper. The most prominent of these differences is more blue-shifted and narrow fluorescence emission spectra for annealed spin-cast films versus films on weighing paper.7 Immediately after spin-casting, it could be seen that the as-spun (AS) state was unique and unlike what was observed for both the TA and SM states on weighing paper substrate (Fig. 8). In the case of BF2tbmOC12, no major differences in solid-state emission properties were observed between thin spin-cast films and thicker films on weighing paper, indicating that this dye is less sensitive to thickness and substrate effects than other BF2bdk materials.7 The AS form seems to represent a combination of the blue and red emissive states, similar to other BF2bdk dye films exhibiting ML, but here there is a large gap in the peak emissions of the TA and SM forms.7 Lifetime measurements were also performed on spin-cast films in all three forms (Table S2, ESI†). As is typical for BF2bdk dyes, the red-shifted, SM emission had a much longer lifetime (τpw0 = 12.12 ns) than the TA emission (τpw0 = 7.03 ns). This, along with the broadening of the peak upon smearing, suggests the formation of excimeric or unique ground-state aggregates.7,20,22
Just as with furan derivatives, the thiophene dye substituted with OC12H25 exhibited switchable emissions in the solid state while its methoxy-substituted counterpart did not. This is presumably due to the disruption of close supramolecular interactions.
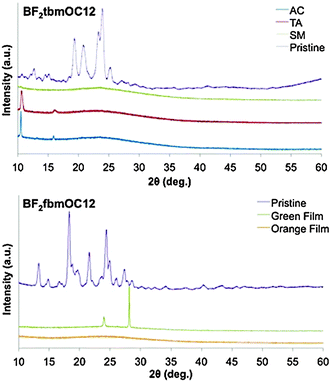
X-ray diffraction of films
X-ray diffraction (XRD) diffractograms were obtained for pristine powders of the BF2tbmOC12 and BF2fbmOC12 dyes and compared to those of the drop cast films on microscope cover glass in various forms in order to glean information about the amorphous versus crystalline states of these dyes (Fig. 9). Drop-cast films of the BF2tbmOC12 dye exhibited behavior typical of ML dye films.7,22 There was no change between the as-cast (AC) and thermally annealed (TA) films, with both exhibiting a relatively strong peak at ∼10° and a much smaller peak at ∼15°. This suggests that the dye film is crystalline both in the AC and TA states. The diffractogram of the smeared film was devoid of peaks, confirming the amorphous nature of the smeared films.In order to probe the thermally responsive behavior of BF2fbmOC12, the powder XRD patterns of orange and green drop-cast films on glass were compared to the diffraction patterns of the pristine powder (Fig. 9). No peaks were observed in the diffraction pattern of the orange film, whereas sharp peaks at ∼24° and ∼28° were detected in the diffraction pattern of the green film. The pattern of the pristine powder also displayed peaks near these same angles. These results indicate that the orange phase generated by melt quenching BF2fbmOC12 is amorphous and the green phase produced via gentle heating of BF2fbmOC12 is crystalline and both are likely to be present in the pristine powder, just as emission spectra and lifetime data suggest.
Conclusion
In summary, four solid-state emissive difluoroboron β-diketonate dyes bearing thiophene and furan substituents as well as short (OMe) and long (OC12H25) alkoxyl chains were synthesized. While the length of the chain had little effect on the optical properties of the dyes in solution, the C12H25 alkoxyl chain afforded both the thiophene and furan substituted dyes unique, switchable solid-state luminescence features. The BF2tbmOC12 dye exhibited high-contrast (Δλmax = 77 nm) reversible ML as both films on weighing paper and glass substrates. Powder XRD revealed the blue-shifted, thermally annealed (TA) form of the dye to be crystalline in nature while the red-shifted, smeared (SM) form was amorphous. Furthermore, this dye could be carried through at least seven cycles of annealing and smearing without any noticeable deterioration in the responsiveness.The BF2fbmOC12 dye showed similar dual emissive behavior as films on glass or weighing paper, but the stimulus required for switching of emission was different. By gently heating the sample a green emissive form could be obtained. An orange emissive form could be obtained via melt quenching. This could be achieved by melting the film and then quickly removing the heat source, allowing the film to cool quickly either while held in the air or submerging in liquid N2. Powder XRD of drop-cast films in both emissive forms revealed the green form to be crystalline with well-defined peaks in the diffractograms while the orange form was amorphous with no peaks. The unique responsiveness could make this dye useful as a quality control indicator for any process requiring intense heating followed by rapid cooling.
Because both dyes bearing OC12H25 alkoxyl chains exhibited switchable emissive behavior while the methoxy-substituted counterparts did not, adding a long alkyl chain substituent to π-stacking, solid-state emitting fluorophores could be an effective strategy for inducing switchable luminescent behavior. Presumably, this is due to the chains disrupting close intermolecular interactions such as π-stacking and thus enabling more available molecular conformations. Finally, the difference in thermally responsive emissive behavior between BF2tbmOC12 and BF2fbmOC12 may be attributed to closer intermolecular interactions in the H-aggregated furan dye causing the material to become trapped in the amorphous state when it is heated to the melting point and then the melt is rapidly quenched. It may also be attributed to a much more stable ordered emissive state for the BF2tbmOC12 material.
Acknowledgements
We thank the National Science Foundation (CHE 1213915) and the National Institutes of Health (R01 CA167250) for support for this research. We thank Christopher DeRosa for assistance and helpful discussions.References
- K. Tanaka and Y. Chujo, NPG Asia Mater., 2015, 7, e223 CrossRef CAS.
- R. Tan, Q. Lin, Y. Wen, S. Xiao, S. Wang, R. Zhang and T. Yi, CrystEngComm, 2015, 17, 6674 RSC.
- C. A. DeRosa, C. Kerr, Z. Fan, M. Kolpaczynska, A. S. Mathew, R. E. Evans, G. Zhang and C. L. Fraser, ACS Appl. Mater. Interfaces, 2015, 7, 23633 CAS.
- T. Butler, W. A. Morris, J. Samonina-Kosicka and C. L. Fraser, Chem. Commun., 2015, 51, 3359 RSC.
- A. G. Mirochnik, B. V. Bukvetskii, E. V. Fedorenko and V. E. Karasev, Russ. Chem. Bull., 2004, 53, 291 CrossRef CAS.
- N. D. Nguyen, G. Zhang, J. Lu, A. E. Sherman and C. L. Fraser, J. Mater. Chem., 2011, 21, 8409 RSC.
- W. A. Morris, T. Liu and C. L. Fraser, J. Mater. Chem. C, 2015, 352 RSC.
- E. Cogné-Laage, J.-F. Allemand, O. Ruel, J.-B. Baudin, V. Croquette, M. Blanchard-Desce and L. Jullien, Chem. – Eur. J., 2004, 10, 1445 CrossRef PubMed.
- Y. L. Chow, C. I. Johansson, Y.-H. Zhang, R. Gautron, L. Yang, A. Rassat and S.-Z. Yang, J. Phys. Org. Chem., 1996, 9, 7 CrossRef CAS.
- K. Ono, K. Yoshikawa, Y. Tsuji, H. Yamaguchi, R. Uozumi, M. Tomura, K. Taga and K. Saito, Tetrahedron, 2007, 63, 9354 CrossRef CAS.
- M. Halik, W. Wenseleers, C. Grasso, F. Stellacci, E. Zojer, S. Barlow, J.-L. Bredas, J. W. Perry and S. R. Marder, Chem. Commun., 2003, 1490 RSC.
- T. Liu, A. D. Chien, J. Lu, G. Zhang and C. L. Fraser, J. Mater. Chem., 2011, 21, 8401 RSC.
- S. Xu, R. E. Evans, T. Liu, G. Zhang, J. N. Demas, C. O. Trindle and C. L. Fraser, Inorg. Chem., 2013, 52, 3597 CrossRef CAS PubMed.
- G. Zhang, J. Lu, M. Sabat and C. L. Fraser, J. Am. Chem. Soc., 2010, 132, 2160 CrossRef CAS PubMed.
- T. Butler, W. A. Morris, J. Samonina-Kosicka and C. L. Fraser, ACS Appl. Mater. Interfaces, 2016, 8, 1242 CAS.
- G. Zhang, G. M. Palmer, M. W. Dewhirst and C. L. Fraser, Nat. Mater., 2009, 8, 747 CrossRef CAS PubMed.
- J. Samonina-Kosicka, C. A. DeRosa, W. A. Morris, Z. Fan and C. L. Fraser, Macromolecules, 2014, 47, 3736 CrossRef CAS PubMed.
- C. A. DeRosa, J. Samonina-Kosicka, Z. Fan, H. C. Hendargo, D. H. Weitzel, G. M. Palmer and C. L. Fraser, Macromolecules, 2015, 48, 2967 CrossRef CAS PubMed.
- M. Kolpaczynska, C. A. DeRosa, W. A. Morris and C. L. Fraser, Aust. J. Chem., 2016, 69, 537 CrossRef CAS.
- X. Sun, X. Zhang, X. Li, S. Liu and G. Zhang, J. Mater. Chem., 2012, 22, 17332 RSC.
- R. Yoshii, K. Suenaga, K. Tanaka and Y. Chujo, Chem. – Eur. J., 2015, 4506 Search PubMed.
- N. D. Nguyen, G.-Q. Zhang, J.-W. Lu, A. E. Sherman and C. L. Fraser, J. Mater. Chem., 2011, 21, 8409 RSC.
- S. J. Evenson, T. M. Pappenfus, M. C. R. Delgado, K. R. Radke-Wohlers, J. T. L. Navarrete and S. C. Rasmussen, Phys. Chem. Chem. Phys., 2012, 14, 6101 RSC.
- S. C. Rasmussen, S. J. Evenson and C. B. McCausland, Chem. Commun., 2015, 51, 4528 RSC.
- C.-T. Poon, D. Wu, W. H. Lam and V. W.-W. Yam, Angew. Chem., Int. Ed., 2015, 54, 10569 CrossRef CAS PubMed.
- M. Mitani, S. Ogata, S. Yamane, M. Yoshio, M. Hasegawa and T. Kato, J. Mater. Chem. C, 2015, 4, 2752 RSC.
- N. J. Greco and Y. Tor, Tetrahedron, 2007, 63, 3515 CrossRef CAS PubMed.
- K. C. Naeem, A. Subhakumari, S. Varughese and V. C. Nair, J. Mater. Chem. C, 2015, 3, 10225 RSC.
- K. Chung, M. S. Kwon, B. M. Leung, A. G. Wong-Foy, M. S. Kim, J. Kim, S. Takayama, J. Gierschner, A. J. Matzger and J. Kim, ACS Cent. Sci., 2015, 1, 94 CrossRef CAS PubMed.
- D. B. G. Williams and M. Lawton, J. Org. Chem., 2010, 75, 8351 CrossRef CAS PubMed.
- G. Zhang, J. P. Singer, S. E. Kooi, R. E. Evans, E. L. Thomas and C. L. Fraser, J. Mater. Chem., 2011, 21, 8295 RSC.
- E. R. Carraway, J. N. Demas, B. A. DeGraff and J. R. Bacon, Anal. Chem., 1991, 63, 337 CrossRef CAS.
- C. A. Heller, R. A. Henry, B. A. McLaughlin and D. E. Bliss, J. Chem. Eng. Data, 1974, 19, 214 CrossRef CAS.
- X. Z. Sun, X. Li, S. Liu and G. Zhang, J. Mater. Chem., 2012, 22, 17332 RSC.
- O. C. Kappe, S. S. Murphree and A. Padwa, Tetrahedron, 1997, 53, 14179 CrossRef.
- S. Shibata, K. Murasugi and K. Kaminishi, IEEE Trans. Parts, Hybrids, Packag., 1976, 12, 223 CrossRef.
- S. Biedermann, P. Tschudin and K. Grob, Anal. Bioanal. Chem., 2010, 398, 571 CrossRef CAS PubMed.
- D.-W. Sun, Advances in Food Refrigeration, Leatherhead Food RA Pub., Leatherhead, 2001 Search PubMed.
- M. Sase, S. Yamaguchi, Y. Sagara, I. Yoshikawa, T. Mutai and K. Araki, J. Mater. Chem., 2011, 21, 8347 RSC.
- X. Zhang, Z. Chi, B. Xu, L. Jiang, X. Zhou, Y. Zhang, S. Liu and J. Xu, Chem. Commun., 2012, 48, 10895 RSC.
- W. Liu, Y. Wang, L. Bu, J. Li, M. Sun, D. Zhang, M. Zheng, C. Yang, S. Xue and W. Yang, J. Lumin., 2013, 143, 50 CrossRef CAS.
- R. Yoshii, A. Hirose, K. Tanaka and Y. Chujo, Chem. – Eur. J., 2014, 20, 8320 CrossRef CAS PubMed.
- R. G. Huber, M. A. Margreiter, J. E. Fuchs, S. von Grafenstein, C. S. Tautermann, K. R. Liedl and T. Fox, J. Chem. Inf. Model., 2014, 54, 1371 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Additional synthetic, spectral and computational details. See DOI: 10.1039/c6qm00008h |
| This journal is © the Partner Organisations 2017 |