Investigation on electrochemical behaviors of NiCo2O4 battery-type supercapacitor electrodes: the role of an aqueous electrolyte
Abstract
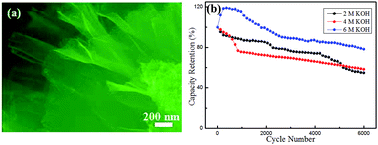
Mesoporous NiCo2O4 flower-like structures are prepared successfully by a facile solvothermal route. The electrochemical performance of the as-synthesized products as electrode materials for battery-type supercapacitors is systematically investigated at various concentrations of potassium hydroxide (KOH) aqueous solution by cyclic voltammetry, galvanostatic charge/discharge and electrochemical impedance spectroscopy. NiCo2O4 supercapacitor electrode in the 6 M KOH electrolyte delivers high specific capacity (122.5 C g−1 at 1 A g−1), excellent rate performance (82.5 C g−1 at 10 A g−1) and cycling stability (21.7% capacity loss after 6000 cycles at 2 A g−1). The results show that KOH electrolyte at high concentrations plays an important role in improving electrochemical performance of NiCo2O4 battery-type supercapacitor electrodes.
- This article is part of the themed collection: Inorganic Chemistry Frontiers HOT articles for 2017


 Please wait while we load your content...
Please wait while we load your content...