Li6Zn3(BO3)4: a new zincoborate featuring vertex-, edge- and face-sharing LiO4 tetrahedra and exhibiting reversible phase transitions†
Abstract
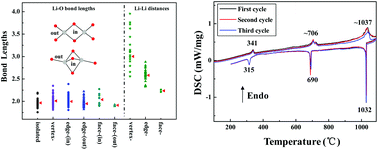
A new lithium zincoborate Li6Zn3(BO3)4 has been successfully prepared via a high-temperature solution method. Li6Zn3(BO3)4 is composed of LiO4 and ZnO4 tetrahedra, and nearly coplanar BO3 units. A face-sharing LiO4 configuration was found in the title compound, and a comparison of Li–O configurations was systemically investigated for all Li-containing borates. Interestingly, Li6Zn3(BO3)4 has two reversible phase transitions at about 341 and 706 °C, which implies that Li6Zn3(BO3)4 is a potential candidate as a phase change material. In situ high-temperature X-ray diffraction (XRD) and differential scanning calorimetry (DSC) analysis were used to verify the two reversible phase transitions. The linear optical properties were researched in detail using ab initio density functional theory (DFT).


 Please wait while we load your content...
Please wait while we load your content...